Abstract
The developmental emergence of learning has traditionally been attributed to the maturation of single brain regions necessary for learning in adults, rather than to the maturation of synaptic interactions within neural systems. Acquisition and retention of a simple form of motor learning, classical conditioning of the eyeblink reflex, depends on the cerebellum and interconnected brainstem structures, including the inferior olive. Here, we combined unit recordings from Purkinje cells in eye regions of the cerebellar cortex and quantitative electron microscopy of the inferior olive to show that the developmental emergence of eyeblink conditioning in rats is associated with the maturation of inhibitory feedback from the cerebellum to the inferior olive. The results are consistent with previous work in adult animals and indicate that the maturation of cerebellar inhibition within the inferior olive may be a critical factor for the formation and retention of learning-specific cerebellar plasticity and eyeblink conditioning.
Recent studies have identified the neural circuitry underlying certain learned responses and determined that memories, although formed and stored in particular brain regions (such as the amygdala and cerebellum), are established through interactions among multiple interconnected brain regions1–9. Studying the developmental assembly of the neural systems underlying learning provides insight into the synaptic interactions that correlate with its ontogeny and may help to elucidate possible limiting factors in forming and storing memories within particular brain regions4,10–12.
Delay eyeblink conditioning, which emerges ontogenetically between postnatal day (P)17 and P24 in rats12–14, is a simple form of motor learning in which repeated pairings of a conditioned stimulus (CS; a tone, for example) and an unconditioned stimulus (US; periorbital stimulation) promote the acquisition of eyeblink conditioned responses15 (CRs). The memory for the eyeblink CR is formed and stored within the cerebellum, but depends on CS input from the mossy/parallel fiber system and US input from the climbing fibers of the inferior olive5,6. Conditioning-specific changes in activity within the cerebellar interpositus nucleus interact with the neural circuitry underlying eyeblink conditioning in two main ways5,6. First, eyeblink CRs are produced by learning-specific increases in interpositus neuronal activity during the CS that drive neurons in the red nucleus, which are connected to motor nuclei in the eyeblink reflex path. Second, feedback from the interpositus nucleus potentiates its own CS input from the pontine nuclei and inhibits its own US input from the inferior olive. In this way, neurons in the interpositus nucleus are endowed with the ability to regulate their own plasticity-inducing CS and US inputs.
The ontogeny of eyeblink conditioning is not related to developmental differences in behavioral responses to the CS or US13 or various conditioning parameters that influence learning12–14, and it has recently been reported that the ontogeny of the eyeblink CR is attributable to the development of memory formation in the cerebellum, rather than to the overt expression of memory16. In addition, microstimulation of the interpositus nucleus elicits eyeblinks in young rats that do not learn16, indicating that the efferent pathway from the interpositus nucleus to the red nucleus is relatively mature at P17. These points indicate that the developmental emergence of eyeblink conditioning is due to the ontogenetic emergence of learning-related cerebellar plasticity, rather than age-related sensory or motor differences.
The inferior olive is the sole source of climbing fibers in the cerebellum and produces all-or-none complex spikes in the Purkinje cells of the cerebellar cortex17 by P15 (refs. 18,19). Cerebellar feedback accounts for more than 90% of the γ-aminobutyric acid (GABAergic) inhibitory terminals within the inferior olive20,21 and determines the topographical organization of climbing fibers in the cerebellar cortex22. Although no consensus has emerged regarding the exact role of inhibitory cerebellar feedback to the inferior olive, recent evidence indicates that interactions between the cerebellum and inferior olive contribute to the acquisition and extinction of the eyeblink CR23–25. It is possible that the ability to regulate climbing fiber activity via cerebellar inhibitory feedback is an important factor underlying the ontogenetic emergence of the eye-blink CR. In the present experiments, we combined unit recording of Purkinje cell complex spikes with electron microscopy in the inferior olive to investigate developmental changes in inhibitory synaptic connections from the cerebellum to the inferior olive.
Results
Inhibition of climbing fiber activity
To investigate cerebellar regulation of climbing fiber activity, we monitored complex spike activity in Purkinje cells from eye regions of lobule HVI of the cerebellar cortex (Fig. 1a, inset) during the ontogeny of eyeblink conditioning. If inhibitory cerebellar feedback to the inferior olive is mature at P17, then increases in cerebellar interpositus neuronal activity, which produce eyeblink CRs5,6,16, should inhibit US-elicited complex spikes at both P17 and P24 (ref. 23). Analyses were performed on 49 Purkinje cell complex spikes from seven P17 rats and 54 complex spikes from seven P24 rats that received paired presentations of the CS and US. We also analyzed 37 complex spikes from five P17 rats and 39 complex spikes from six P24 rats that received five sessions of explicitly unpaired presentations of the CS and US. All analyses were performed on complex spike activity from the fifth session of training.
