Short abstract
The retinoblastoma family consists of the well-studied tumor suppressor pRb/p105 and two recently identified proteins, pRb2/p130 and p107. Members of the family can bind and repress transcription factors and other regulatory proteins and have roles in growth suppression, differentiation and apoptosis.
Abstract
The destiny of a cell - whether it undergoes division, differentiation or death - results from an intricate balance of many regulators, including oncoproteins, tumor-suppressor proteins and cell-cycle-associated proteins. One of the better-studied tumor suppressors is the retinoblastoma protein, known as pRb or p105. Two recently identified proteins, pRb2/p130 and p107, show structural and functional similarities to pRb, and these proteins and their orthologs make up the retinoblastoma (Rb) family. Members of the family have been found in animals and plants, and a related protein is known in the alga Chlamydomonas. Members of the Rb family are bound and inactivated by viral proteins and, in turn, bind cellular transcription factors and repress their function, and can also form complexes with cyclins and cyclin-dependent kinases and with histone deacetylases. They are found in the nucleus and their subnuclear localization depends on binding to the nuclear matrix. Members of the family form part of a signal-transduction pathway called the Rb pathway, which is important in cell-cycle regulation and have roles in growth suppression, differentiation and apoptosis in different organisms and cell types.
Gene organization and evolutionary history
Gene structure and chromosomal localization
The three human genes encoding members of the retinoblastoma (Rb) family share some features that are similar to other housekeeping genes, including a lack of the canonical TATA or CAAT boxes found in the promoters of most differentially expressed genes, the presence of a GC-rich zone immediately surrounding the main transcription-initiation site, the presence of multiple consensus sequences for binding the Sp1 transcription factor, and the presence of multiple transcription start sites [1,2,3].
The human RB gene (which encodes a protein of 105 kDa and is also called p105) was first identified when both familial and sporadic retinoblastoma, a form of malignant tumor of the retina, were found to be associated with deletions at 13q14 [4,5,6]. The RB transcript is encoded by 27 exons dispersed over about 200 kilobases (kb) of genomic DNA; the exons range from 31 to 1,889 base pairs (bp) in length and the introns range from 80 bp to 60 kb.
The human p107 gene (which encodes a protein of 107 kDa and is also known as RBL1) is located at chromosome 20q11.2, a region of special interest because of its association with some myeloid disorders. The arrangement of the p107 gene is similar to that of the other members of the Rb family. It is composed of 22 exons that vary in length from 50 to 840 bp, spanning approximately 100 kb of genomic DNA.
The human Rb2 gene (which encodes a protein of 130 kDa and is also called RBL2) maps to chromosome 16q12.2. The Rb2 messenger RNA is 4.6 kb in length; the gene consists of 22 exons and spans over 50 kb of genomic DNA, and the 21 introns vary in size from 82 bp to almost 9 kb.
The three Rb-family proteins are also known as 'pocket proteins', after their conserved pocket region, which is composed of two conserved domains (A and B) separated by a spacer (Figure 1). The pocket is important for the binding of other proteins (see below). The exons encoding domain A of the RB gene (exons 11-17), domain B (20-23), and the spacer region between domains A and B (18 and 19) are very similar in all members of the family. Interestingly, amino-acid residues that are identical between p107 and pRb2 are also found in the same exonic positions. This feature is not shared with RB, suggesting a closer evolutionary relationship between the p107 and Rb2 genes [7]. Additionally, the spacer regions of Rb2 and p107 show higher similarity to each other than to RB [8].
Figure 1.
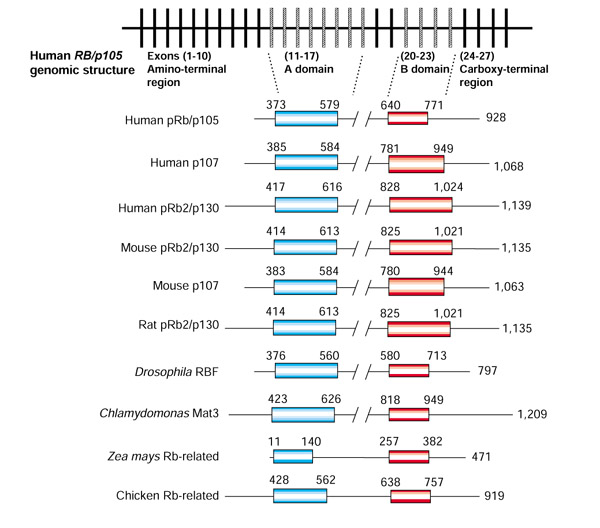
Comparison of the genomic structure of human retinoblastoma genes and of the functional domains in different Rb-related proteins. Boxes indicate exons of the human RB gene; hatched boxes indicate exons encoding domains A and B. Adapted from [7].
Evolutionary history
The Rb-family proteins are fairly well conserved over a range of species. The arrangement of helices in domain A of pRb strongly resembles the cyclin-box folds found in cyclin A and the transcription factor TFIIB [9]; the Rb-family proteins may therefore have arisen in evolution by a tandem duplication of this fold. A phylogenetic tree of pRb protein sequences is shown in Figure 2.
Figure 2.
