Abstract
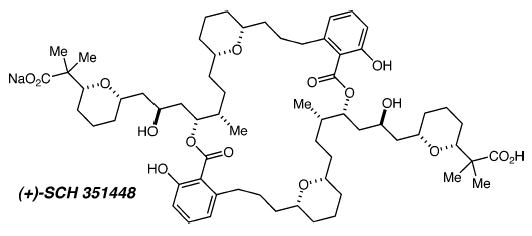
An efficient and stereocontrolled total synthesis of (+)-SCH 351448, a novel activator of low-density lipoprotein receptor promoter, has been achieved with a longest linear sequence of 21 steps. Key steps include applications of the recently developed asymmetric allyl- and crotylsilane reagents and a new protodesilylative version of the tandem silylformylation/allylsilylation reaction, which provides an efficient synthesis of 1,5-syn-diols.
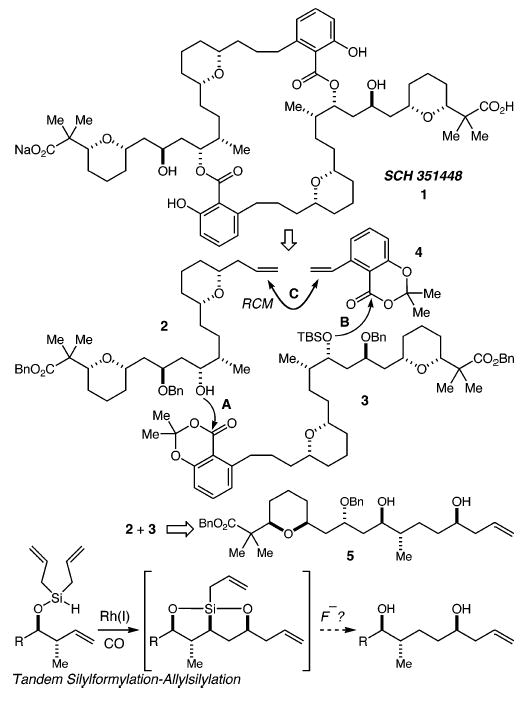
In 2000, researchers at the Schering-Plough Research Institute and Duke University reported the isolation and structure elucidation of a dimeric polyketide they termed SCH 351448 (1).1 The isolation of SCH 351448 was guided by its activation of low-density lipoprotein receptor (LDL-R) promoter. This intriguing biological activity2 and the novel structure have combined to elicit attention from the synthetic community,3 and two total syntheses have been recorded.4,5 Our retrosynthetic analysis envisioned the coupling of alcohol 2 with ester 3 (step A, Scheme 1), followed by deprotection of the tert-butyldimethylsilyl (TBS) group and coupling of the resultant alcohol with ester 46 (step B). Finally, ring-closing metathesis (RCM, step C) would be followed by hydrogenation of the alkene product accompanied by deprotection of the benzyl ethers and esters to provide the natural product. Fragments 2 and 3 could arise from a common intermediate 5. Our tandem silylformylation–allylsilylation methodology7 seemed well-suited to the synthesis of the 1,5-syn-diol in 5 but would require a previously unexplored protodesilylation workup in place of the standard oxidative procedure for triol synthesis.
Scheme 1.