Fig. 1.
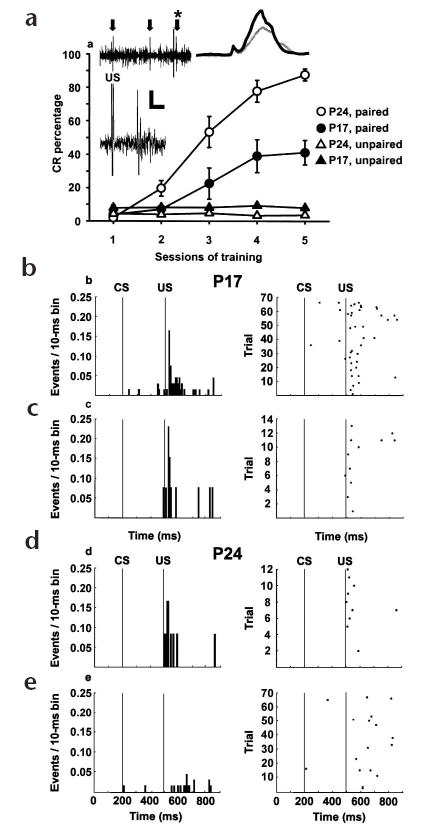
Learning-related inhibition of complex spikes parallels the development of eyeblink conditioning. (a) Eyeblink CRs for P17 and P24 rats that received paired or unpaired CS–US presentations. Inset, left, slow and fast sweep (asterisk in slow sweep) of raw US-elicited complex spike activity from a Purkinje cell in an eye region of lobule HVI (arrows indicate complex spikes); scale bars = 105 ms, 55 μV; 18 ms, 40 μV. Inset, right, averaged integrated EMG activity from CR trials for the P17 (gray) and P24 (black) rats from which complex spikes in b–e were recorded. (b–e) Histograms (left) and rasters (right) of single complex spikes from a P17 rat on trials without (b) and with (c) CRs and a P24 rat on trials without (d) and with (e) CRs.
Rats from both age groups that received paired presentations of the CS and US showed significantly more CRs than their unpaired control groups on sessions 3–5 (Fig. 1a; F4,84 = 5.851, P < 0.01). P17 rats that received paired training showed significantly fewer CRs than P24 rats that received paired training between sessions 3 and 5. According to previous studies, the few eyeblink CRs seen in P17 rats are produced by increases in cerebellar interpositus neuronal activity16. If cerebellar feedback to the inferior olive is mature at P17, then complex spike activity immediately after the US should be inhibited when both age groups show CRs23. Consistent with the hypothesis that cerebellar feedback to the inferior olive is immature at P17, complex spikes in P17 rats occurred immediately after the US on trials when rats did not (Fig. 1b) or did (Fig. 1c) display eyeblink CRs. In P24 rats, on the other hand, the synchronous complex spike responses immediately after the US seen on trials without CRs (Fig. 1d) were inhibited on trials with CRs (Fig. 1e).
The mean complex spike activity from all P17 and P24 rats that received paired training shows that inhibition of complex spikes during CR trials was also present at the population level at P24, but not at P17 (Fig. 2a). The small burst of complex spike activity during the tone on trials with eyeblink CRs, which may be related to sensory or motor coding26, is consistent with previous recording studies in the inferior olive27. An ANOVA on the percentage of trials (with or without a CR) during which a complex spike occurred during each of the first four 50-ms bins after the US revealed a significant age × response type × bin interaction (F3,606 = 3.946, P < 0.02). We attribute this to a decrease in the probability of a complex spike occurring in the first 50 ms after the US during CR trials in P24 rats, but not in P17 rats (P17: CR−, 45.7 %, CR+, 43.02%; P24: CR−, 41.6%, CR+, 11.4%). In addition, the onset and peak latencies of complex spike activity after the US in P24 rats, but not in P17 rats, were significantly greater on trials with CRs than trials without CRs (Fig. 2b; onset latency: F1,202 = 6.055, P < 0.02; peak latency: F1,202 = 39.505, P < 0.001). That is, increased neuronal activity within the cerebellum, which produces eyeblink CRs in both age groups, significantly inhibited US-elicited complex spikes in P24 rats, but not P17 rats. Moreover, many of the complex spikes that did respond after the US during CR trials in P24 rats occurred at latencies too long to be directly evoked by the US28–30.
