Abstract
Discrepant effects of drugs on behavior maintained by temporal-discrimination procedures make conclusive statements about the neuropharmacological bases of timing difficult. The current experiment examined the possible contribution of a general, drug-induced disruption of stimulus control. Four pigeons responded on a three-component multiple schedule that included a fixed-interval 2-min, temporal discrimination, and color-matching component. Under control conditions, response rates and choice responses during the first two components showed evidence of control by time, and accuracy for color matching was high in the third component. Morphine administration flattened the distribution of fixed-interval responding and produced a general disruption of accuracy in the temporal-discrimination component, whereas accuracy in the color-matching component was relatively unaffected. Analysis of the psychophysical functions from the temporal-discrimination component indicated that morphine decreased accuracy of temporal discrimination by decreasing overall stimulus control, rather than by selectively affecting timing. These results suggest the importance of determining the neurophysiological bases of stimulus control as it relates to temporal discrimination.
Keywords: morphine, timing, stimulus control, temporal discrimination, key peck, pigeons
The neurophysiological processes underlying temporal regulation of behavior and accurate discrimination of temporal duration have been of increasing interest in recent years. Research with both humans and nonhumans has led to the formation of several theoretical accounts that attempt to explain the environmental and neurophysiological underpinnings of accurate temporal discrimination. For example, prominent theoretical accounts of the neurophysiological basis of timing, such as the generalized timing model (e.g., Matell, Meck, & Nicolelis, 2003), hypothesize elaborate neurologically based information processing systems. According to these models, accurate discrimination of duration is governed by internal-clock mechanisms composed of pacemakers and accumulators. Based on input from these systems interacting with memory for recent temporal events, an organism is able to accurately discriminate and respond based on temporal stimuli.
Although models of temporal processing have led to productive research aimed at describing the neuroanatomical correlates of temporal discrimination (e.g., Gibbon, Malapani, Dale, & Gallistel, 1997; Meck, 1996), conclusive statements about the neural and biochemical basis of timing remain elusive (see Gibbon et al., 1997). A growing body of research has provided support for the role that dopamine and other neurotransmitters play in accurate timing of relevant temporal events (e.g., Buhusi, 2003; Hinton & Meck, 1997; Meck, 1996). In spite of these advances, further work is needed to understand more precisely how neuropharmacological and behavioral processes contribute to accurate discrimination of temporal stimuli (see Richelle & Lejuene, 1998).
To complicate matters, a growing number of discrepant findings have been reported in which the same drug, or drugs from the same pharmacological class, produced different effects on behavior maintained by a variety of temporal-discrimination procedures (e.g., Chiang et al., 2000; Frederick & Allen, 1996; Knealing & Schaal, 2002; Odum, Lieving, & Schaal, 2002; Santi, Coppa, & Ross, 2001). For example, some researchers have reported that amphetamine results in overestimation of time (e.g., Chiang et al., 2000; Meck, 1996), whereas some have found generalized disruption of timing or even underestimation of time (e.g., Chiang et al., 2000). The reasons for these discrepant results are unclear. To date, analyses have suggested that variables such as species, sex, route of drug administration, and procedural variations cannot fully account for the discrepant outcomes (Çevik, 2003; Odum, 2002; Odum et al., 2002; Odum & Ward, 2004).
One possible explanation for these discrepant results is that drug administration results in a general decrease in discrimination of all types of stimuli, rather than in selective changes in the neuropharmacological mechanisms responsible for timing. If this were the case, then, following drug administration, choice responses in temporal-discrimination procedures may no longer be under the functional control of the presented sample durations. In fact, drugs have been reported to affect performance on a variety of discrimination procedures. For example, in one early study Berryman, Cumming, Nevin, and Jarvik (1964) reported that sodium pentobarbital dose-dependently decreased accuracy in a color matching-to-sample task in pigeons. In a more recent study, Andrews and Holtzman (1988) assessed the effects of morphine and amphetamine on performance in a visual discrimination procedure in rats. In their procedure, responses to one of two levers were reinforced if a stimulus light had been briefly flashed above the lever at the beginning of the trial. In this procedure, although amphetamine had relatively little effect, morphine produced a dose-dependent decrease in accuracy.
In addition to these studies, others have reported that drugs from a variety of pharmacological classes have disrupted performance on discrimination procedures in rats (e.g., Grilly, Genovese, & Nowak, 1980; Koek & Slangen, 1983, 1984), pigeons (e.g., Berryman, Jarvik, & Nevin, 1962; Eckerman, Lanson, & Berryman, 1978; Picker, Massie, & Dykstra, 1987), and monkeys (e.g., Dykstra, 1979; Ridley, Baker, & Weight, 1980). These results, when considered along with the discrepant results in the timing literature mentioned above, suggest the importance of experimental preparations and methods that clearly can distinguish selective effects of drugs on timing from effects that occur as a result of a more general disruption of stimulus control.
Few studies have attempted to assess simultaneously the effects of drugs on accuracy of temporal and other types of discriminations. Santi, Weise, and Kuiper (1995) assessed the effects of amphetamine on performance on a temporal discrimination and a visual symbolic matching-to-sample procedure in pigeons. Amphetamine disrupted accuracy for temporal discrimination more than accuracy for symbolic matching-to-sample. In addition, contrary to prominent theoretical predictions (e.g., Meck, 1996), amphetamine did not produce overestimation of time. Santi et al. suggested that their results were due to disruption of attention to the temporal sample stimuli rather than selective changes in timing.
The present experiment further examined how drugs simultaneously affect the stimulus control engendered by temporal and color samples. We used a multiple schedule in which we assessed the effects of morphine on performance during fixed-interval, temporal discrimination, and color-matching components. Morphine was used because it has been shown to disrupt performance in a temporal-discrimination procedure (e.g., Odum & Ward, 2004) and also has been shown to disrupt performance in a visual discrimination procedure by decreasing the discriminability of the sample stimuli (Koek & Slangen, 1984). We reasoned that if the neuropharmacological effects of morphine are specific to timing, then we should see clear disruption of temporal discrimination, with little or no disruption of color matching. If, however, morphine produces a general disruption in stimulus control, then we should see changes in accuracy for color matching as well as temporal discrimination. In addition, performance during the temporal-discrimination component was analyzed using a method suggested by Blough (1996), which can distinguish drug effects due to changes in timing from those effects due to disruption of stimulus control.
Method
Subjects
Four experimentally naive White Carneau pigeons served as subjects. Pigeons were maintained at 80% ± 15 g of their free-feeding weight by postsession feeding as needed. Between sessions, pigeons were individually housed in a temperature-controlled colony under a 12∶12 hr light/dark cycle and had free access to water and digestive grit.