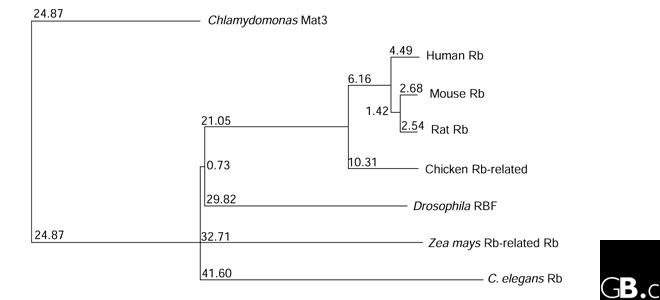
Phylogenetic tree illustrating the diversity of pRb in eight representatives of different phyla and kingdoms. Numbers are branch lengths, which correspond to the estimated evolutionary distance between protein sequences. The tree was constructed using ClustalW.
Mammals
Homologs of the three human Rb-family proteins have also been found in mice. The mouse pRb2 protein has a 43-amino-acid deletion in the pocket domain compared with the human homolog and other members of the family. This region (starting at residue 211 in human pRb2 [10,11]) is highly conserved in human pRb2 and p107, showing 70% identity over the 43 amino acids, but human pRb2 and pRb are only 50% identical over 21 amino acids of the region [12]. The corresponding region in mouse p107 binds and represses the transcription factor Sp1, but the significance of this deletion in mouse pRb2 remains unclear [13]. In both humans and mice, pRb2 shows a higher identity in amino-acid sequence to p107 than to pRb. Regions conserved between pRb2 and pRb are limited to the A and B domains of the pocket region, but conserved regions between pRb2 and p107 appear throughout the entire length of the protein, especially in the amino-terminal region, suggesting that the amino-terminal region could be very important for their functions [12]. Domains A and B and the carboxy-terminal region are highly conserved between the human and mouse p107 proteins. Domains A and B exhibited 90.6% and 89.4% identity respectively, and the carboxy-terminal region showed 91.5% similarity. With the exception of the 100% identity found in the string of amino acids stretching from position 782 to 889 in the B domain of human and mouse p107, the highest level of homology (94%) was found in the amino-terminal domain [14].
Rat pRb2 is almost 90% identical in amino-acid sequence to human pRb2 [15,16]. The 4.87 kb cDNA contains an 1,135 amino-acid open reading frame with high homologies to the human and mouse Rb2 and a partial homology to Rb. Rat pRb2 and rat pRb are conserved only in the pocket region and are only 32% identical in this region [16].
Other vertebrates
Comparison of chicken Rb-family proteins with those of mouse, human and Xenopus reveals a 66% amino-acid identity in the A and B domains of the pocket region but only 33% identity in the spacer between A and B [17]. A 20-amino-acid sequence at the carboxyl terminus is completely conserved in all the aforementioned Rb-family sequences, but its biological function is not yet clear. Although the chicken Rb family proteins demonstrate great similarity to the pRb homologs in mice, human and Xenopus in multiple regions, they also possess characteristics that are unique to each species. The region near the amino terminus is the most variable in Rb proteins in these four species. Chicken and Xenopus pRb each contain a unique and shorter amino terminus than the mouse and human homologs [17,18]. There are no known homologs of human p107 or pRb2 in Xenopus or chicken.
Invertebrates
The Drosophila RBF protein is intriguing, as it has structural features that resemble all three members of the Rb family, suggesting that the RBF gene may have evolved from a common ancestor of the human Rb-family genes. Paradoxically, the nucleotide sequence of RBF is more similar to human p107 and RB2 genes than it is to the Rb gene, but the RBF protein sequence has a higher percentage identity with pRb than with p107 or pRb2. The highly conserved spacer domain found in both p107 and pRb2 is absent in RBF, as is a long insertion in the B segment of the pocket domain, which is present in pRb2 and p107 but not in pRb [19].
The nematode Caenorhabditis elegans has a protein called LIN-35 that has significant sequence similarity with the human Rb pocket proteins [20]. LIN-35 shows 20% identity to human pRb2, 19% to p107, 15% to pRb, and 16% to Drosophila RBF [20]. The highest conservation is found in domains A and B, but the spacer region is not as highly conserved; it is short, as in human pRb. Because LIN-35 is not particularly similar in sequence to any one of the human Rb family proteins, LIN-35 may have diverged from an ancestor common to the Rb family proteins.
Plants
Until recently, it was thought that the Rb family proteins were peculiar to vertebrates [21], but in 1998 a homolog was cloned in a plant [22], and Rb homologs have now been found in maize, tobacco, Chenopodium rubrum (red goosefoot) and Arabidopsis. The conservation of Rb and of other components of the Rb pathway in plants suggests that Rb may have an important role in the development of all multicellular organisms, not just animals. The highest level of identity with human Rb-family proteins (20-35%) is found in the pocket region [23].
There is no evidence of an Rb pathway in any unicellular organism, but the mat3 gene of the unicellular green alga Chlamydomonas reinhardtii, which belongs to the land plant lineage, has a domain structure homologous to Rb [22]. It contains a pocket region with two domains separated by a spacer and also has the sequence Leu-X-Cys-X-Glu (LxCxE in the single-letter amino-acid code, where x indicates a non-conserved amino acid), which is characteristic of the Rb-family proteins and is thought to be a peptide-binding site (see Characteristic structural features). Unlike mammalian Rb-family mutants, however, mat3 mutants do not have a shortened G1 phase, do not enter S phase prematurely, and can exit the cell cycle and differentiate normally, indicating that this Chlamydomonas gene has a different role from that of animal Rb-like genes [24].