Fig. 2.
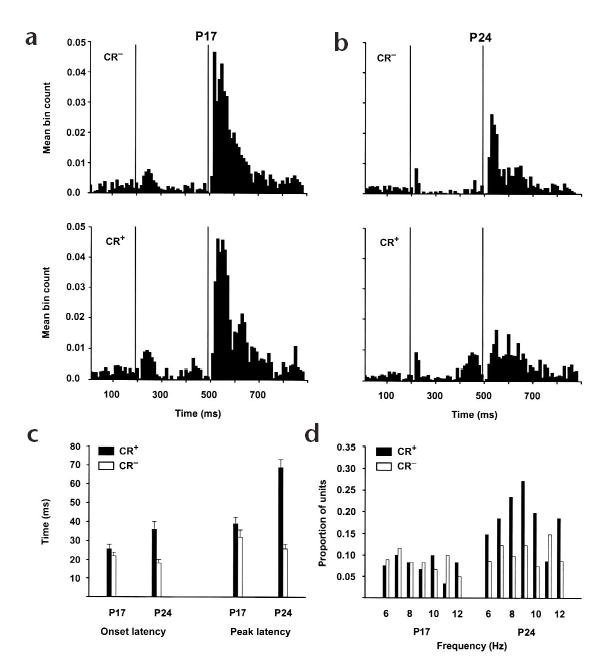
Learning-related changes in complex spike response magnitude, onset latency, peak latency and rhythmicity parallel the ontogeny of eyeblink conditioning. (a, b) Mean neuronal activity for all complex spikes recorded from Purkinje cells in lobule HVI in the P17 (a) and P24 (b) paired groups during the fifth session of conditioning on trials without (top) and with (bottom) CRs. Vertical lines indicate the onsets of the CS and US, respectively. (c) Mean onset and peak latencies for complex spikes recorded during the fifth conditioning session in the paired groups on trials with (black bars) and without (white bars) CRs. (d) Distribution of the proportions of complex spikes recorded during the fifth conditioning session in the paired groups that exhibited significant autocorrelations at each frequency (time lag−1) during trials with (black bars) and without (white bars) CRs.
Climbing fiber rhythmicity
It is possible that many of the long (>50 ms) latency complex spikes that occurred after the US during trials with CRs were produced by rhythmic oscillations in inferior olive neurons. Interactions among somatic and dendritic membrane conductances produce a prominent 8–12 Hz rhythm in action potentials and subthreshold membrane voltage oscillations of inferior olive neurons31—a rhythm that is also present in complex spike activity patterns22,28,30. Hyperpolarization of olivary neurons deinactivates a Ca2+ conductance, which increases the amplitude of membrane voltage oscillations such that previously subthreshold oscillations become large enough to produce action potentials in olivary neurons31 and therefore complex spikes in Purkinje cells. Cerebellar inhibition during eyeblink CRs may therefore increase the rhythmicity of olivary neurons by increasing the amplitude of subthreshold membrane voltage oscillations32 despite inhibiting responses immediately after the US. The periorbital US resets each olivary neuron’s rhythm, such that rhythmic patterns of activity after the US will occur relative to US onset28,30,31. To investigate whether cerebellar inhibition during CRs influenced rhythmic firing in complex spikes, we calculated the autocorrelation coefficients of the cumulative mean activity for each complex spike in all paired and unpaired groups (10-ms bins).