Apparatus
Four BRS/LVE sound-attenuating chambers were used. Chambers were constructed of painted metal with aluminum front panels. The chambers measured 35 cm across, 30.7 cm deep, and 35.8 cm high. Each front panel had three translucent plastic keys that could be lit from behind with red, green, yellow, and blue light. Keys also could be lit with a variety of horizontal and vertical line stimuli, and required a force of at least 0.10 N to record a response. Keys were 2.6 cm in diameter and 24.6 cm from the floor. A lamp (28 V, 1.1 W) mounted 4.4 cm above the center key served as a houselight. A rectangular opening 9 cm below the center key provided access to a solenoid-operated hopper filled with pelleted pigeon chow. During hopper presentations, the opening was lit with white light and the houselight and keylights were extinguished. White noise and chamber ventilation fans masked extraneous noise. Contingencies were programmed and data collected by a microcomputer located in an adjacent room using Med Associates® interfacing and software.
Procedure
Pretraining
Experimental sessions occurred 7 days a week at approximately the same time. Following magazine training, the pigeons were exposed to an autoshaping procedure (Brown & Jenkins, 1968). During these sessions, all key colors and stimuli were presented in the key locations in which they would appear during the experiment. Following three sessions of autoshaping, the pigeons reliably pecked all key colors and stimuli to be used in the experiment. Key pecking was then maintained on a fixed-interval (FI) schedule of food delivery of progressively increasing duration until an FI 2-min schedule was reached. During these sessions, the center key was lit with three black vertical lines on a white background. The FI 2-min schedule was in effect for six sessions prior to multiple-schedule training.
Multiple Schedule Training
The procedure was a three-component multiple schedule that included FI 2-min, temporal discrimination, and color-matching components. During initial training, these components were presented in random order with the requirement that each be presented 14 times during the session and no component occur more than two times in a row. Components were separated by a 30-s intercomponent interval (ICI) during which all keylights and the houselight were extinguished. Pecks to the keys during the ICI had no programmed consequences. To allow time for drug absorption prior to selected sessions, all sessions began with a 10-min chamber blackout. Following the blackout, the houselight and center key were lit to begin the session. Temporal discrimination and color-matching components were preceded by the lighting of the center key with three black horizontal lines on a white background. This key served as a trial-ready stimulus to ensure that the pigeon was attending to the sample. A peck to the center key randomly produced either a temporal discrimination or color-matching trial.
Temporal-discrimination Component
A peck to the center key darkened the keylight and turned off the houselight for a period of 2 or 8 s. This blackout duration constituted the temporal sample for each trial. Sample durations were randomly selected each trial with the constraint that each sample duration be presented an equal number of times during the session. Following the sample presentation, the left and right keys were lit different colors, each color corresponding to either a short or a long sample duration. The location of each color (left or right key) was randomly determined from trial to trial (e.g., Stubbs, 1968). A peck to the key that was the color that corresponded to the duration of the temporal sample (short or long) resulted in 3-s access to food. A peck to the key that was the other color produced a 3-s blackout. Key colors were counterbalanced across pigeons in case of a systematic drug-induced color bias (see, e.g., Wenger, McMillan, Moore, & Williamson, 1995). For Pigeons P211 and P212, the colors during the temporal-discrimination component were green and red. For P211, green corresponded to a short sample duration and red to a long duration. This color assignment was reversed for P212. For Pigeons P213 and P214, the colors during the temporal-discrimination component were blue and yellow. For P213, yellow corresponded to a short sample duration and blue to a long duration. This color assignment was reversed for P214.
Color-matching Component
A peck to the center key extinguished the trial-ready stimulus and lit the key with a color sample for 2 s. The center key then was extinguished and the side keys were lit different colors. The location of each color (left or right key) varied randomly from trial to trial. A peck to the key that matched the sample color led to 3-s access to food, and a peck to the key that did not match the sample color led to a 3-s blackout. Key colors during this component were counterbalanced. For Pigeons P211 and P212, the colors during the color-matching component were blue and yellow. For P213 and P214, the colors were green and red.
FI Component
The center key was lit with three black vertical lines on a white background. The first peck after 2 min resulted in 3-s access to food.
After color-matching and temporal discrimination accuracies were at least 80% over the last 10 sessions, intermediate sample durations of 3, 4, 4.5, 5.5, 6, and 7 s were inserted into the temporal-discrimination component. Sample durations of less than 5 s were considered short and sample durations of more than 5 s were considered long. Correct categorization of the intermediate sample durations was reinforced. A 3-min limited hold was instituted during each component. If a response did not occur within 3 min, all keylights and the houselight were extinguished and a 30-s ICI occurred, after which a new component was randomly selected. The number of component presentations of each type was changed to eight FI, 40 temporal discrimination, and eight color-matching components. Each temporal sample duration was thus presented five times during each session. Sessions ended after 56 trials or 90 min, whichever occurred first. Sessions usually ended after 56 trials in approximately 70 min.
Correction Procedure
During training, when temporal discrimination or color-matching accuracy was low because of a pronounced color or side bias, a correction procedure was instated. In this procedure, a peck to the incorrect side key was followed by a 3-s blackout. The entire trial was then repeated, with the same sample duration or color, and the side keys lit with the same colors in the same positions. This process continued until a correct choice ended the trial in food. All pigeons experienced the correction procedure at some point during training. The correction procedure was not in effect during the drug-testing phase.
Morphine Tests
Drug testing began for individual pigeons when accuracy in the temporal discrimination and color-matching components was high and stable and rates of responding and the index of curvature (a measure of temporal patterning; Fry, Kelleher, & Cook, 1960) during the FI were stable (without any evident trend or unusual variability) as judged by visual inspection over the last 10 sessions. Responding met these criteria within 112 to 164 sessions, across pigeons.
Morphine sulfate (Sigma) was dissolved in 0.9% saline and administered in a volume of 1.0 ml/kg of the 80% free-feeding body weight. Morphine and vehicle were administered via intramuscular injections into the breast immediately before the pigeon was placed in the experimental chamber. To accustom the pigeons to the injection procedure, they were given one to three preliminary injections of saline. Results of these injections were excluded from the analyses.
Following the preliminary injections, morphine and vehicle were given in the following order: 1.0 mg/kg, 3.0 mg/kg, 0.56 mg/kg, 5.6 mg/kg, and saline. These doses were chosen because they produce a wide range of effects of morphine (e.g., Odum & Ward, 2004). Tests were separated by at least three consecutive baseline sessions not preceded by an injection. The session immediately preceding a morphine or vehicle session was designated as a control session. Dose-effect curves were determined with all doses before any dose was repeated. The effects of saline and each drug dose were determined four times for each pigeon. Data from the color matching and temporal-discrimination components during sessions preceded by drug administration were included in analyses only if at least half of the presented components of each type were completed. For Pigeon P211, data were excluded on these grounds for one session following administration of 3.0 and 5.6 mg/kg. For Pigeon P212, data were excluded for one session following administration of 3.0 mg/kg and for two sessions following administration of 5.6 mg/kg. For Pigeon P213, data were excluded for 5.6 mg/kg, as this pigeon responded during only one session following administration of this dose. Data from all sessions were included for Pigeon P214.
