Abstract
Background
A previously reported association between prolonged lactation and maternal mortality has generated concern that breast-feeding may be detrimental for HIV-positive women.
Methods
As part of a trial conducted in Lusaka, Zambia, 653 HIV-positive women were randomly assigned either to a counseling program that encouraged abrupt cessation of breast-feeding at 4 months (group A) or to a program that encouraged prolonged breast-feeding for the duration of the woman’s own informed choice (group B). We examined whether mortality up to 2 years post-partum increased with breast-feeding for a longer duration.
Results
There was no difference in mortality 12 months after delivery between 326 HIV-positive women randomly assigned to short breast-feeding [group A: 4.93%; 95% confidence interval (CI), 2.42–7.46] versus 327 women assigned to long breast-feeding (group B: 4.89%; 95% CI, 2.38–7.40). Analysis based on actual practice, rather than random assignment, also demonstrated no increased mortality due to breast-feeding.
Conclusions
Although HIV-related mortality was high in this cohort of untreated HIV-positive women, prolonged lactation was not associated with increased mortality.
Keywords: maternal mortality, HIV/AIDS, breastfeeding, women, lactation, prognosis, Zambia
Introduction
In the context of the HIV/AIDS pandemic, breast-feeding is both a protector of and a hazard for infant health in low resource settings [1]. An unanticipated finding from a trial in Nairobi, Kenya added the maternal dimension to this predicament. This study observed a three-fold increased risk of mortality among HIV-positive women randomized to breast- compared to formula-feeding [2]. Although the Kenyan observation has not been replicated in at least three observational studies [3–5], this has not settled the question and concern about the effect of lactation on maternal health continues to be voiced [6]. A true risk may be hidden in observational studies if breast-feeding is less common or shorter among sicker women, raising the importance of investigating the question using an experimental design. To date, there are no other randomized studies of this question.
We report here the results of a trial of early cessation of breastfeeding on mortality of HIV-positive women in Lusaka, Zambia. We investigated whether mortality among HIV-positive women is increased with prolonged breast-feeding.
Methods
Data on maternal outcomes were collected as part of the Zambia Exclusive Breast-feeding Study (ZEBS) designed to evaluate the effect of short exclusive breast-feeding on infant outcomes (mortality and HIV transmission) [7]. In brief, HIV-positive women attending prenatal care services at two sites in Lusaka, Zambia, were recruited. Women were given single-dose nevirapine and were counseled about risks and benefits of infant feeding options. Women who intended to breastfeed were eligible to be enrolled. Once enrolled, women were encouraged to exclusively breastfeed until 4 months. Half were randomized, when their child reached at least 1 week of age, to a counseling program that encouraged abrupt cessation of breastfeeding at 4 months (group A). The other half were randomized to a program that encouraged continued exclusive breastfeeding to 6 months with gradual introduction of weaning foods thereafter (group B). The duration of breastfeeding after 4 months in group B was determined by each woman’s personal informed choice. The study was approved by the Institutional Review Boards of the investigators’ institutions. Written informed consent was obtained from all participants.
At enrollment, blood was drawn for CD4 T-cell counts (FACSCount system; BD Immunocytometry Systems, San Jose, California, USA), measurement of hemoglobin (Hemocue system; Hemocue, Lake Forest, California, USA) and plasma was frozen for later quantitative HIV RNA tests (Roche Amplicor, version 1.5; Roche, Branchburg, New Jersey, USA). Sociodemographic information and a medical and obstetric history were obtained, and the woman’s weight and height were measured.
Women were followed through delivery, and if their newborn was alive and being breast-fed at 1 week post-partum, and the mother was still willing to continue in the study, the mother–child pair was randomized. Mothers and infants were followed at regular clinic visits to 24 months post-partum. At each study visit detailed questionnaires were completed, including questions about whether the child was still being breast-fed, and if not, the age when breast-feeding stopped. Home visits were interspersed between clinic visits to ensure frequent contact. Missed visits were tracked by a team of outreach workers. Information from hospital records and/or family was sought for maternal or child deaths. Mothers of children who died were encouraged to continue in the study to collect maternal health outcomes.
The analysis was restricted to 710 women who had delivered a live-born infant more than 12 months before this analysis. Eleven women who re-enrolled with second pregnancies were excluded. To compare characteristics between randomly assigned groups, chi-squared tests were used for categorical and t-tests for continuous variables. The mortality of the mothers from the time of the study child’s birth was compared between the two randomly assigned feeding groups using Kaplan–Meier methods and the log-rank test. Stratum-specific mortality analyses were performed using the Cox proportional hazards model [8]. Kaplan–Meier methods were also used to describe breast-feeding practices by group, based on the mother’s report of the age when all breastfeeding stopped.