If increased cerebellar inhibition during CRs influenced rhythmic firing patterns in the inferior olive, then, when not completely inhibited23,27, complex spikes should occur in clusters 80–120 ms apart more frequently on trials with CRs than on trials with no CRs (Fig. 1e; complex spikes 80–120 ms apart occur with a rhythmicity of about 8–12 Hz). At P24, complex spikes showed statistically significant peaks in their autocorrelograms at 80–120 ms more frequently on trials with CRs than on trials without CRs (CS period: χ2 = 839.42, P < 0.01; US period: χ2 = 152.84, P < 0.01). In contrast, complex spikes in P17 rats showed only a small CR-related increase in 8–12 Hz rhythmicity after the US (χ2 = 47.808, P < 0.05). Moreover, the proportion of complex spikes showing CR-related increases in rhythmicity was significantly higher in P24 rats than in P17 rats (χ2 = 452.79, P < 0.01; Fig. 2d). There were no relationships between eyeblink responses and complex spike activity in the unpaired control groups. Purkinje cell complex spike activity in all P17 rats showed slightly higher spontaneous complex spike firing rates in the baseline period, which may be related to immature cerebellar feedback to the inferior olive22 (P17 = 1.24 Hz; P24 = 0.91 Hz; F1,175 = 61.197, P < 0.01). In summary, experiment 1 showed that P17 rats generally do not show two forms of learning-related complex spike activity that depend on inhibitory feedback from the cerebellum to the inferior olive22,23,28.
Addition of inhibitory synapses in the DAO
In experiment 2, we used quantitative electron microscopy to explore the possibility that the inability of P17 cerebellar neurons to inhibit complex spike activity, despite occasionally producing eyeblink CRs, is due to a lack of inhibitory synapses within the dorsal accessory olive (DAO). The DAO is the nucleus within the inferior olivary complex that provides the necessary and sufficient information about the US during eyeblink conditioning5,6 and provides most of the climbing fibers to lobule HVI of the cerebellar cortex33. Quantitative electron microscopy, combined with systematic random sampling34 and the stereological physical disector35, was used to obtain unbiased and reliable estimates of the total number of excitatory (+) and inhibitory (−) axodendritic (D) and axospinous (S) synapses20,21,36 (Fig. 3a) and excitatory and inhibitory multiple synapse boutons (MSB; Fig. 3b) in the DAO of eight P17 rats and six P24 rats.
Fig. 3.
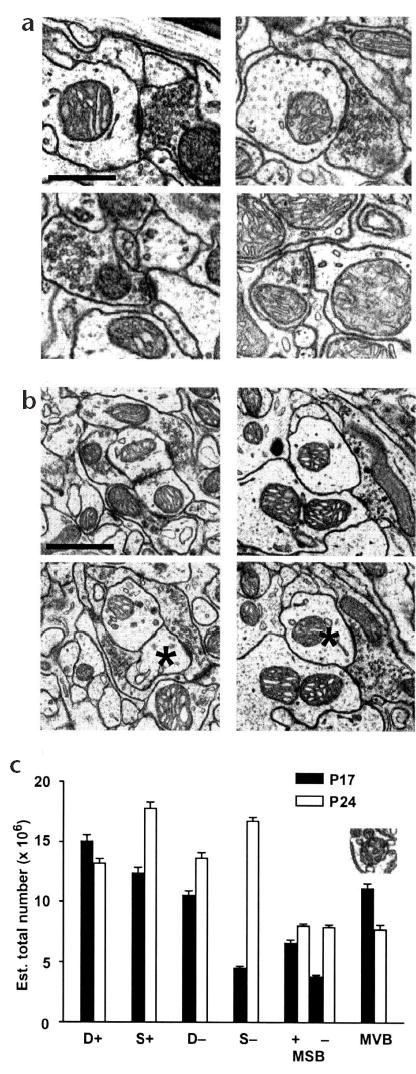
Addition of inhibitory synapses parallels the development of eyeblink conditioning. (a) Excitatory axodendritic (top left) and axospinous (bottom left) synapses; inhibitory axodendritic (top right) and axospinous (bottom right) synapses. Scale bar, 0.2 μm. (b) Emergence of excitatory (left) and inhibitory (right) axodendritic synapses (asterisks) between the look-up (top) and reference (bottom) sections to form excitatory and inhibitory multiple synapse boutons. Scale bar, 0.3 μm. (c) Estimated mean total number (+s.e.m.) of excitatory axodendritic (D+) and axospinous (S+) synapses; inhibitory axodendritic (D−) and axospinous (S−) synapses; excitatory (MSB+) and inhibitory (MSB−) multiple synapse boutons; and multivesicular bodies (MVB). Inset, multivesicular body.