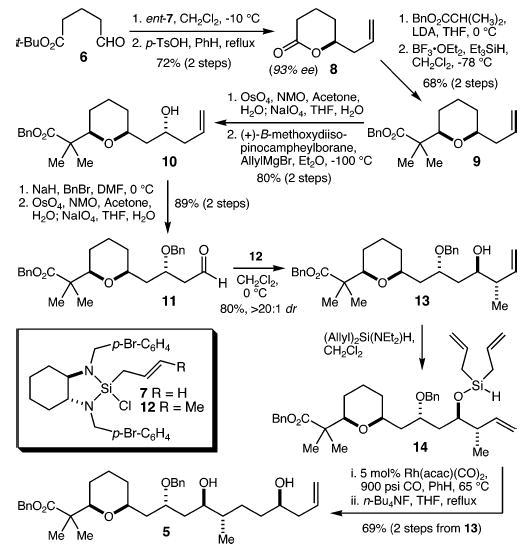
Asymmetric allylation of aldehyde 6 using our recently developed silane reagent ent-78 followed by lactonization with p-TsOH provided lactone 8 in 72% yield (Scheme 2). The ee of the allylation product was found to be 93%. Addition of the lithium enolate derived from benzyl isobutyrate to lactone 8, and cis diastereoselective (>20:1) reduction of the resulting lactol9 gave tetrahydropyran 9 in 68% yield (two steps). Oxidative cleavage of the alkene to the corresponding aldehyde was followed by asymmetric allylation using Brown’s protocol10 (≥10:1 dr) to give alcohol 10 in 80% yield (two steps). Protection of alcohol 10 as a benzyl ether and oxidative cleavage of the alkene gave aldehyde 11 in 89% yield (two steps). Asymmetric crotylation employing the enantiomer of crotylsilane 1211 gave alcohol 13 as a single diastereomer (>20:1) in 80% yield. Treatment of 13 with diallyl-diethylaminosilane6 provided silyl ether 14, which was immediately subjected to the rhodium-catalyzed tandem silylformylation–allylsilylation reaction (5 mol % Rh(acac)(CO)2, 900 psi CO, PhH, 65 ° C).7 Upon ventilation of the high-pressure reaction apparatus, the residue was treated with n-Bu4NF in THF (reflux) to provide diol 5 as a single diastereomer in 69% yield from 13. While this protodesilylative version of our tandem silylformylation–allylsilylation reaction represents a relatively straightforward extension of the methodology, the difficulties encountered by Hoveyda in attempting a protodesilylation in a related β-hydroxysiloxane system12 had given us cause for concern. That the reaction in the present system is indeed smooth provides a direct synthesis of saturated 1,5-syn-diols from homoallylic alcohols.
Scheme 2.
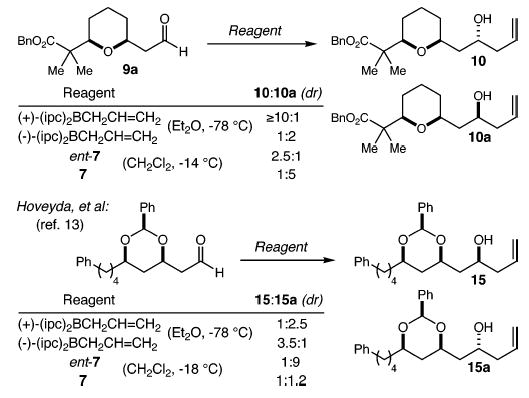
The use of the Brown allylation protocol for the synthesis of 10 is worthy of further comment. When the same allylation was performed using allylsilane ent-7, the diastereoselectivity was poor (2.5:1) (Scheme 3). When the respective enantiomeric allyl reagents were employed, alcohol 10a was the major product with 2:1 and 5:1 dr for the Brown reagent and allylsilane 7, respectively. As shown, similar observations have been recorded by Hoveyda using a different chiral β-alkoxyaldehyde.13 It therefore appears, at least with these two aldehydes, that the two protocols are complementary: moderate to good selectivity for the 1,3-syn product can be realized with the Brown protocol, while moderate to good selectivity for the 1,3-anti product may be secured with our allylsilane reagents. It should be noted that with β-benzyl-oxyaldehydes, the allyl- and crotylsilane reagents 7 and 12 display excellent reagent control, overwhelming any substrate bias, as demonstrated both by the conversion of 11 to 13 and by a similar set of experiments in our earlier work.8
Scheme 3.