To determine whether a lack of complete adherence to randomly assigned feeding practice had obscured any differences, in addition to ‘intent-to-treat’ analyses, the analyses were repeated based on actual practice and adjusted for potential confounders within each group. Proportional hazards models were used to estimate the relative hazard of women’s mortality as a function of breast-feeding as a time-dependent variable lagged by 28 days. The lag was included to avoid bias towards an apparent increased risk associated with no breast-feeding if women stopped breast-feeding due to illness or other reasons soon before their death.
Results
Of 710 HIV-positive women with live-born infants, 653 (92%) were randomized. Five women (0.7%) died shortly after birth [septicemia (n = 2), malaria (n = 2) and severe bleeding (n = 1]]. Twenty-eight (3.9%) infants died before randomization (four of these were born to mothers who also died shortly after delivery). Eight mothers were not randomized because they elected not to breast-feed and 20 withdrew consent for the study before randomization. The 326 women randomized to group A were similar to the 327 randomized to group B but breastfeeding duration after 4 months differed substantially between the two groups (Table 1).
Table 1.
Baseline characteristics of 653 HIV-positive women in Lusaka, Zambia, assigned to group A (early cessation of breast-feeding) or to group B (continued breast-feeding).
| Group A (n = 326) | Group B (n = 327) | P-value | |
|---|---|---|---|
| Age (years) mean (SD) | 25.8 (5.14) | 26.2 (5.39) | 0.34 |
| CD4 cell count × 106/l | |||
| Median | 331 | 354 | 0.59 |
| N (%) < 200 × 106/l | 72 (22.1) | 70 (21.4) | 0.83 |
| Plasma HIV RNA copies/mla | |||
| Median | 42,010 | 46,751 | 0.38 |
| N (%) > 100 000 copies/ml | 100 (30.9) | 112 (34.4) | 0.34 |
| Hemoglobin g/dl | |||
| Mean (SD) | 10.6 (1.53) | 10.6 (1.53) | 0.57 |
| N (%) < 10 g/dl | 91 (28.0) | 94 (28.8) | 0.83 |
| Body mass index 1 month post-partuma | |||
| Mean (SD) | 21.7 (3.67) | 21.3 (2.81) | 0.13 |
| N (%) < 18.5 | 61 (19.1) | 51 (15.9) | 0.30 |
| Water source | |||
| N (%) Tap inside home | 23 (7.1) | 21 (6.4) | 0.75 |
| Education | |||
| N (%) Some high school | 139 (42.6) | 147 (45.0) | 0.55 |
| Employed | |||
| N (%) Full-time job | 27 (8.3) | 18 (5.5) | 0.16 |
| Food security | |||
| N (%) No food at home > 1 day in last month | 83 (25.5) | 85 (26.0) | 0.88 |
| Still breastfeeding:b | |||
| 4 months | 91.2 | 92.7 | |
| 5 months | 31.5 | 90.4 | |
| 12 months | 21.7 | 72.6 | |
| Median breastfeeding duration (months) | 4 | 15 | < 0.0001 |
Viral load measurements were available for 324 women in group A and 326 women in group B. Height and weight measurements were available for 320 women in group A and 320 women in group B.
Probability of still breastfeeding per 100 women by the indicated month calculated using Kaplan–Meier.
Until 4 months post-partum, a period over which the study-assigned feeding practices did not differ, mortality rates in the two groups were similar: 2.7% [95% confidence interval (CI), 0.8–4.5] in group A and 2.7% (95% CI, 0.9–4.5) in group B. Cumulatively up to 12 months, a period over which group A was counseled to stop breast-feeding and feeding practices between the two groups diverged substantially, mortality rates in the two groups remained highly similar: 4.9% (95% CI, 2.4–7.5) in group A and 4.9% (95% CI, 2.4–7.4) in group B. In the period up to 24 months post-delivery, there continued to be no evidence of increased mortality in group B relative to group A (Fig. 1; P = 0.38). If the analysis was restricted to women surviving past 4 months, there was still no difference between the groups (P = 0.30).
Fig. 1. Mortality after delivery among 653 HIV-positive women in Lusaka, Zambia.