Results
Figures 1 and 2 show the effects of morphine on FI performance. Figure 1 shows overall response rates during the FI during control sessions and as a function of morphine. Saline had no systematic effect on overall rates of key pecking. Morphine decreased rates of pecking somewhat, although in some cases the decreases were relatively small. Figure 2 shows the index of curvature (Fry et al., 1960) for all pigeons during control sessions and as a function of increasing morphine dose. The index of curvature is a measure of the proportional distribution of responses across fixed intervals. Fixed intervals were divided into ten 12-s bins. The number of responses that occurred in each bin was summed across the session for the FI component. The index of curvature then was calculated for each session using the following formula from Fry et al. (1960):
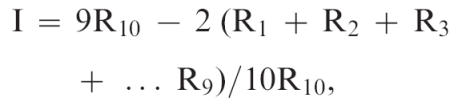 |
where R1 is the total number of responses occurring in the first bin, R2 is the total number of responses occurring in the first and second bin, R3 is the total number of responses occurring in the first, second, and third bins, and so on until R10, which is the total number of responses occurring in all bins. Calculated in this way, the possible range of the index is −0.90 (if all responses occurred in the first bin) through 0 (if an equal number of responses occurred in each bin) to +0.90 (if all responses occurred in the last bin).
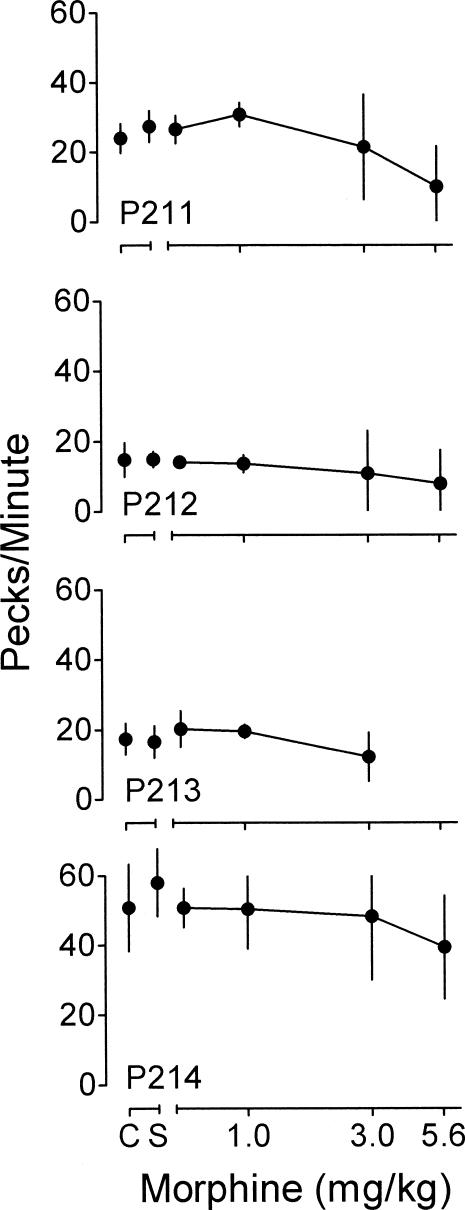
Figure 1. Mean pecks per minute during the FI 2-min component as a function of morphine for each pigeon.
Unconnected points show means for all control (C) and saline (S) sessions. Lines connect points showing the mean across doses of morphine. Vertical bars represent one standard deviation above and below the mean.
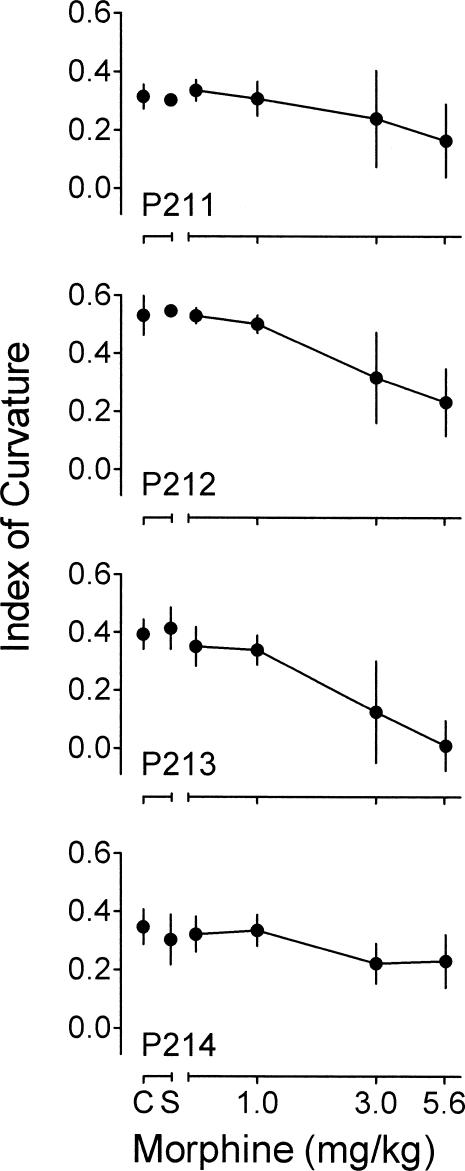
Figure 2. Mean index of curvature (degree of temporal patterning) during the FI 2-min component as a function of morphine for each pigeon.
See text for calculation. Other details as in Figure 1.
During control sessions, the index of curvature was positive, indicating that relatively more responses occurred later in the interval. Saline had no systematic effect on the index. Morphine dose-dependently decreased the index of curvature for all pigeons, with the largest decreases occurring for Pigeons P212 and P213. The decrease in the index indicates that, under morphine, relatively more responses occurred earlier in the intervals compared to control performance.