The proportion of inhibitory synapses relative to the total number of synapses in the DAO showed a developmental increase from 35.5% in P17 rats to 49.5% in P24 rats (t8,6 = 13.821, P < 0.001), which indicates that the DAO at P17 has disproportionately fewer inhibitory synapses than the DAO at P24. Moreover, by P24 the DAO has approximately equal numbers of inhibitory and excitatory synapses (Fig. 3c). The developmental transition to balanced synaptic innervation in the DAO is attributable to three main events (Fig. 3c): (i) excitatory axodendritic synapses, which are initially overproduced at P17 (preplanned comparison, t8,6 = 2.657, P < 0.02), are pruned and perhaps transformed into excitatory axospinous synapses by P24 (preplanned comparison, t8,6 = 7.415, P < 0.001); (ii) there is a dramatic developmental increase in the number of inhibitory synapses between P17 and 24 (F1,24 = 71.505, P < 0.001), including a more than three-fold increase in the number of inhibitory axospinous synapses (preplanned comparison, t8,6 = 27.089, P < 0.001); and (iii) there is an addition of inhibitory terminals forming synapses with multiple olivary dendrites and dendritic spines (F1,24 = 34.03, P < 0.001). Moreover, there were significantly more dendritic multivesicular bodies (MVB), which are involved in membrane recycling and transport37,38, at P17 than at P24 (Fig. 3c; t8,6 = 5.007, P < 0.001). In sum, experiment 2 showed that there was a substantial addition of inhibitory synapses within the DAO between P17 and P24, which may be added by the production of inhibitory multiple synapse boutons. Together, experiments 1 and 2 show that the ability of cerebellar inhibition to regulate the activity of inferior olive neurons increases developmentally and is likely attributable to a massive increase in the total number of inhibitory synapses.
Discussion
The present findings indicate that inhibitory feedback from the cerebellum to the inferior olive substantially matures during the developmental time period in which the delay eyeblink CR emerges. Climbing fiber input from the inferior olive is thought to provide a teaching signal to the cerebellum for adaptive learned movements1–3,5,6,9,23–25,39,40. Inhibitory projections from the cerebellum to the inferior olive are thought to provide feedback that learning about a particular stimulus (such as a periorbital US) has occurred in the cerebellum (eyeblink CRs)3,5,6,9,23–25,39,40. The results of experiments 1 and 2 indicate that cerebellar neurons at P17 are largely unable to inhibit olivary neurons and are therefore unable to regulate climbing fiber activity.
Much empirical evidence indicates that an essential component of the memory for the eyeblink CR is formed and stored in the cerebellar interpositus nucleus and depends on CS input from the mossy fibers of the pontine nuclei and US input from the climbing fibers of the inferior olive 5,6,9,23–25,39. The present experiments were conducted to explore the possibility that immature cerebellar feedback to the inferior olive was a possible limiting factor influencing the developmental emergence of the eyeblink CR.
The first experiment showed that even when cerebellar interpositus neurons drove eyeblink CRs, P17 rats were unable to inhibit US-evoked complex spikes in Purkinje cells. Because fewer interpositus neurons exhibited learning-related increases in activity in P17 rats compared to P24 rats16, the inability to inhibit climbing fiber activity at P17 might have been due to the development of cerebellar plasticity. Alternatively, cerebellar feedback to the inferior olive may not have been mature at P17, interfering with the ability of cerebellar neurons to inhibit climbing fiber activity. The results of experiment 2 indicate that cerebellar feedback to the inferior olive matures over the time period in which the eyeblink CR emerges, and suggest that immature cerebellar feedback to the inferior olive probably prevents learning-related changes in interpositus neuronal activity from regulating climbing fibers at P17.
Recent simulation and experimental studies indicate that cerebellar feedback to the inferior olive helps to maintain an equilibrium between long-term potentiation (LTP) and depression (LTD) in the cerebellum9,24,25 and may provide insight into the possible effects of weak cerebellar feedback to the inferior olive on the formation and retention of cerebellar plasticity during eyeblink conditioning. In particular, cerebellar feedback to the inferior olive helps to establish and preserve discrete learning-related changes due to the convergence of the CS and US during training. The inability of the interpositus neurons to regulate olivary neuronal activity in P17 rats may interfere with learning in two main ways: (i) the complex spikes in the cerebellar cortex, which supply a massive inhibitory influence to the interpositus neurons17, cannot be inhibited and may limit activity-dependent plasticity in the cerebellar interpositus nucleus; and (ii) the lack of inhibitory feedback may fail to regulate bidirectional plasticity (LTD and LTP) within the cerebellum9,24,25. Recent evidence suggests that the failure to regulate bidirectional plasticity within the cerebellum prevents discrete learning-related synaptic changes from being established during conditioning23,24, or alternatively, may actively erase discrete cerebellar plasticity immediately following conditioning25,41,42.