Characteristic structural features
The three pocket proteins consist of an amino-terminal domain, a pocket region composed of two conserved domains (A and B, residues 373-771 in human pRb) separated by a spacer region, and a carboxy-terminal domain. The pocket domain is responsible for interaction of the protein with transcription factors, cyclins, and cyclin-dependent kinases (CDKs), and for its functional activity [1,6,8,14,25,26]. The pRb2 and p107 proteins are thought to be more closely related to each other than they are to pRb. Some amino acids present in the B region of pRb are lacking in p107 and in pRb2. Conversely, p107 and pRb2 share a motif in the spacer region, which is absent in the pRb sequence. This enables them to form a strong binding site for cyclin A-Cdk2 and cyclin E-Cdk2 [8,14,26,27,28,29]. Additionally, pRb2 and p107 share a sequence near the amino terminus that is missing in pRb. A 20-amino-acid sequence at the carboxyl terminus is completely conserved in most Rb-family homologs, but its biological function has not been yet clarified.
The crystal structure of human pRb domain A shows that it is composed of nine α helices, two of them forming a hydrophobic core and the remaining seven surrounding this core [9]. In domain A, 47 amino acids are completely conserved between the three human Rb-family proteins, of which 21 are polar and 26 are non-polar. The majority of the conserved non-polar residues interact to stabilize the tertiary structure of the proteins. Interactions of the polar conserved residues suggest that they also have a role in stabilizing the tertiary structure. The A and B domains of the pRb pocket region have a cyclin-fold structural motif that is also common to cyclins and the transcription factor TFIIB. The pRb pocket domain also has a β hairpin, an extended tail, and eight additional helices. The cyclin folds of the B domain are more similar to the cyclin folds in cyclin B and TFIIB than they are to the cyclin folds of the A domain [30].
Both domains A and B are required for interactions with viral oncoproteins and cellular transcription factors [9,30]. The pocket region can also bind proteins that lack the LxCxE motif, such as the E2F family of transcription factors [31,32,33,34,35]. In pRb2 and p107 the spacer region can bind cyclin A-Cdk2 and cyclin E-Cdk2 complexes [36,37,38,39]. The surface residues of pRb that are conserved across species and with human p107 and pRb2 proteins cluster in two regions: the LxCxE-binding site in the B domain and the interface between the A and B domains. The conservation of this interface suggests that it may participate in binding to E2F or to proteins that may mediate transcriptional repression by pRb. Conservation of regions within the LxCxE binding site across species indicates its structural and functional importance. The four residues that meet the backbone of the peptide are identical in pRb homologs from human, newt, chicken, fruit fly and maize and in the human p107 and pRb2 [30].
A dozen distinct phosphorylation sites have been found in the spacer region, but the exact number of serine and threonine residues of pRb that can be phosphorylated during the G1 phase remains undefined [40]. Phosphorylation of pRb is important because it can influence its relationship with interacting proteins [40]. Ten of the potential phosphorylation sites are fully conserved between the three members of the Rb family in the rat: four in the amino-terminal region, five in the carboxy-terminal region, and one in the spacer region [16].
Localization and function
Subcellular distribution
Rb-family proteins are found in the nucleus. High-resolution deconvolution microscopy studies have revealed that, during G1 and S phases, the three pocket proteins are found in perinucleolar foci [41]. A recent study reported that some mechanisms of control of the cell cycle correlate, at least in part, with the compartmentalization of Rb proteins within the nucleus [42]. For example, the cell-cycle-dependent binding of pRb2 and p107 to the E2F4 transcription factor changes as a function of their subnuclear localization. Specifically, in the nucleoplasm, pRb2-E2F4 complexes are more numerous during G0 and G1 phases, whereas in the nucleolus they increase in S phase. In contrast, p107-E2F4 complexes in the nucleoplasm are more numerous in S phase than in G0 or G1 phases, and no cell-cycle change is observed in the nucleolus [42].
Additionally, pRb2, p107, E2F4 and the complexes between pRb2 and the histone deacetylase HDAC1 are all associated with the inner nuclear matrix, and they localize to sites different from pRb. The nuclear matrix, which is composed of chromatin and filamentous structures, is an integral part of nuclear structure and undergoes profound reorganization during DNA replication, gene expression and mitotis [43]. Recently, it has been shown that pRb is associated with the nuclear matrix only during G0 and G1 phases [44], whereas pRb2 and p107 associate with the nuclear matrix in a phase-independent manner [42]. According to Mancini et al. [44], pRb is distributed widely throughout the matrix, particularly at the nuclear periphery and in nucleolar remnants, whereas the core filaments of the matrix contain no detectable pRb. A significantly larger amount of pRb2, p107, E2F4, and their complexes were found in interchromatin than in heterochromatin regions [42]. Because active transcriptional sites are confined to the less-condensed interchromatin regions, it is not surprising that both Rb-related proteins and E2Fs, possibly associated with HDAC1, are more numerous in these regions.
The phosphorylation status of pRb2 and p107 regulates their association with different parts of the nuclear matrix. In extracts from G0/G1-phase cells, pRb2 and p107 are primarily in a hypophosphorylated state; in S-phase extracts, p107 remains hypophosphorylated but pRb2 is hyperphosphorylated, weakly bound to the nuclear matrix and inactivated. This suggests that the repressional control exhibited by pRb2 could be more intricate than that of pRb because the interaction of pRb2 with the nuclear matrix is modulated by phosphorylation as the cell moves from G1 to S phase. Nuclear structure may bring specific sequences together with transcriptional factors in both normal and transformed cells [45]; the association of p107 and pRb2 with the inner nuclear matrix is therefore a promising new area of research.