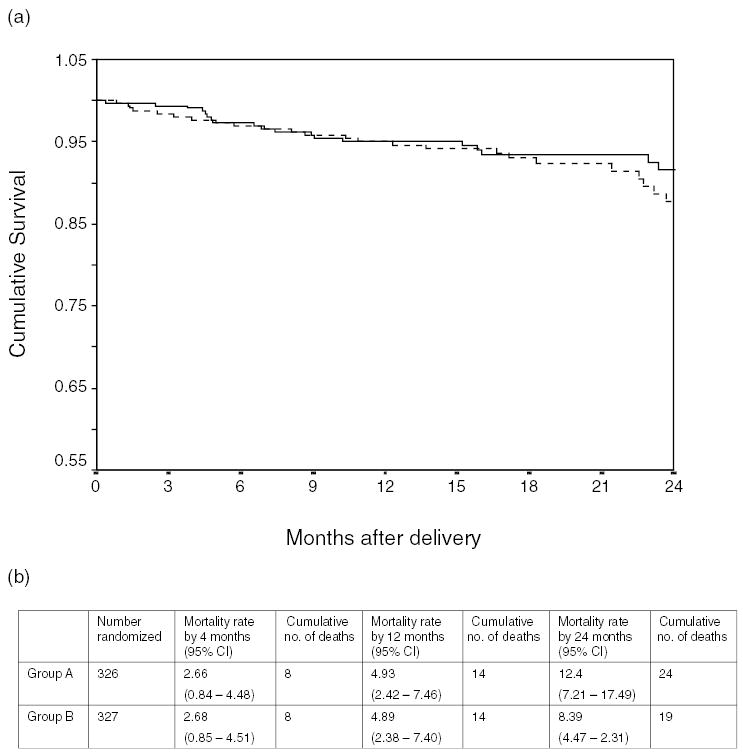
(a) Kaplan–Meier curves for women randomized to cessation of breastfeeding at 4 months (group A: dashed line) or to continued breastfeeding (group B: solid line) and (b) Kaplan–Meier cumulative probabilities of mortality at 4,12, and 24 months post-delivery among women randomized to groups A and B. Group A, early cessation of breast-feeding; group B, continued breast-feeding. CI, confidence interval.
Causes of death were similar between the groups. In group A, 22 of 24 deaths had a known cause: pulmonary tuberculosis (n = 9), diarrhea (n = 8), malaria (n = 4), meningitis (n = 3), sepsis (n = 3) and pneumonia (n = 1). In group B, 16 of 19 deaths had a known cause: pulmonary tuberculosis (n = 5), diarrhea (n = 5), malaria (n = 4), anemia (n = 3), meningitis (n = 2), pneumonia (n = 2) and sepsis (n = 1) (> 1 cause could be given). Cohort retention was 80% to 12 months and 73% to 24 months and did not differ by random assignment. Neither were other factors, including CD4 cell counts, viral load, body mass index (BMI) or hemoglobin, associated with retention.
To investigate if prolonged breast-feeding was associated with increased mortality in sub-groups of women at highest risk of HIV-related mortality, the association between randomly assigned feeding practice and mortality up to 24 months was examined within strata based on baseline CD4 cell count, viral load, hemoglobin measured during pregnancy and BMI measured at 1 month post-partum. Group assignment was not associated with mortality in any stratum. For example, among women with CD4 cell counts < 200 × 106 cells/l the cumulative mortality rate by 12 months was 14.9% (95% CI, 6.4–23.5) in group A; and 11.0% (95% CI, 3.3–18.7) in group B (P = 0.39).
There was no evidence that those women in group A who did not comply with the counseling to stop breast-feeding had higher mortality. Rather, in unadjusted analyses, no breast-feeding was associated with significantly higher mortality than breast-feeding [relative hazard (RH), 8.7; 95% CI, 1.59–48.10]. The association was reduced towards the null (RH, 1.23; 95% CI, 0.23–6.51) after adjustment for baseline CD4 cell count, viral load, BMI and child mortality. In group B, the higher mortality associated with not breast-feeding was also reduced towards the null (RH, 2.87; 95% CI, 0.92–8.99) after adjustment for the same variables.
Discussion
In our randomized study of HIV-positive women in Lusaka, Zambia, mortality rates among women randomized to early cessation of breastfeeding were near identical to those randomized to continued breast-feeding despite marked differences in breast-feeding duration between the groups. Failure to fully adhere to random assignment is unlikely to have diluted associations towards the null since, once confounding was taken into account, analyses based on actual practice yielded the same conclusion.
Our results contradict the observation from the Kenyan trial [2], but are consistent with the three observational studies of the question [3–5]. We speculate that the original Kenyan result was a chance observation. Moreover, much of the excess mortality they observed in the breastfeeding arm occurred soon after delivery. As most of these deaths are due to obstetric causes, it is unlikely that breastfeeding, hypothesized to pose a cumulative nutritional burden on the HIV-positive mother, could have affected these. There was also an imbalance in intrauterine transmission between the two arms in their study suggesting that sicker women may have been over-represented in the breastfeeding arm [9].