A recent study shows that temporarily inactivating cerebellar feedback to the inferior olive disrupts Kamin’s blocking effect23. The authors suggest that preventing the cerebellum from regulating its own olivary input during phase-2 training allows information about the US to enter into associations with a new, redundant CS. Although it is possible that this study is analogous to the immature cerebellar feedback to the inferior olive in P17 rats, it is important to note that cerebellar feedback to the inferior olive was temporarily inactivated, whereas the cerebellar feedback in P17 rats is more analogous to chronic inactivation24,25. Our results suggest that the cerebellum’s chronic inability to regulate its own plasticity-inducing olivary inputs at P17, due to fewer inhibitory synapses, may have a critical role in obstructing the emergence or preservation of learning-related cerebellar plasticity, leading to weaker eyeblink conditioning.
Although the maturation of cerebellar feedback to the inferior olive is associated with the ontogeny of eyeblink conditioning, it is likely that there are other developmental factors influencing the ontogeny of the eyeblink CR. For example, previous studies have shown that CS inputs to the cerebellum exhibit a developmental increase in CS activation from P17 to P24 (ref. 16 and D.A.N. & J.H.F. Developmental changes in eyeblink conditioning and neuronal activity in the cerebellar cortex. Soc. Neurosci. Abstr. 640.3, 2001). In addition, the level of postsynaptic depolarization induced by immature cerebellar synapses might be insufficient to activate cellular cascades critical for the formation or preservation of cerebellar plasticity40,43. The development of CS pathway inputs to the cerebellum and the development of US pathway regulation are probably both critical factors influencing the formation of learning-specific plasticity in the infant cerebellum. Although the one-to-one relationship between climbing fibers and Purkinje cells is established around P15 (refs. 18,19), it is possible that some Purkinje cells are still innervated by multiple climbing fibers at P17, which has been shown to affect motor coordination and eyeblink conditioning45,46. It is also possible that there are developmental changes in modulatory inputs to the cerebellum (for example, cholinergic and noradrenergic inputs) that influence the ontogeny of eyeblink conditioning. The myriad developmental factors influencing the ontogeny of eye-blink conditioning are still under investigation.
Our present findings, coupled with empirical and theoretical work showing that cerebellar inhibition in the inferior olive regulates plasticity within the cerebellum9,23–25,39,40, indicate that cerebellar inhibition within the inferior olive may be a major factor underlying the developmental emergence of learning. In sum, our data suggest that the ontogeny of a simple form of motor learning (eyeblink conditioning) may depend critically on the ability to modify the teaching signal46,47 through cerebellar regulation of climbing fiber activity1–3,5,6,9,23–25,39,40,48.
Methods
Subjects
The subjects were twenty P17–18 and nineteen P24–25 Long-Evans rat pups. Sex was counterbalanced.
Surgery
Rats were fitted with electromyogram (EMG) and neuronal recording electrodes and a bipolar US electrode as described previously16,27. Treatment of the animals and surgical procedures were in accordance with the National Institutes of Health Guidelines and were approved by the University of Iowa Animal Care and Use Committee.
Conditioning
The paired group received five training sessions over two days at approximately 4-h intervals on days 17 and 18 or days 24 and 25. Each session consisted of 80 paired presentations of the tone CS (2.0 kHz, 300 ms, 85 dB SPL) and the periorbital stimulation US (6 ms, 4 mA) with an interstimulus interval of 294 ms, 10 CS-alone trials and 10 US-alone trials. The intertrial interval averaged 30 s (range 18–42 s). The unpaired control group received five sessions consisting of 180 explicitly unpaired presentations of the CS and US over two days with an inter-trial interval that averaged 15 s. Behavioral data were examined from computer records of EMG responses.
Neuronal recording procedure
The neuronal activity was recorded to disk as described previously16,27. A template-matching program was used to identify all of the spikes with similar waveform characteristics16,27. The complex spikes examined in the present study were considered to be from eye regions in that they exhibited short-latency responses to periorbital stimulation.