Tissue expression patterns
The three Rb-family members vary in their expression patterns in different tissues at various stages of the cell cycle: pRb is abundant during all phases of the cell cycle, showing only slight variations in expression levels but significant differences in its phosphorylation status; pRb2 is detectable at high levels in non-proliferating cells; and p107 expression is lost in cells that have withdrawn from the cell cycle, but is high throughout the proliferative cell cycle [31,46,47,48]. The pRb protein is ubiquitously expressed in normal cells and tissues. All three pocket proteins are highly expressed in some differentiated cells, although the pattern of expression is cell-type-specific. In neurons and in skeletal muscle cells there is a high expression of pRb2, whereas p107 shows higher expression levels in breast and prostate epithelial cells [49].
Functions
The retinoblastoma protein was originally described as a tumor suppressor, as it was found to be mutated in many forms of cancer. The region to which the human p107 gene maps (20q11.2) is not normally mutated in tumor cells, but a fraction of human myelogenous leukemias contain deletions of this region [1]. Mutations or deletions within the region containing Rb2 (16q12.2) have been described several times in human neoplasias, including breast, hepatic, ovarian, and prostatic cancers, suggesting that it is also a tumor suppressor [15]. Even though the pocket proteins are highly similar in many ways, each member of the family has distinct functions and has a non-redundant role [39]. Pocket-protein functions sometimes appear redundant, however, such as when the loss of one family member by mutation is totally or partially compensated for by the activity of another family member [50,51,52,53].
Growth-suppressive properties
The three Rb-family members can inhibit cell growth, acting on the cell cycle between G0 and S phases, primarily through binding and inactivation of transcription factors [54]. The growth-suppressive activity of the Rb-family members is cell-type-specific: for example, the C33A human cervical carcinoma cell line is inhibited by overexpression of p107 [25] and pRb2 [55], but not by pRb, whereas the T98G human glioblastoma cell line is sensitive to the growth-suppressive effects of pRb2 yet is unresponsive to that of pRb and p107 [25,56]. Saos-2 human osteosarcoma cells are growth-arrested in the G0/G1 phase of the cell cycle by all of the Rb-family members [25,56,57]. Together, these findings indicate that there are some fundamental differences in the molecular pathways by which the different Rb-family proteins exert control over the cell cycle.
The Rb family and differentiation
The pRb protein has an integral role in various differentiation processes, such as adipogenesis, myogenesis and hematopoiesis [58,59]. Studies on cellular differentiation have shown an interaction between pRb and several differentiation-specific transcription factors, such as the basic helix-loop-helix transcription factor MyoD, nuclear factor activated by interleukin-6 (NF-IL6) and the HMG-box-containing represser HBP1 [60,61,62]. For example, members of the MyoD family associate with pRb, and the binding of pRb to MyoD is thought to induce activation of genes that are specific for myogenic development. This is supported by the finding that cell lines lacking a functional RB are unable to convert into myogenic cells [63].
Perhaps the most convincing evidence of the importance of pRb in cellular differentiation and specialization comes from the studies of RB knockout mice. Homogeneous germline disruptions of the RB gene cause death by day 14 of gestation, associated with gross defects in the development of the hematopoietic and central nervous systems [64,65,66].
The Rb family and apoptosis
In addition to the canonical role of RB as a tumor suppressor gene, it has been recently discovered that pRb also acts as an anti-apoptotic factor. The evidence implies that transforming growth factor β1 (TGF-β1) induces apoptosis by suppressing pRb expression [67], and the active hypophosphorylated form of pRb inhibits the apoptotic function of interferon γ (IFN-γ) [68]. Experiments performed with RB-/- mice demonstrated that widespread cell death occurs in tissues that normally express high levels of pRb, such as liver, ocular lens, nervous system, and skeletal muscle tissue [64,65,66,69]. The p107 protein could also have an anti-apoptotic effect: RB-/-p107-/- double-knockout mouse embryos have more extensive apoptosis in their central nervous system and liver than single mutant RB-/- embryos [70]. Further studies are needed, though, to clarify the role of pRb2 in this context.
The Rb family and angiogenesis
Proper vascularization is necessary for the formation of a tumor mass and for invasion of other tissues during metastasis [71]. New blood vessels form a network in the tumor mass that provides the nourishment and substrates necessary for the progression of tumorigenesis. In fact, if a tumor is not nourished by supports derived from the blood vessels, its diameter is limited to 1-2 mm [72]. The vascularization mechanism is controlled by the highly balanced activities of angiogenic and anti-angiogenic molecules, which act in opposition to each other [72,73]. Two of the major factors regulating angiogenesis are the vascular endothelial growth factor (VEGF) and the multifunctional protein thrombospondin-1 (TSP-1). Recent evidence shows that pRb2, like the oncoprotein Ras and the tumor suppressor p53, is involved in angiogenesis [74,75,76]. Enhanced expression of pRb2 through virus-mediated gene transfer in tumors grown in nude mutant mice downregulates VEGF expression, contributing to the inhibition of tumor formation [76]. To date, no reports have been published on the role of the other Rb family members in angiogenesis.
Frontiers
During the past ten years our understanding of cell-cycle events has increased exponentially. Most of the work on the Rb family so far has focused on the development of assays that enhance our understanding of the key cell-cycle players. With the advent of proteomics, the next steps will be to study the interactions among different proteins and to discern the different protein-expression profiles that occur in normal and diseased tissues. These studies will help to find novel diagnostic and prognostic markers, as well as new and more specific targets for future molecular therapies.