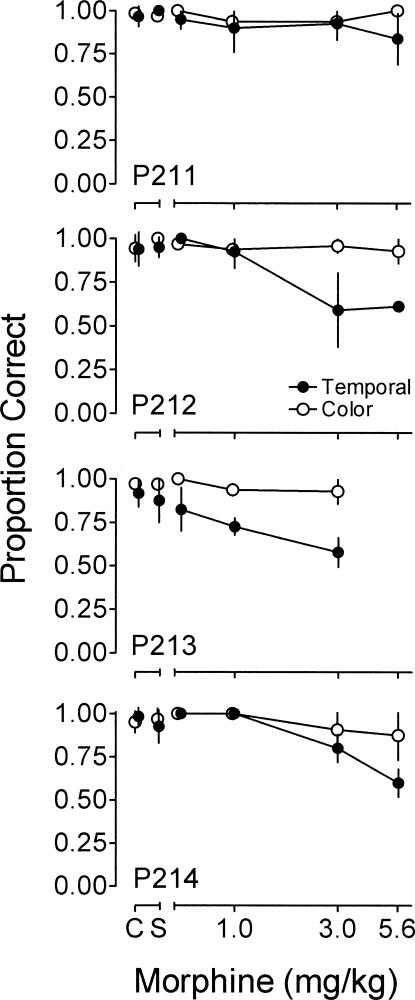
Although control accuracy in both the color matching and temporal-discrimination components was above 0.8, accuracy for temporal-discrimination was slightly lower than for color matching. Because the level of stimulus control during control conditions has been shown to moderate the disruptive effects of drugs (e.g., Ksir, 1975), we compared the effects of morphine on temporal discrimination and color matching at similar levels of control accuracy. Figure 3 shows the proportion correct for the temporal discrimination and color-matching components as a function of morphine dose for each pigeon. Data shown from the temporal-discrimination component reflect only trials with 2 and 8 s sample durations. Control proportion correct in both components was between 0.8 and 1.0 for all pigeons. There was no systematic difference in accuracy between the color matching and temporal-discrimination components. Saline had no systematic effect on accuracy in either component. Overall, morphine decreased accuracy during temporal-discrimination trials, whereas accuracy for color matching was relatively unaffected. This effect was less apparent for Pigeon P211. Examination of the accuracy data with all temporal-discrimination trials included showed that the overall effects of morphine were the same, although the overall level of accuracy for temporal discrimination remained below that for color matching at all doses of morphine.
Figure 3. Proportion correct during the color matching (unfilled circles) and portions of the temporal-discrimination (filled circles) components as a function of morphine for each pigeon.
Points showing accuracy for temporal discrimination reflect only accuracy for trials with 2- and 8-s sample durations. Points for temporal discrimination and color-matching accuracy are offset slightly on the x axis for clarity. Other details as in Figure 1. Data are not shown for Pigeon P213 following administration of 5.6 mg/kg morphine because this pigeon responded following only one administration of this dose.
To assess whether the effects of morphine on accuracy during the temporal discrimination and color-matching components were statistically significantly different, a line was fit using linear regression to the data relating the proportion correct in the temporal discrimination and color-matching components to the dose of morphine. Control and saline data were excluded from this analysis. Data were pooled across pigeons. The slope of the function relating proportion correct in the temporal-discrimination component to the dose of morphine was −0.055, whereas the slope of the function for the proportion correct in the color-matching component was −0.011. These slopes were significantly different, F(1,112) = 12.60, p = 0.00057, when compared using analysis of covariance as described by Zar (1999). These results show that morphine decreased accuracy of discrimination of the temporal sample endpoints, whereas accuracy of color matching was relatively unaffected.
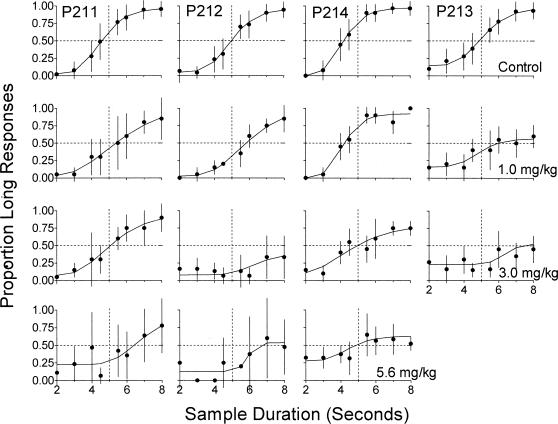
Figures 4 and 5 present a detailed analysis of the effects of morphine on performance in the temporal-discrimination component. The data in Figure 4 are expressed as the mean proportion of responses to the long key color as a function of sample duration. The data were fit using a cumulative Gaussian function with four parameters: upper and lower asymptotes, standard deviation (SD), and mean. The fits were obtained with the SOLVER tool of the EXCEL 5.0 spreadsheet program. Blough (1996) noted that if the means of the fitted functions are different, averaging functions can lead to a reduction of the slope of the mean function. To avoid this artifact, we averaged the proportion long response data from each determination of each dose of morphine and fit one Gaussian function to the average data from each dose for each pigeon.
Figure 4. Mean proportion of responses to the long key color during control (top row) and morphine sessions (lower rows) as a function of sample duration for each pigeon during the temporal-discrimination component.
Dotted lines indicate the bisection of .5 responses to the key color corresponding to the long sample duration, and the duration (5 s) that was midway between the short and long sample duration categories. Vertical bars represent one standard deviation above and below the mean. Data are not shown for Pigeon P213 following administration of 5.6 mg/kg morphine because this pigeon responded following only one administration of this dose.
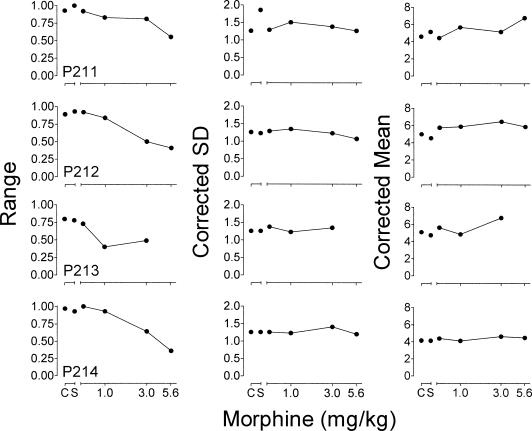
Figure 5. Parameter estimates from the fit of the cumulative Gaussian function (see text for details) to mean proportion long pecks from the temporal-discrimination component for each pigeon as a function of morphine.
The left column shows the range (upper asymptote – lower asymptote) of the function. The center column shows corrected estimates of the standard deviation (SD) of the function. The right column shows corrected estimates of the mean of the function, or the time at which .5 of the pecks were to the key color corresponding to the long category (point of subjective equality; PSE). Unconnected points show parameter estimates for control (C) and saline (S) data. Lines connect data points showing parameter estimates across doses of morphine. Data are not shown for Pigeon P213 following administration of 5.6 mg/kg morphine because this pigeon responded following only one administration of this dose.
In an analysis of these functions first introduced by Heinemann, Avin, Sullivan, and Chase (1969) and described in detail by Blough (1996), the parameters of the model reflect three sources of error in discrimination procedures: overall stimulus control, sensitivity, and bias. The degree of overall stimulus control is reflected in the range of the function (upper asymptote – lower asymptote). In the current temporal-discrimination procedure, if stimulus control was perfect, the values of the upper and lower asymptotes would be 1 and 0, respectively. The resulting range of the function would be 1.0, indicating perfect discrimination of the endpoints (2 and 8 s) of the temporal sample continuum. The SD is a measure of the slope of the function and reflects the degree of sensitivity to the differences between the sample stimuli in the short and long categories, with greater SDs indicating decreased sensitivity. The mean of the function is the duration at which the proportion of responses to the long key color is .5 (the point of subjective equality; PSE). This parameter is a measure of the degree of bias, and is affected by shifts in the psychophysical curve. Leftward and rightward shifts in the curve change the mean of the function and indicate bias for the key color associated with short and long sample durations, respectively.