Data analysis
Significant differences were evaluated by Tukey’s honestly significant difference (HSD) test (all P < 0.05). Repeated measures analysis of variance (ANOVA) was performed on the CR percentage. Mean cumulative peristimulus histograms of the firing rates of each complex spike were created for the entire period of the conditioning trial (1 s) for each trial type (with or without CRs) from the fifth training session of all groups. Cumulative mean activity for each individual complex spike (10-ms bins) was analyzed during the last 150 ms of the CS period and during a 150-ms period immediately following the US to determine peak and onset latencies and to calculate the autocorrelations. Onset latencies were defined as the first bin with activity significantly above baseline. Peak latencies were defined as the bin with the highest value after US onset. Significant peaks in the autocorrelograms had to be greater than one standard deviation (s.d.) above the average level of the autocorrelogram from a 300 ms post-CS onset or post-US onset period.
Histology
On the day after training, the rats were killed with a lethal injection of sodium pentobarbital (90 mg/kg), transcardially perfused with 100 ml of physiological saline followed by 300 ml of 3% formalin. Brains were post-fixed, sectioned at 50 μm and stained with cresyl violet.
Electron microscopy
The sampling procedure was modified from a previous study34 to obtain four systematic random sampling fields from each rat. Briefly, rats were deeply anesthetized with pentobarbital (120 mg/kg) and transcardially perfused with 0.1 M phosphate buffer (pH 7.2), followed by 2% paraformaldehyde/2.5% glutaraldehyde in 0.1 M phosphate buffer. Brains were post-fixed in the skull overnight, and 9–12 consecutive 175 μm thick coronal slabs were taken throughout the entire rostral–caudal extent of the DAO. The position of the first cut within the first 175 μm interval was selected randomly, and the subsequent cuts were placed at systematic 175 μm intervals from each other. Tissue slabs were rinsed in 0.1 M sodium cacodylate, post-fixed in 1% OsO4/1.5% potassium ferrocyanide, en bloc stained with 2.5% uranyl acetate, dehydrated through a series of acetones, embedded in Epon (Pelco International, Redding, California), and then cured at 60 °C for 2 d. DAO volume was estimated using the Cavalieri principle on a projection microscope at 50.5× by point counting. The cumulative medial–lateral and dorsal–ventral lengths of the DAO were measured and divided into four systematic random intervals to demarcate the lateral and dorsal edge of each sampling field, respectively. Ribbons of 40–45 serial sections (silver-gray interference color) were mounted on 5% Formvar-coated copper slotted grids (Electron Microscopy Sciences, Fort Washington, Pennsylvania), stained with uranyl acetate and lead citrate, and viewed on a Hitachi H-600 (Hitachi, Pleasanton, California) transmission electron microscope with a rotational grid holder at an initial magnification of 8,000×. The same tissue area was photographed in all sections of each series. After that, a micrograph of a calibration grid was taken. Photomicrographs were prepared at a final magnification of 22,400×. Mean section thickness was determined using Small’s method of minimal folds measured directly on the negative (magnification 50,000×). Each series was assigned a code number to be decoded after completion of all analyses.
Excitatory synapses were identified by the presence of rounded or spherical vesicles and an asymmetric postsynaptic density20,21,36. Inhibitory synapses were identified by the presence of pleomorphic or flattened vesicles and a symmetric postsynaptic density20,21,36. Synapses within the middle 32 serial sections (to ensure unequivocal synapse identification34) were counted using the stereological physical disector and an unbiased counting frame34,35. Numerical density was calculated for each rat as the mean number of synapses or multivesicular bodies counted per disector divided by the mean disector volume. The total number of these structural elements was calculated for each rat as the product of the mean numerical density and the estimated DAO volume.
Acknowledgments
We thank Y. Geinisman and J. Kleim for advice regarding electron microscopy procedures, J. Disterhoft and C. Weiss for comments on a previous version of this manuscript, the staff at the Central Microscopy Research Facility at the University of Iowa, and A. Muckler, H. Halverson, C. Rabinak and J. Sweet for technical assistance. Supported by a grant (NS38890 to J.H.F.) and a predoctoral fellowship (NS41713 to D.A.N.) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Marr D. A theory of cerebellar cortex. J Physiol (Lond) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 3.Ito, M. The Cerebellum and Neural Control (Raven, New York, 1984).