Acknowledgments
Acknowledgements
T.T. is supported by funding from "Dottorato in Patologia Diagnostica e Quantitativa", University of Siena, Italy, and by a fellowship from the "Fondazione Aldo Gini", Padova.
References
- Ewen ME, Xing YG, Lawrence JB, Livingston DM. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991;66:1155–1164. doi: 10.1016/0092-8674(91)90038-z. Cloning of the cDNA for human p107, a retinoblastoma-related protein and its chromosomal mapping. [DOI] [PubMed] [Google Scholar]
- Baldi A, Boccia V, Claudio PP, De Luca A, Giordano A. Genomic structure of the human retinoblastoma-related Rb2/p130 gene. Proc Natl Acad Sci USA. 1996;93:4629–3462. doi: 10.1073/pnas.93.10.4629. Characterization of the genomic structure of human RB-related gene RB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong FD, Huang HJ, To H, Young LJ, Oro A, Bookstein R, Lee EY, Lee WH. Structure of the human retinoblastoma gene. Proc Natl Acad Sci USA. 1989;86:5502–5506. doi: 10.1073/pnas.86.14.5502. Characterization of the genomic structure of the human Retinoblastoma gene RB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung YK, Murphree AL, T'Ang A, Qian J, Hinrichs SH, Benedict WF. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987;236:1657–1661. doi: 10.1126/science.2885916. Evidence of identifiable structural changes of the RB gene in cancers including, in some cases, homozygous internal deletions with corresponding truncated transcripts. [DOI] [PubMed] [Google Scholar]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. Evidence of the presence of mutations in the retinoblastoma gene in human retinoblastoma and osteosarcoma. [DOI] [PubMed] [Google Scholar]
- Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987;235:1394–1399. doi: 10.1126/science.3823889. Cloning of the cDNA for human pRb, the retinoblastoma protein. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Hanafusa H, Takimoto H, Ohgama Y, Akagi T, Shimizu K. Structure of the human retinoblastoma-related p107 gene and its intragenic deletion in a B-cell lymphoma cell line. Gene. 2000;251:37–43. doi: 10.1016/s0378-1119(00)00193-1. Characterization of the genomic structure of the human retinoblastoma-related gene p107. [DOI] [PubMed] [Google Scholar]
- Mayol X, Grana X, Baldi A, Sang N, Hu Q, Giordano A. Cloning of a new member of the retinoblastoma gene family (pRb2) which binds to the E1A transforming domain. Oncogene. 1993;8:2561–2566. cDNA cloning of human pRb2, which binds to the adenovirus E1A. [PubMed] [Google Scholar]
- Kim HY, Cho Y. Structural similarity between the pocket region of retinoblastoma tumour suppressor and the cyclin-box. Nat Struct Biol. 1997;4:390–395. doi: 10.1038/nsb0597-390. Determination of the X-ray crystal structure of the pocket region of pRb. [DOI] [PubMed] [Google Scholar]
- LeCouter JE, Whyte PF, Rudnicki MA. Cloning and expression of the Rb-related mouse p130 mRNA. Oncogene. 1996;12:1433–1440. cDNA cloning and expression of mouse pRb2. [PubMed] [Google Scholar]
- Pertile P, Baldi A, De Luca A, Bagella L, Virgilio L, Pisano MM, Giordano A. Molecular cloning, expression, and developmental characterization of the murine retinoblastoma-related gene Rb2/p130. Cell Growth Differ. 1995;6:1659–1664. cDNA cloning and developmental characterization of mouse pRb2. [PubMed] [Google Scholar]
- Chen G, Guy CT, Chen HW, Hu N, Lee EY, Lee WH. Molecular cloning and developmental expression of mouse p130, a member of the retinoblastoma gene family. J Biol Chem. 1996;271:9567–9572. cDNA cloning and developmental characterization of mouse pRb2. [PubMed] [Google Scholar]
- Datta PK, Raychaudhuri P, Bagchi S. Association of p107 with Sp1: genetically separable regions of p107 are involved in regulation of E2F- and Sp1-dependent transcription. Mol Cell Biol. 1995;15:5444–5452. doi: 10.1128/mcb.15.10.5444. The Rb-related protein p107 has been shown to be a regulator of the transcription factor E2F. The authors provide evidence for a novel interaction between p107 and the transcription factor Sp1, showing that distinct regions of p107 are involved in the control of Sp1 and E2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Graham C, Lacy S, Duncan AM, Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. Isolation by cDNA cloning of the human pRb2, purified through coimmunoprecipitation with E1A. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Bell DW, Testa JR, Mayol X, Baldi A, Grana X, Klinga-Levan K, Knudson AG, Giordano A. The retinoblastoma-related gene, RB2, maps to human chromosome 16q12 and rat chromosome 19. Oncogene. 1993;8:3465–3468. Chromosomal mapping of the human Rb-related gene Rb2. [PubMed] [Google Scholar]
- Sawada Y, Nomura H, Endo Y, Umeki K, Fujita T, Ohtaki S, Fujinaga K. Cloning and characterization of the rat p130, a member of the retinoblastoma gene family. Biochim Biophys Acta. 1997;1361:20–27. doi: 10.1016/s0925-4439(97)00037-9. cDNA cloning and characterization of rat pRb2. [DOI] [PubMed] [Google Scholar]
- Feinstein R, Bolton WK, Quinones JN, Mosialos G, Sif S, Huff JL, Capobianco AJ, Gilmore TD. Characterization of a chicken cDNA encoding the retinoblastoma gene product. Biochim Biophys Acta. 1994;1218:82–86. doi: 10.1016/0167-4781(94)90103-1. Cloning of chicken retinoblastoma cDNA. [DOI] [PubMed] [Google Scholar]