Figure 4 shows that the control data (top row) were well described by the cumulative Gaussian functions, which accounted for an average of 99.3% of the variance across pigeons. The functions had an average mean of 4.7 s, indicating that pigeons accurately discriminated the passage of time. Morphine (lower rows) tended to dose-dependently decrease the proportion of long responses following long sample durations, with an increase in variability in the functions and across determinations at higher doses. Particularly at higher doses, morphine also increased the proportion of long responses following short sample durations. In most cases, the proportion of responses to the long key color tended to increase as a function of increasing sample duration, a result that indicates some control of choice behavior by the temporal dimension of the stimuli. In addition, in most cases the functions remained roughly ogival, albeit somewhat flattened, across increasing doses of morphine.
Figure 5 shows dose-effect curves of the parameters derived from fitting the function to the mean proportion long response data from each pigeon. The left panel shows the range of the psychophysical curves during control sessions and as a function of morphine. During control sessions, the range was between .80 and .97, indicating a relatively high level of overall stimulus control. Saline administration had no systematic effect on the range. Morphine dose-dependently decreased the range for all pigeons. This result indicates a dose-dependent decrease in stimulus control.
To determine the extent to which decreases in stimulus control were responsible for the decreased accuracy in the temporal-discrimination component, we examined the effects of morphine on the measures of sensitivity and bias (i.e., SD and mean). Interpreting these measures can be problematic because, as Blough (1996) noted, decreases in the range also can change the estimates of the other parameters. Because of this relation between these parameters, SD changes due to decreased stimulus control are confounded with those due to changes in sensitivity (see e.g., Blough, 1996). To determine the actual effects of morphine on sensitivity of temporal discrimination in this procedure, it is necessary to obtain an unconfounded estimate of the slope of the function (i.e., SD). To obtain this estimate, it is necessary to remove the influence of changes in the range from the estimate of the SD. Accordingly, we employed an asymptote correction first introduced by Heinemann et al. (1969).
In calculating this correction, Heinemann et al. (1969) assumed that, given perfect stimulus control, the asymptotes of the psychophysical functions should fall at zero and unity. They further suggested that failure of the asymptotes to fall at these expected values indicates that choice behavior on some trials is not governed by the presented stimuli but is instead governed by some other, unspecified stimuli. The asymptote correction gives the probability of a response with the assumption that choice behavior on the current trial is governed by the sample stimuli. In other words, the correction gives an estimate of the parameters of the model with the assumption of perfect stimulus control (i.e., range value of 1.0). The resulting estimate of the SD is considered to represent a more accurate measure of the effects of morphine on sensitivity of temporal discrimination.
The center column of Figure 5 shows the SD of the corrected psychophysical curves during control sessions and across doses of morphine. The control SD was 1.26 for all 4 pigeons, indicating relatively high sensitivity to the temporal distribution of the sample stimuli in the short and long categories. Saline had little effect on the SD with the exception of a large increase for Pigeon P211. Morphine slightly increased the SD at some doses for some pigeons and decreased it for others. Overall, morphine had no systematic effect on the SDs. These results suggest that when the influence of overall stimulus control is controlled for, sensitivity of temporal discrimination was not systematically affected by morphine.
In addition to changing the SD, increases or decreases in the range also can affect the estimate of the mean. Therefore, the asymptote correction described above was applied to obtain unconfounded estimates of the mean. The right column of Figure 5 shows the means of the corrected psychophysical curves during control sessions and across doses of morphine. The control means were between 4.2 and 5.1 s, indicating accurate estimation of the passage of time. Administration of saline had no systematic effect on the mean. Across pigeons, morphine had no systematic effect on the mean. These results suggest that when the influence of stimulus control was controlled for, morphine did not systematically shift the curves either to the left or the right, indicating no systematic bias for the key color associated with short or long samples.
To examine further the effects of morphine on choice behavior in the temporal-discrimination component, we calculated response latencies. Table 1 shows the latencies to peck the trial-ready stimulus and temporal-sample comparisons during all control and saline sessions and as a function of morphine dose for all pigeons. Under control conditions, pecks to the trial-ready stimulus occurred on average 1.5 to 4.3 s after the stimulus was presented. Saline had relatively little effect on response latencies. Morphine increased the response latency and standard deviation for 3 of 4 pigeons, particularly at the highest doses. For Pigeon P214, morphine had no appreciable effect on the latency to respond to the trial-ready stimulus, and for Pigeon P211 morphine decreased latency at the three lowest doses but increased latency at the highest dose. Responses to the temporal sample comparisons under control conditions usually occurred within 2.5 s of choice-key illumination. Saline had relatively little effect on the latencies. Across doses, morphine had no systematic effect on the choice-response latencies. Latencies decreased slightly for 2 pigeons (P211 and P212), and showed little change for the other 2 pigeons. Taken together, these results show that although morphine increased the latency to peck the trial-ready stimulus at higher doses, the latency to peck a short or long comparison key was not affected.
Table 1. Mean latency (in seconds) for responses to trial-ready stimuli and temporal choice comparisons during all control and saline sessions and as a function of morphine dose for all pigeons. Numbers in parentheses are standard deviations of the mean. Data are not shown for Pigeon P213 following administration of 5.6 mg/kg morphine because this pigeon responded following only one administration of this dose.