- 4.Carew TJ. Developmental assembly of learning in Aplysia. Trends Neurosci. 1989;12:389–394. doi: 10.1016/0166-2236(89)90078-7. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 7.Kandel ER, Pittenger C. The past, the future and the biology of memory storage. Philos Trans R Soc Lond B Biol Sci. 1999;354:2027–2052. doi: 10.1098/rstb.1999.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 9.Medina JF, Repa CJ, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- 10.Diamond, A. The Development and Neural Bases of Higher Cognitive Functions Vol. 8 (NY Academy of Sciences, New York, 1990). [DOI] [PubMed]
- 11.Carew, T.J., Menzel, R. & Shatz, C.J. Mechanistic Relationships Between Development and Learning (Wiley, New York, 1998).
- 12.Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- 13.Stanton ME, Freeman JH, Jr, Skelton RW. Eye-blink conditioning in the developing rat . Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- 14.Stanton, M.E. & Freeman, J.H. Jr. Developmental studies of eye-blink conditioning in a rat model. in Eye-blink Classical Conditioning: Animal (eds. Woodruff-Pak, D.S. & Steinmetz, J.E.) 105–134 (Kluwer, Amsterdam, 2000).
- 15.Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning research with the rabbit. Prog Psychobiol Physiol Psychol. 1983;10:197–275. [Google Scholar]
- 16.Freeman JH, Jr, Nicholson DA. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. J Neurosci. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles, J.C., Ito, M. & Szentogothai, J. The Cerebellum as a Neuronal Machine (Springer, Heidelberg, 1967).
- 18.Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- 19.Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Zeeuw CI, Holstege JC, Ruigrok TJH, Voogd J. Ultrastructural study of the GABAergic, cerebellar and meso-diencephalic innervation of the cat medial accessory olive: anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- 21.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 22.Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- 24.Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- 25.Medina JF, Mauk MD. Simulations of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. J Neurosci. 1999;19:7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh JP, Lang EJ, Sugihara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson DA, Freeman JH., Jr Developmental changes in eye-blink conditioning and neuronal activity in the inferior olive. J Neurosci. 2000;20:8218–8226. doi: 10.1523/JNEUROSCI.20-21-08218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llinas R, Sasaki K. The functional organization of the olivocerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci. 1989;1:587–602. doi: 10.1111/j.1460-9568.1989.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 29.Lang, E.J. & Rosenbluth, J. Role of myelination in the development of a uniform olivocerebellar conduction time. J. Neurophysiol. (in press). [DOI] [PubMed]
- 30.Bloedel JR, Ebner TJ. Rhythmic discharge of climbing fibre afferents in response to natural peripheral stimuli in the cat. J Physiol (Lond) 1984;352:129–146. doi: 10.1113/jphysiol.1984.sp015282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol (Lond) 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loewenstein Y, Yarom Y, Sompolinsky H. The generation of oscillations in networks of electrically coupled cells. Proc Natl Acad Sci USA. 2000;98:8095–8100. doi: 10.1073/pnas.131116898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 34.Geinisman Y, Gundersen HJ, van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;25:805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- 35.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 36.Peters, A., Palay, S.L. & deF. Webster, H. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells (Oxford Univ. Press, New York, 1991).
- 37.Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson RF, Thompson JK, Kim JJ, Krupa DJ, Shinkman PG. The nature of reinforcement in cerebellar learning. Neurobiol Learn Mem. 1998;70:150–176. doi: 10.1006/nlme.1998.3845. [DOI] [PubMed] [Google Scholar]
- 40.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 41.Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 42.Spoelstra J, Schweighofer N, Arbib MA. Cerebellar learning of accurate predictive control for fast-reaching movements. Biol Cybernet. 2000;82:321–333. doi: 10.1007/s004220050586. [DOI] [PubMed] [Google Scholar]
- 43.Freeman JH, Jr, Nicholson DA. Ontogenetic changes in the neural mechanisms of eyeblink conditioning. Integr Physiol Behav Sci. 2001;36:15–35. doi: 10.1007/BF02733945. [DOI] [PubMed] [Google Scholar]
- 44.Aiba A, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 45.Chen C, et al. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKCγ mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 46.Rescorla, R.A. & Wagner, A.R. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. in Classical Conditioning II: Current Research and Theory (eds. Black, A.H. & Prokasy, W.F.) 64–99 (Appleton-Century-Crofts, New York, 1972).
- 47.Fanselow MS. Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron. 1998;20:625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 48.Hesslow G, Ivarsson M. Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Exp Brain Res. 1996;110:36–46. doi: 10.1007/BF00241372. [DOI] [PubMed] [Google Scholar]