- Destree OH, Lam KT, Peterson-Maduro LJ, Eizema K, Diller L, Gryka MA, Frebourg T, Shibuya E, Friend SH. Structure and expression of the Xenopus retinoblastoma gene. Dev Biol. 1992;153:141–149. doi: 10.1016/0012-1606(92)90098-2. Characterization of the Xenopus Retinoblastoma gene. [DOI] [PubMed] [Google Scholar]
- Du W, Vidal M, Xie JE, Dyson N. RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev. 1996;10:1206–1218. doi: 10.1101/gad.10.10.1206. Discovery of an RB-related gene in Drosophila. [DOI] [PubMed] [Google Scholar]
- Lu X, Horvitz HR. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell. 1998;95:981–991. doi: 10.1016/s0092-8674(00)81722-5. lin-35 in C. elegans encodes a protein similar to the tumor suppressors pRb, p107 and pRb2. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. A general review on retinoblastoma proteins. [DOI] [PubMed] [Google Scholar]
- Gutierrez C. The retinoblastoma pathway in plant cell cycle and development. Curr Opin Plant Biol. 1998;1:492–497. doi: 10.1016/s1369-5266(98)80041-1. A review of the retinoblastoma protein in plants. [DOI] [PubMed] [Google Scholar]
- de Jager SM, Murray JA. Retinoblastoma proteins in plants. Plant Mol Biol. 1999;41:295–299. doi: 10.1023/a:1006398232003. Description of the retinoblastoma proteins in plants. [DOI] [PubMed] [Google Scholar]
- Umen JG, Goodenough UW. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev. 2001;15:1652–1661. doi: 10.1101/gad.892101. Discovery of a retinoblastoma-related protein in Chlamydomonas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. Discovery of the growth-suppressive properties of p107. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Demetrick D, Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993;7:2378–2391. doi: 10.1101/gad.7.12a.2378. Characterization of human pRb2 by cDNA cloning and two-hybrid protein interaction screening in yeast. [DOI] [PubMed] [Google Scholar]
- Lees E, Faha B, Dulic V, Reed SI, Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. An analysis of the association of cyclin E-Cdk2 and cyclin A-Cdk2 complexes with p107 and E2F. [DOI] [PubMed] [Google Scholar]
- Faha B, Ewen ME, Tsai LH, Livingston DM, Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992;255:87–90. doi: 10.1126/science.1532458. This article and [29] describe the interaction of p107 and cyclin A. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Faha B, Harlow E, Livingston DM. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. See [28]. [DOI] [PubMed] [Google Scholar]
- Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–865. doi: 10.1038/36038. Structure of the retinoblastoma pocket domain bound to a peptide from human papillomavirus E7. [DOI] [PubMed] [Google Scholar]
- Paggi MG, Baldi A, Bonetto F, Giordano A. Retinoblastoma protein family in cell cycle and cancer: a review. J Cell Biochem. 1996;62:418–430. doi: 10.1002/(SICI)1097-4644(199609)62:3%3C418::AID-JCB12%3E3.0.CO;2-E. A general review on retinoblastoma proteins and cancer. [DOI] [PubMed] [Google Scholar]
- Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. E2F appears to be a functional target for pRb. The disruption of this E2F-pRb interaction may be a common mechanism of action for the oncoproteins encoded by DNA tumor viruses. [DOI] [PubMed] [Google Scholar]
- Qin XQ, Chittenden T, Livingston DM, Kaelin WG., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992;6:953–964. doi: 10.1101/gad.6.6.953. Identification of growth-suppressive domain in pRb. All naturally occurring RB mutations known to be compatible with stable protein expression map to the T/E1A and cellular protein-binding region (the domain). When full-length pRb and certain truncated forms were synthesized in human RB-/- cells, the authors found that the minimal region necessary for overt growth suppression extended from residue 379 to 928. A functional pocket domain and sequences extending from the carboxy-terminal boundary of the pocket to the carboxyl terminus of the protein were both necessary for growth suppression. Both sets of sequences were also required for E2F binding; hence, the two functions may be linked. [DOI] [PubMed] [Google Scholar]
- Qian Y, Luckey C, Horton L, Esser M, Templeton DJ. Biological function of the retinoblastoma protein requires distinct domains for hyperphosphorylation and transcription factor binding. Mol Cell Biol. 1992;12:5363–5372. doi: 10.1128/mcb.12.12.5363. The biological function of pRb requires phosphorylation. The authors constructed a panel of deletion mutants of pRb expression vectors and used a biological assay for pRb that measures growth inhibition and morphologic changes in pRb-transfected Saos-2 cells to correlate structural alterations of the pRb coding region with function. They identified two regions of pRb that are required for E2F binding and for hyperphosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert SW, Chellappan SP, Horowitz JM, Nevins JR. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. The identification of a feedback regulatory loop between E2F and pRb. [DOI] [PubMed] [Google Scholar]
- De Luca A, MacLachlan TK, Bagella L, Dean C, Howard CM, Claudio PP, Baldi A, Khalili K, Giordano A. A unique domain of pRb2/p130 acts as an inhibitor of Cdk2 kinase activity. J Biol Chem. 1997;272:20971–20974. doi: 10.1074/jbc.272.34.20971. This paper shows that a portion of Rb2/p130 acts as an inhibitor of Cdk2 kinase activity. [DOI] [PubMed] [Google Scholar]