| Subject | Response | Control | Saline | Morphine dose |
|||
| 0.56 mg/kg | 1.0 mg/kg | 3.0 mg/kg | 5.6 mg/kg | ||||
| P211 | Trial ready | 2.37 (0.30) | 2.44 (0.54) | 1.51 (0.18) | 1.32 (0.29) | 1.21 (0.31) | 4.71 (3.91) |
| Choice | 2.22 (0.21) | 2.20 (0.11) | 1.73 (0.06) | 1.72 (0.16) | 1.94 (0.27) | 2.21 (0.14) | |
| P212 | Trial ready | 4.28 (1.20) | 4.28 (2.04) | 4.32 (0.51) | 4.63 (1.14) | 5.63 (4.92) | 6.53 (3.10) |
| Choice | 2.59 (0.24) | 2.54 (0.25) | 2.47 (0.44) | 2.16 (0.14) | 2.10 (0.39) | 1.94 (0.16) | |
| P213 | Trial ready | 3.20 (0.73) | 3.02 (0.38) | 3.36 (0.52) | 2.10 (0.20) | 5.05 (5.21) | |
| Choice | 1.18 (0.13) | 1.36 (0.31) | 1.22 (0.11) | 1.20 (0.15) | 1.38 (0.21) | ||
| P214 | Trial ready | 1.42 (0.40) | 1.30 (0.11) | 1.50 (0.50) | 1.10 (0.17) | 1.10 (0.23) | 1.22 (0.53) |
| Choice | 1.33 (0.12) | 1.33 (0.18) | 1.30 (0.10) | 1.31 (0.10) | 1.32 (0.10) | 1.40 (0.04) | |
Discussion
The baseline performance in the FI and temporal-discrimination components indicated discriminative control of behavior by time. The index of curvature from the FI component was positive, indicating that relatively more responses occurred later in the interval. This pattern of responding is typical of that usually seen in FI-schedule performance (Ferster & Skinner, 1957). During the temporal-discrimination component, the functions fitted to the proportion long response data were roughly ogival in shape, with an average mean (4.7 s) between the arithmetic and geometric means of the sample duration endpoints. The parameters derived from fitting the cumulative Gaussian functions to the data (i.e., range, SD, mean) showed that under control conditions, choice behavior was under the control of the temporal dimension of the sample stimuli. In addition, baseline proportion correct in the color-matching component was between .94 and .98 for all pigeons, indicating relatively high discrimination between the two sample colors. These results show that baseline performance during each component of the multiple schedule was typical of performance when these procedures are arranged separately.
Morphine dose-dependently decreased the index of curvature. This result is consistent with results from other studies of the effects of morphine on FI performance (e.g., Odum & Schaal, 2000; Rhodus, Elsmore, & Manning, 1974). Also similar to results from other studies, overall response rates during the FI were decreased as a function of increasing morphine dose (e.g., Odum & Schaal, 2000).
During the temporal-discrimination component, morphine decreased the proportion of responses to the key color corresponding to a long sample duration following long samples and increased the proportion of long choices following short sample durations, especially at higher doses. Morphine also dose-dependently decreased the range of the psychophysical functions, indicating a decrease in stimulus control. Although morphine decreased the measure of stimulus control, it did not have a systematic effect on the measures of sensitivity or bias (i.e., SD and mean) once unconfounded estimates of these parameters were obtained using the asymptote correction (Blough 1996; Heinemann et al., 1969). This result indicates that administration of morphine was not associated with changes in sensitivity and did not result in over or underestimation of the duration of the temporal samples. Taken together, these results indicate that although morphine decreased overall stimulus control, choice behavior was still under the remaining discriminative control of the temporal samples. Choice behavior in the color-matching component was relatively unaffected by morphine administration.
Morphine decreased the index of curvature during the FI, which resulted partly from increases in response rates early in the interval and partly from decreases in response rates later in the interval. Response rate changes of this sort have been interpreted as reflecting overestimation of time (e.g., Killeen, 1991; McAuley & Leslie, 1986; Meck, 1996). However, morphine produced no systematic effects on the measures of timing in the temporal-discrimination component. These apparently conflicting results can be resolved by appealing to another interpretation of the effects of drugs on behavior maintained by FI schedules. Drug-induced changes in response rates can be considered under the rubric of rate constancy (e.g., Byrd, 1979, 1981; Gonzalez & Byrd, 1977). The rate-constancy concept states that drugs can reduce variability in behavior. As drug doses increase, response rates tend to converge toward a more uniform rate. This type of convergence in response rates during the FI is what might be expected if administration of drugs resulted in a general loss of stimulus control. Therefore, rather than being considered as evidence of overestimation of time, the response rate changes observed in the FI component can be interpreted as resulting from a morphine-induced disruption of stimulus control.
Somewhat surprising is the fact that morphine disrupted the measure of stimulus control (i.e., accuracy) during the temporal-discrimination component, but had little effect on accuracy during the color-matching component. One possible explanation is that the effects of drugs on behavior maintained by discrimination procedures depend on the difficulty of the discrimination. In the current experiment, the color-matching component was a matching-to-sample task, whereas the temporal-discrimination component consisted of a symbolic matching-to-sample (SMTS) task. The conditional discrimination required in the temporal-discrimination component could be considered more difficult and, therefore, could have been more easily disrupted by morphine.
Although this interpretation is appealing, the results of another experiment suggest it may not be correct. In the study conducted by Santi et al. (1995), one trial type required pigeons to match short (2 s) and long (8 s) sample durations to red and green comparison stimuli, whereas the second trial type required pigeons to match red and green samples to vertical and horizontal line comparisons. Both types of trials consisted of SMTS tasks, yet amphetamine decreased accuracy during temporal-discrimination trials and not during color-matching trials. Santi et al.'s results suggest that the selective disruption of temporal discrimination in the current experiment was not due to the more difficult nature of the conditional discrimination in the temporal-discrimination component.
Further consideration of the two discrimination tasks used in the present experiment may provide an answer. In the color-matching component, sample stimuli consisted of two colors, whereas the temporal-discrimination component presented a continuum of eight sample durations. Inspection of the control psychophysical functions (Figure 4) shows that intermediate sample durations in the short and long categories were not always categorized as such. This result is evidence of stimulus generalization, which often occurs when stimuli to be discriminated vary along a continuum (e.g., Guttman & Kalish, 1956; see Honig & Urcuioli, 1981, for review). Thus being embedded in the context of the temporal sample continuum could have rendered discrimination for the temporal sample endpoints more easily disrupted by morphine than discrimination of the two colors in the color-matching component. It is possible that had color matching been assessed along a continuum, we would have seen similar changes in accuracy between the color matching and temporal-discrimination components. Future experiments should examine this possibility.
One final possibility is that temporal discrimination may be more susceptible to disruption by pharmacological manipulations than color discrimination. The results of an experiment by Bradley and Blough (1993) suggest that some categories of discriminations may indeed be more easily disrupted by drugs than others. In separate experimental conditions, pigeons discriminated between wavelength and luminance samples, which were varied along a continuum in a SMTS procedure. Morphine decreased accuracy more for discrimination of luminance than for discrimination of wavelength. In addition, morphine produced decreases of overall stimulus control during the luminance, but not the wavelength, condition. Thus the differential effect of morphine on accuracy in the color-matching and temporal-discrimination components in the present experiment may have resulted partly from an increased vulnerability of temporal discrimination to the disruptive effects of morphine.
The results of the current experiment once again highlight the discrepancies in the literature on the effects of drugs on timing. Specifically, our results suggest that the effects of drugs on behavior maintained by some temporal-discrimination procedures may have little to do with the neuropharmacological correlates of timing performance. Rather, disruption of temporal discrimination by some drugs may be a result of a general decrease in stimulus control. Decreases in stimulus control also have been observed when behavior maintained by discrimination procedures is subjected to nonpharmacological disruption, such as delivering food during the intertrial interval (e.g., Blough, 1998; Nevin, Milo, Odum, & Shahan, 2003). In addition, Sutton and Roberts (2002) reported results consistent with a loss of overall stimulus control when they assessed temporal discrimination during a divided-attention task (Experiments 1 and 2), and when they illuminated a distracter light during probe trials in a temporal-discrimination procedure (Experiment 3).