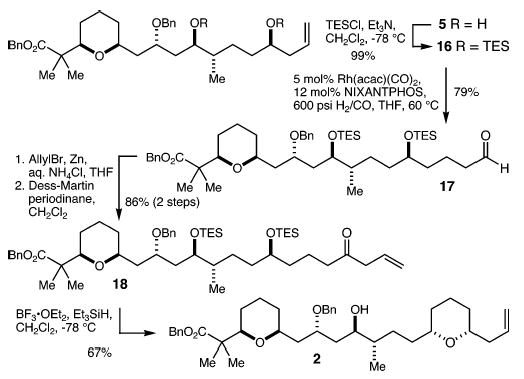
Protection of diol 5 as the bis-triethylsilyl (TES) ether 16 (99%) was followed by hydroformylation using the linear-selective Nixantphos ligand14 to give aldehyde 17 in 79% yield (Scheme 4). Barbier-type allyl addition to 17 using the conditions of Luche15 and subsequent oxidation with the Dess–Martin periodinane16 then produced allyl ketone 18 in 86% overall yield (two steps). Silyl ether deprotection and diastereoselective (>20:1) lactol reduction9 were accomplished by treatment of 18 with Et3SiH and BF3βOEt2, leading to tetrahydropyran 2 in 67% yield.
Scheme 4.
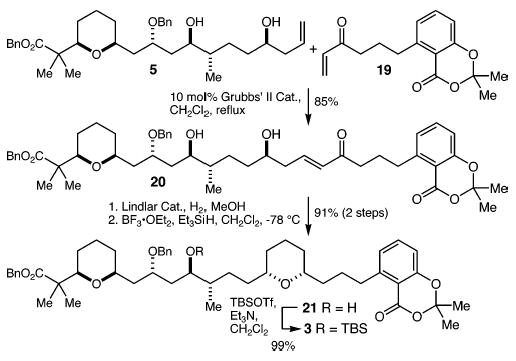
Cross metathesis17 between 5 and enone 196 proceeded smoothly using the second-generation Grubbs catalyst18 to deliver enone 20 in 85% yield (Scheme 5). Conjugate reduction was accomplished by hydrogenation over Lindlar’s catalyst, and the resulting lactol was reduced with Et3SiH and BF3·OEt2 to give tetrahydropyran 21 as a single diastereomer9 (>20:1) in 91% yield (two steps). Finally, protection of the alcohol as its tert-butyldimethylsilyl (TBS) ether proceeded to give 3 in 99% yield.
Scheme 5.
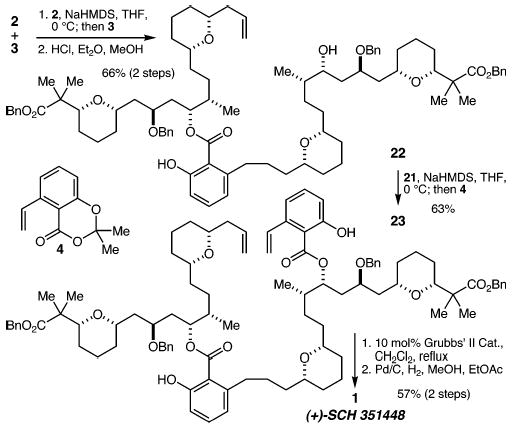
With fragments 2 and 3 in hand, we were positioned to investigate their coupling (Scheme 6). Thus, deprotonation of alcohol 2 with sodium hexamethyldisilazide (NaHMDS) and addition of acetonide 3 led to the desired ester product, and methanolysis of the TBS ether then produced alcohol 22 in 66% yield (two steps). Deprotonation of 22 with 2.5 equiv of NaHMDS and treatment with acetonide 46 provided bis-benzoyl ester 23 in 63% yield. RCM then proceeded smoothly using the second-generation Grubbs catalyst,18 and the macrocycle product was subjected to hydrogenation over Pd/C, resulting in reduction of the alkene, both benzyl ethers, and both benzyl esters. After workup with 4 M HCl saturated with NaCl,4 (+)-SCH 351448 was obtained in 57% yield (two steps). The spectral data for our synthetic material matched those of the natural compound.