Maternal depletion, as a result of the combined effects of lactation, HIV infection and poor nutritional intake, was postulated to explain risks posed by breast-feeding in the Kenyan study [2]. Our study population would be expected to be at high risk of this phenomenon since many had advanced HIV disease, low BMI and a quarter reported not having had food at home at least one day in the previous month. However, even within high-risk subgroups, there was no trend towards excess mortality associated with prolonged breast-feeding. The depletion hypothesis was compelling because HIV disease is associated with severe wasting and the association between weight loss and lactation is promoted (in affluent societies) as one of the benefits of breast-feeding [10]. However, a review of the effects of lactation on maternal weight reveals that the magnitude of weight loss is small and is not observed in all populations [11,12]. In poorly-nourished populations, slower rates of weight loss or even weight gain are observed during lactation suggesting adaptive compensatory mechanisms that may lower the nutritional cost of pregnancy and lactation [12]. One such mechanism may involve changes to nutrient partitioning for milk production in favor of conservation of body reserves at the expense of milk quality [11–13]. Our data suggest that any effect of prolonged breast-feeding on maternal weight does not translate into increased mortality.
In unadjusted statistical analyses, there was a strong association between higher mortality and stopping breastfeeding in our data that was reduced towards the null only after adjustment simultaneously for several confounders, including, but not limited to, maternal CD4 cell count. The ‘healthy breast-feeder’ effect has been previously noted [4] and demonstrates how easily observational data can be misinterpreted. Thus our finding of no association in a randomized, rather than non-experimental, study design carries particular salience.
If antiretroviral drugs used during breast-feeding reduce risks of postnatal transmission then, from the child’s perspective, any advantage of shortening or avoiding breast-feeding becomes moot. This, in turn, elevates the question of the effect of breast-feeding on maternal health to renewed prominence. We find no increased mortality due to prolonged breast-feeding among HIV-positive mothers living in socio-economically disadvantaged communities and with poor access to adequate daily nutrition. Counseling offered to HIV-positive pregnant women who are learning about their HIV status as part of programs to prevent mother-to-child HIV transmission should include this reassuring information.
Acknowledgments
This study was supported in part from grants from the National Institute of Child Health and Human Development HD 39611 and HD 40777. G.M.A. is an Elizabeth Glaser Pediatric AIDS Foundation Scientist.
References
- 1.Humphrey J, Iliff P. Is breast not best? Feeding babies born to HIV-positive mothers: bringing balance to a complex issue. Nutr Rev. 2001;59:119–127. doi: 10.1111/j.1753-4887.2001.tb06999.x. [DOI] [PubMed] [Google Scholar]
- 2.Nduati R, Richardson BA, John G, Mbori-Ngacha D, Mwatha A, Ndinya-Achola J, et al. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet. 2001;357:1651–1655. doi: 10.1016/S0140-6736(00)04820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutsoudis A, Coovadia H, Pillay K, Kuhn L. Are HIV-infected women who breastfeed at increased risk of mortality? AIDS. 2001;15:653–655. doi: 10.1097/00002030-200103300-00019. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML, Read J, Leroy V, Dabis F. Mortality among HIV-infected mothers and children’s feeding modality: The Breast-feeding and HIV International Transmission Study (BHITS).Second IAS Conference on HIV and Pathogenesis, Paris, France: July 2003.
- 5.Sedgh G, Spiegelman D, Larsen U, Msamanga G, Fawzi WW. Breastfeeding and maternal HIV-1 disease progression and mortality. AIDS. 2004;18:1043–1049. doi: 10.1097/00002030-200404300-00013. [DOI] [PubMed] [Google Scholar]
- 6.Sande MA, Ronald A. Treatment of HIV/AIDS: do the dilemmas only increase? JAMA. 2004;292:266–268. doi: 10.1001/jama.292.2.266. [DOI] [PubMed] [Google Scholar]
- 7.Thea DM, Vwalika C, Kasonde P, Kankasa C, Sinkala M, Semrau K, et al. Issues in the design of a clinical trial with a behavioral intervention–the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–365. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Collett D. Modelling Survival Data in Medical Research New York: Chapman-Hall, 1994.
- 9.Newell ML. Does breastfeeding really affect mortality among HIV-1 infected women? Lancet. 2001;357:1634–1635. doi: 10.1016/s0140-6736(00)04857-1. [DOI] [PubMed] [Google Scholar]
- 10.Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. 1997;17:19–36. doi: 10.1146/annurev.nutr.17.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann PE, Sherriff JL, Mitoulas LR. Homeostatic mechanisms that regulate lactation during energetic stress. J Nutr. 1998;128:394S–399S. doi: 10.1093/jn/128.2.394S. [DOI] [PubMed] [Google Scholar]
- 12.Winkvist A, Rasmussen KM, Lissner L. Associations between reproduction and maternal body weight: examining the component parts of a full reproductive cycle. Eur J Clinl Nutr. 2003;57:114–127. doi: 10.1038/sj.ejcn.1601502. [DOI] [PubMed] [Google Scholar]
- 13.Prentice AM, Lunn PG, Watkinson M, Whitehead RG. Dietary supplementation of lactating Gambian women. II. Effect on maternal health, nutritional status and biochemistry. Hum Nutr – Clin Nutr. 1983;37:65–74. [PubMed] [Google Scholar]