- Zhu L, Enders G, Lees JA, Beijersbergen RL, Bernards R, Harlow E. The pRB-related protein p107 contains two growth suppression domains: independent interactions with E2F and cyclin/cdk complexes. EMBO J. 1995;14:1904–1913. doi: 10.1002/j.1460-2075.1995.tb07182.x. p107 contains two growth suppression domains. One domain corresponds to the sequences needed for interaction with the transcription factor E2F, and the other corresponds to the interaction domain for cyclin A or cyclin E complexes. In cervical carcinoma cell line C33A, which was previously shown to be sensitive to p107 but resistant to pRb growth suppression, only the cyclin-binding domain is active as a growth suppressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Harlow E, Dynlacht BD. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev. 1995;9:1740–1752. doi: 10.1101/gad.9.14.1740. p107 uses a domain related to the cyclin-dependent kinase inhibitor p21 to bind cyclin/Cdk2. [DOI] [PubMed] [Google Scholar]
- Claudio PP, De Luca A, Howard CM, Baldi A, Firpo EJ, Koff A, Paggi MG, Giordano A. Functional analysis of pRb2/p130 interaction with cyclins. Cancer Res. 1996;56:2003–2008. An analysis of the interaction of pRb2 with cyclins and its functional meaning. [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. The pRb protein acts to constrain the G1-S transition in mammalian cells. Phosphorylation of pRb in G1 phase inactivates its growth-inhibitory function, allowing for cell-cycle progression. Complete phosphorylation of pRb, inactivation of E2F binding, and activation of E2F transcription occur only after sequential action of at least two distinct G1 cyclin kinase complexes, such as cyclin D-Cdk4/6 and cyclin E-Cdk2 complexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 2000;14:2855–2868. doi: 10.1101/gad.842600. Nuclear organization of DNA replication in mammalian cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini N, Trimarchi C, Claudio PP, Stiegler P, Marinelli F, Maltarello MC, La Sala D, De Falco G, Russo G, Ammirati G, et al. pRb2/p130 and p107 control cell growth by multiple strategies and in association with different compartments within the nucleus. J Cell Physiol. 2001;189:34–44. doi: 10.1002/jcp.1135. Ultrastructural distribution of pRb2 and p107 and their association with nuclear matrix. [DOI] [PubMed] [Google Scholar]
- Loidl P, Eberharter A. Nuclear matrix and the cell cycle. Int Rev Cytol. 1995;162B:377–403. doi: 10.1016/s0074-7696(08)62622-4. A general review on the relationship between nuclear matrix and the cell cycle. [DOI] [PubMed] [Google Scholar]
- Mancini MA, Shan B, Nickerson JA, Penman S, Lee WH. The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc Natl Acad Sci USA. 1994;91:418–422. doi: 10.1073/pnas.91.1.418. This paper shows that the retinoblastoma gene product is a nuclear-matrix-associated protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, Montecino M, van Wijnen AJ, Stein JL, Lian JB. Nuclear structure-gene expression interrelationships: implications for aberrant gene expression in cancer. Cancer Res. 2000;60:2067–2076. A review describing the emerging evidence for functional interrelationships of nuclear structure and gene expression. A linkage is suggested between tumor-related modifications in nuclear organization and compromised gene regulation during the onset and progression of cancer. [PubMed] [Google Scholar]
- Kondo T, Higashi H, Nishizawa H, Ishikawa S, Ashizawa S, Yamada M, Makita Z, Koike T, Hatakeyama M. Involvement of pRB-related p107 protein in the inhibition of S phase progression in response to genotoxic stress. J Biol Chem. 2001;276:17559–17567. doi: 10.1074/jbc.M009911200. This paper describes the involvement of p107 in the inhibition of S-phase progression. [DOI] [PubMed] [Google Scholar]
- Baldi A, De Luca A, Claudio PP, Baldi F, Giordano GG, Tommasino M, Paggi MG, Giordano A. The RB2/p130 gene product is a nuclear protein whose phosphorylation is cell cycle regulated. J Cell Biochem. 1995;59:402–408. doi: 10.1002/jcb.240590311. pRb2 is a nuclear protein and it is cell-cycle-regulated by phosphorylation. [DOI] [PubMed] [Google Scholar]
- Cinti C, Leoncini L, Nyongo A, Ferrari F, Lazzi S, Bellan C, Vatti R, Zamparelli A, Cevenini G, Tosi GM, Claudio PP, Maraldi NM, Tosi P, Giordano A. Genetic alterations of the retinoblastoma-related gene RB2/p130 identify different pathogenetic mechanisms in and among Burkitt's lymphoma subtypes. Am J Pathol. 2000;156:751–760. doi: 10.1016/s0002-9440(10)64941-3. The first evidence of a genetic alteration in pRb2 in Burkitt's lymphomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi A, Esposito V, De Luca A, Fu Y, Meoli I, Giordano GG, Caputi M, Baldi F, Giordano A. Differential expression of Rb2/p130 and p107 in normal human tissues and in primary lung cancer. Clin Cancer Res. 1997;3:1691–1697. Immunohistochemical expression of pRb2 and p107 in normal tissues and primary lung cancers. pRb2 is expressed at low levels in most aggressive lung tumors. [PubMed] [Google Scholar]
- Wiman KG. The retinoblastoma gene: role in cell cycle control and cell differentiation. Faseb J. 1993;7:841–845. doi: 10.1096/fasebj.7.10.8393817. A review describing the role of the retinoblastoma protein in cell-cycle control and differentiation. [DOI] [PubMed] [Google Scholar]