The similarities between the effects of morphine on the measure of stimulus control in the current experiment and the reported effects of pharmacological and nonpharmacological manipulations on measures of stimulus control in other experiments suggest a common mechanism underlying stimulus control in temporal and other types of discriminations. For example, attention to relevant dimensions of antecedent stimuli is considered to be an important factor in the establishment and maintenance of overall stimulus control (see McIlvane, Dube, & Callahan, 1996, for discussion). The decrease in the measure of stimulus control observed in the current experiment could be interpreted as resulting from a disruption of attention to the sample stimuli (see Blough, 1996; Heinemann et al., 1969; Santi et al., 1995). A disruption of attention to the sample stimuli, however, likely would result in increased choice response latencies. The fact that choice response latencies were not systematically affected by administration of morphine (see Table 1) may be problematic for this account.
The interpretation of the decreases in accuracy during the temporal-discrimination component in the present experiment as resulting from decreases in stimulus control must be tempered in light of the results from the color-matching component. A morphine-induced general disruption of stimulus control would be expected to decrease accuracy during the color-matching component as well. That morphine had no apparent effect on accuracy during this component is troublesome for a stimulus control account of these results.
The asymptote correction used to derive the measure of stimulus control in the present experiment has not been used widely in the analysis of data from temporal-discrimination procedures (but see Church & Gibbon, 1982). It is possible, therefore, that this method of data analysis may not be suitable to characterize accurately the effects of drugs on behavior maintained by temporal-discrimination procedures. Even so, the results from the temporal-discrimination component are meaningful on other grounds. Examination of the uncorrected psychophysical functions (Figure 4) shows that morphine administration flattened the functions, a result that indicates a generalized disruption of temporal discrimination. This disruption was not accompanied by any systematic leftward or rightward shifts in the functions, indicating that pigeons did not over or underestimate the duration of the samples. Thus the results of the present experiment, whether interpreted in terms of a decrease of stimulus control or a disruption of timing, can be added to the growing number of results that appear to be discrepant with the predictions of current models of the neuropharmacology of timing (e.g., Meck, 1996).
The results of the present experiment and analysis suggest the utility of experimental preparations and data-analysis techniques that can clearly separate the effects of drugs on stimulus control from their effects on timing. Although characterization of data in terms of theoretical constructs (e.g., attention) will be ultimately less informative than determining the underlying neurophysiological mechanisms responsible for successful performance during discrimination procedures, such characterization may provide a bridge between the behavioral and neuroscience disciplines in the search for the neurophysiological basis of stimulus control. Experimental methods and techniques developed in the field of neuroscience could be combined with the methodology already employed by behavioral researchers to further reveal the neurophysiological foundations of basic behavioral phenomena. Of particular importance in relation to the results of the present experiment will be the isolation of those neurophysiological systems responsible for the establishment and maintenance of stimulus control during temporal-discrimination procedures. Perhaps this avenue will help to synthesize the so far unresolved discrepancies in the timing literature into an accurate and complete account of the neuropharmacology of timing.
Acknowledgments
The authors thank Jason Martin for his assistance in the computer programming and conduct of this experiment, as well as Timothy Shahan and Christopher Podlesnik for their comments on a previous draft of this manuscript. Clive Wynne, Erin McClure, and Katie Saulsgiver provided valuable suggestions with data analysis and helpful comments on a previous draft of this manuscript. Portions of these data were presented at the 2001 annual meeting of the Southeastern Association for Behavior Analysis in Wilmington, North Carolina and the 2002 annual meeting of the Association for Behavior Analysis in Toronto, Canada.
References
- Andrews J.S, Holtzman S.G. Effects of d-amphetamine, morphine, naloxone, and drug combinations on visual discrimination in rats. Psychopharmacology. 1988;94:172–177. doi: 10.1007/BF00176840. [DOI] [PubMed] [Google Scholar]
- Berryman R, Cumming W.W, Nevin J.A, Jarvik M.E. Effects of sodium pentobarbital on complex operant discriminations. Psychopharmacologia. 1964;6:388–398. doi: 10.1007/BF00404247. [DOI] [PubMed] [Google Scholar]
- Berryman R, Jarvik M.E, Nevin J.A. Effects of pentobarbital, lysergic acid diethylamide and chlorpromazine on matching behavior in the pigeon. Psychopharmacologia. 1962;3:60–65. doi: 10.1007/BF00413108. [DOI] [PubMed] [Google Scholar]
- Blough D.S. Error factors in pigeon discrimination and delayed matching. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:118–131. [Google Scholar]
- Blough D.S. Context reinforcement degrades discriminative control: A memory approach. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:185–199. doi: 10.1037//0097-7403.24.2.185. [DOI] [PubMed] [Google Scholar]
- Bradley D.V, Blough P.M. Visual effects of opiates in pigeons. Psychopharmacology. 1993;111:117–122. doi: 10.1007/BF02257417. [DOI] [PubMed] [Google Scholar]
- Brown P.L, Jenkins H.M. Autoshaping of the pigeon's key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi C.V. Dopaminergic mechanisms of interval timing and attention. In: Meck W.H, editor. Functional and neural mechanisms of interval timing. New York: CRC Press; 2003. pp. 317–338. [Google Scholar]
- Byrd L.D. The behavioral effects of cocaine: Rate dependency or rate constancy. European Journal of Pharmacology. 1979;56:355–362. doi: 10.1016/0014-2999(79)90266-8. [DOI] [PubMed] [Google Scholar]
- Byrd L.D. Quantification in behavioral pharmacology. In: Thompson T, Dews P.B, McKim W.A, editors. Advances in behavioral pharmacology (Vol. 3, pp. 75–90) New York: Academic Press; 1981. [Google Scholar]
- Çevik M.Ö. Effects of methamphetamine on duration discrimination. Behavioral Neuroscience. 2003;117:774–787. doi: 10.1037/0735-7044.117.4.774. [DOI] [PubMed] [Google Scholar]
- Chiang T.J, Al-Ruwaitea A.S.A, Mobini S, Ho M.Y, Bradshaw C.M, Szabadi E. The effect of d-amphetamine on performance on two operant timing schedules. Psychopharmacology. 2000;150:170–184. doi: 10.1007/s002130000422. [DOI] [PubMed] [Google Scholar]