Scheme 6.
The stereocontrolled synthesis of (+)-SCH 351448 was achieved in 32 total steps (including the syntheses of 4 and 19) with a longest linear sequence of 21 steps (2.1% overall yield) from 6. The synthesis of the stereochemistry-rich fragment 5 was accomplished in an efficient 11 steps and 19% overall yield from 6, featured two applications of our asymmetric allyl-/crotylation reagents (6→8 and 11→13), and inspired the development of a simple protodesilylative modification of the tandem silylformylation–allylsilylation reaction for the synthesis of 1,5-syn-diols (14→5).
Supplemental Material
Acknowledgments
This work was supported by a grant from the NIH (NIGMS, GM58133). We are grateful to Amgen and Merck Research Laboratories for research support as well.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and stereochemical proofs. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hegde VR, Puar MS, Dai P, Patel M, Gullo VP, Das PR, Bond RW, McPhail AT. Tetrahedron Lett. 2000;41:1351. [Google Scholar]
- 2.a Brown MS, Goldstein JL. Science. 1986;232:34. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]; b Brown MS, Goldstein JL. Cell. 1997;89:331. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 3.a Bhattacharjee A, Soltani O, De Brabander JK. Org Lett. 2002;4:481. doi: 10.1021/ol016938u. [DOI] [PubMed] [Google Scholar]; b Soltani O, De Brabander JK. Angew Chem, Int Ed. 2005;44:1696. doi: 10.1002/anie.200462577. [DOI] [PubMed] [Google Scholar]
- 4.Kang EJ, Cho EJ, Lee YE, Ji MK, Shin DM, Chung YK, Lee E. J Am Chem Soc. 2004;126:2680. doi: 10.1021/ja0315309. [DOI] [PubMed] [Google Scholar]
- 5.Soltani O, De Brabander JK. Org Lett. 2005;7:2791. doi: 10.1021/ol0510769. [DOI] [PubMed] [Google Scholar]
- 6.Synthesis of this compound is detailed in Supporting Information.
- 7.a Zacuto MJ, Leighton JL. J Am Chem Soc. 2000;122:8587. [Google Scholar]; b Zacuto MJ, O’Malley SJ, Leighton JL. Tetrahedron. 2003;59:8889. [Google Scholar]
- 8.Kubota K, Leighton JL. Angew Chem, Int Ed. 2003;42:946. doi: 10.1002/anie.200390252. [DOI] [PubMed] [Google Scholar]
- 9.Lewis MD, Cha KC, Kishi Y. J Am Chem Soc. 1982;104:4976. [Google Scholar]
- 10.Brown HC, Jadhav PK. J Am Chem Soc. 1983;105:2092. [Google Scholar]
- 11.Hackman BM, Lombardi PJ, Leighton JL. Org Lett. 2004;6:4375. doi: 10.1021/ol0480731. [DOI] [PubMed] [Google Scholar]
- 12.Hale MR, Hoveyda AH. J Org Chem. 1992;57:1643. [Google Scholar]
- 13.Gillingham DG, Kataoka O, Garber SB, Hoveyda AH. J Am Chem Soc. 2004;126:12288. doi: 10.1021/ja0458672. See Supporting Information. [DOI] [PubMed] [Google Scholar]
- 14.van der Veen LA, Keeven PH, Schoemaker GC, Reek JNH, Kamer PCJ, van Leeuwen PWNM, Lutz M, Spek AL. Organometallics. 2000;19:872. [Google Scholar]
- 15.Petrier C, Luche JL. J Org Chem. 1985;50:910. [Google Scholar]
- 16.a Dess DB, Martin JC. J Org Chem. 1983;48:4156. [Google Scholar]; b Dess DB, Martin JC. J Am Chem Soc. 1991;113:7277. [Google Scholar]
- 17.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 18.a Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]; b Sanford MS, Love JA, Grubbs RH. J Am Chem Soc. 2001;123:6543. doi: 10.1021/ja010624k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