- Church R.M, Gibbon J. Temporal generalization. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:165–186. [PubMed] [Google Scholar]
- Dykstra L.A. Effects of morphine, diazepam, and chlorpromazine on discrimination of electric shock. Journal of Pharmacology and Experimental Therapeutics. 1979;209:297–303. [PubMed] [Google Scholar]
- Eckerman D.A, Lanson R.N, Berryman R. Effects of sodium pentobarbital on symbolic matching and symbolic oddity performance. Bulletin of the Psychonomic Society. 1978;11:171–174. [Google Scholar]
- Ferster C.B, Skinner B.F. Schedules of reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Frederick D.L, Allen J.D. Effects of selective dopamine D1- and D2-agonists and antagonists on timing performance in rats. Pharmacology, Biochemistry, and Behavior. 1996;53:759–764. doi: 10.1016/0091-3057(95)02103-5. [DOI] [PubMed] [Google Scholar]
- Fry W, Kelleher R.T, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. Journal of the Experimental Analysis of Behavior. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale C.L, Gallistel C.R. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F.A, Byrd L.D. Mathematics underlying the rate-dependency hypothesis. Science. 1977;196:546–550. doi: 10.1126/science.402028. [DOI] [PubMed] [Google Scholar]
- Grilly D.M, Genovese R.F, Nowak M.J. Effects of morphine, d-amphetamine, and pentobarbital on shock and light discrimination performance in rats. Psychopharmacology. 1980;70:213–217. doi: 10.1007/BF00435317. [DOI] [PubMed] [Google Scholar]
- Guttman N, Kalish H.I. Discriminability and stimulus generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Heinemann E.G, Avin E, Sullivan M.A, Chase S. Analysis of stimulus generalization with a psychophysical method. Journal of Experimental Psychology. 1969;80:215–224. [Google Scholar]
- Hinton S.C, Meck W.H. The ‘internal clocks’ of circadian and interval timing. Endeavour. 1997;21:3–8. doi: 10.1016/s0160-9327(96)10022-3. [DOI] [PubMed] [Google Scholar]
- Honig W.K, Urcuioli P.J. The legacy of Guttman and Kalish (1956): 25 years of research on stimulus generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P.R. Behavior's time. In: Bower G.H, editor. The psychology of learning and motivation. New York: Appleton-Century-Crofts; 1991. pp. 295–334. [Google Scholar]
- Knealing T.W, Schaal D.W. Disruption of temporally organized behavior by morphine. Journal of the Experimental Analysis of Behavior. 2002;77:157–169. doi: 10.1901/jeab.2002.77-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Slangen J.L. Effects of d-amphetamine and morphine on discrimination: Signal detection analysis and assessment of response repetition in the performance deficits. Psychopharmacology. 1983;80:125–128. doi: 10.1007/BF00427954. [DOI] [PubMed] [Google Scholar]
- Koek W, Slangen J.L. Effects of d-amphetamine and morphine on delayed discrimination: Signal detection analysis and assessment of response repetition in the performance deficits. Psychopharmacology. 1984;83:346–350. doi: 10.1007/BF00428543. [DOI] [PubMed] [Google Scholar]
- Ksir C. Scopolamine and amphetamine effects on discrimination: Interaction with stimulus control. Psychopharmacologia. 1975;43:37–41. doi: 10.1007/BF00437612. [DOI] [PubMed] [Google Scholar]
- Matell M.S, Meck W.H, Nicolelis M.A.L. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- McAuley F, Leslie J.C. Molecular analysis of the effects of d-amphetamine on fixed interval schedule performance of rats. Journal of the Experimental Analysis of Behavior. 1986;45:207–219. doi: 10.1901/jeab.1986.45-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane W.J, Dube W.V, Callahan T.D. Attention: A behavioral analytic perspective. In: Lyon G.R, Krasnegor N.A, editors. Attention, memory, and executive function. Baltimore: Paul H. Brookes; 1996. pp. 97–117. [Google Scholar]
- Meck W.H. Neuropharmacology of timing and time perception. Brain Research: Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Nevin J.A, Milo J, Odum A.L, Shahan T.A. Accuracy of discrimination, rate of responding, and resistance to change. Journal of the Experimental Analysis of Behavior. 2003;79:307–321. doi: 10.1901/jeab.2003.79-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum A.L. Behavioral pharmacology and timing. Behavioural Processes. 2002;57:107–120. doi: 10.1016/s0376-6357(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Odum A.L, Lieving L.M, Schaal D.W. Effects of d-amphetamine in a temporal discrimination procedure: Selective changes in timing or rate dependency? Journal of the Experimental Analysis of Behavior. 2002;78:195–214. doi: 10.1901/jeab.2002.78-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum A.L, Schaal D.W. The effects of morphine on fixed-interval patterning and temporal discrimination. Journal of the Experimental Analysis of Behavior. 2000;74:229–243. doi: 10.1901/jeab.2000.74-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum A.L, Ward R.D. The effects of morphine on the production and discrimination of interresponse times. Journal of the Experimental Analysis of Behavior. 2004;82:197–212. doi: 10.1901/jeab.2004.82-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker M, Massie C.A, Dykstra L.A. Evaluation of the effects of opioid agonists and antagonists under a delayed matching-to-sample procedure in pigeons. Psychopharmacology. 1987;93:230–236. doi: 10.1007/BF00179940. [DOI] [PubMed] [Google Scholar]
- Rhodus D.M, Elsmore T.F, Manning F.J. Morphine and heroin effects on multiple fixed-interval schedule performance in rats. Psychopharmacologia. 1974;40:147–155. [Google Scholar]
- Richelle M, Lejeune H. Are we close to decyphering the timer's enigma? Current Psychology of Cognition. 1998;17:867–879. [Google Scholar]
- Ridley R.M, Baker H.F, Weight M.L. Amphetamine disrupts successive but not simultaneous visual discrimination in the monkey. Psychopharmacology. 1980;67:241–244. doi: 10.1007/BF00431263. [DOI] [PubMed] [Google Scholar]
- Santi A, Coppa R, Ross L. Effects of the dopamine D2 agonist, quinpirole, on time and number processing in rats. Pharmacology, Biochemistry, and Behavior. 2001;68:147–155. doi: 10.1016/s0091-3057(00)00452-4. [DOI] [PubMed] [Google Scholar]
- Santi A, Weise L, Kuiper D. Amphetamine and memory for event duration in rats and pigeons: Disruption of attention to temporal samples rather than changes in the speed of the internal clock. Psychobiology. 1995;23:224–232. [Google Scholar]
- Stubbs A. The discrimination of stimulus duration by pigeons. Journal of the Experimental Analysis of Behavior. 1968;11:223–238. doi: 10.1901/jeab.1968.11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J.E, Roberts W.A. The effect of nontemporal information processing on time estimation in pigeons. Learning and Motivation. 2002;33:123–140. [Google Scholar]
- Wenger G.R, McMillan D.E, Moore E, Williamson A.P. Disruption of temporal discrimination by drugs of abuse: I. Unmasking of a color bias. Behavioural Pharmacology. 1995;6:297–310. [PubMed] [Google Scholar]
- Zar J.H. Biostatistical analysis (4th ed.) Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]