- Sage J, Mulligan GJ, Attardi LD, Miller A, Chen S, Williams B, Theodorou E, Jacks T. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–3050. doi: 10.1101/gad.843200. This article confirms the essential role of the Rb family in the control of the G1-S transition; the three Rb family members are placed downstream of multiple cell-cycle control pathways, and the link between loss of cell-cycle control and tumorigenesis is confirmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Zacksenhaus E, Gallie BL, Phillips RA. The retinoblastoma gene family is differentially expressed during embryogenesis. Oncogene. 1997;14:1789–1797. doi: 10.1038/sj.onc.1201014. An article reporting differential expression of pRb, p107 and pRb2 during embryogenesis. Members of the family have distinct but overlapping roles, with p107 and pRb possibly having redundant functions in the central nervous system and liver. [DOI] [PubMed] [Google Scholar]
- Chen PL, Riley DJ, Lee WH. The retinoblastoma protein as a fundamental mediator of growth and differentiation signals. Crit Rev Eukaryot Gene Expr. 1995;5:79–95. A review describing mouse models created through gene knockout and transgenic methods. [PubMed] [Google Scholar]
- Paggi MG, Giordano A. Who is the boss in the retinoblastoma family? The point of view of Rb2/p130, the little brother. Cancer Res. 2001;61:4651–4654. A general review on the retinoblastoma protein family. [PubMed] [Google Scholar]
- Lacy S, Whyte P. Identification of a p130 domain mediating interactions with cyclin A/cdk 2 and cyclin E/cdk 2 complexes. Oncogene. 1997;14:2395–2406. doi: 10.1038/sj.onc.1201085. Identification of a pRb2 domain mediating the interaction with cyclin A-Cdk2 or cyclin E-Cdk2. Using GST-pRb2 fusion proteins representing various regions of pRb2, baculovirus-produced cyclin A-Cdk2 and cyclin E-Cdk2 complexes were found to interact with residues within the spacer region. Cyclin E was able to bind the pRb2 spacer region in the presence or absence of Cdk2 whereas cyclin A binding was dependent upon the presence of Cdk2. [DOI] [PubMed] [Google Scholar]
- Claudio PP, Howard CM, Baldi A, De Luca A, Fu Y, Condorelli G, Sun Y, Colburn N, Calabretta B, Giordano A. p130/pRb2 has growth suppressive properties similar to yet distinctive from those of retinoblastoma family members pRb and p107. Cancer Res. 1994;54:5556–5560. The first evidence for the growth-suppressive properties of pRb2. [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. Functional assessment of how G1- and S-phase cyclins act as regulators of pRb function in the cell cycle by promoting pRb phosphorylation. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Giordano A. Synergistic role of E1A-binding proteins and tissue-specific transcription factors in differentiation. J Cell Biochem. 1997;67:423–431. doi: 10.1002/(sici)1097-4644(19971215)67:4<423::aid-jcb1>3.0.co;2-u. A review that describes the complex relationship between tissue-specific transcription factors and genes regulating the cell cycle. [DOI] [PubMed] [Google Scholar]
- Condorelli GL, Testa U, Valtieri M, Vitelli L, De Luca A, Barberi T, Montesoro E, Campisi S, Giordano A, Peschle C. Modulation of retinoblastoma gene in normal adult hematopoiesis: peak expression and functional role in advanced erythroid differentiation. Proc Natl Acad Sci USA. 1995;92:4808–4812. doi: 10.1073/pnas.92.11.4808. The RB gene plays an erythroid- and stage-specific role in normal adult hematopoiesis, particularly at the level of late erythroid hematopoietic progenitor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo K, Wills KN, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. Describes the role of pRb in myocyte survival. [PubMed] [Google Scholar]
- Carnac G, Fajas L, L'Honore A, Sardet C, Lamb NJ, Fernandez A. The retinoblastoma-like protein p130 is involved in the determination of reserve cells in differentiating myoblasts. Curr Biol. 2000;10:543–546. doi: 10.1016/s0960-9822(00)00471-1. This paper shows that pRb2 preferentially accumulates during muscle differentiation in reserve cells. The authors propose that pRb2, by blocking cell-cycle progression and differentiation, could be part of a specific pathway that defines a pool of reserve cells during terminal differentiation. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. A general review on the functional complexities of E2F and Rb families. [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. This paper shows that pRb has an important role in the production and maintenance of the terminally differentiated phenotype of muscle cells. [DOI] [PubMed] [Google Scholar]
- Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–294. doi: 10.1038/359288a0. A knockout of pRb shows that it is essential for normal mouse development. [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. The knockout of the retinoblastoma protein, showing that heterozygous animals are not predisposed to retinoblastoma, but some display pituitary tumors arising from cells in which the wild-type RB allele is absent. Embryos homozygous for the mutation die between days 14 and 15 of gestation, exhibiting neuronal cell death and defective erythropoiesis. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. doi: 10.1038/359328a0. This paper describes the knockout of the retinoblastoma protein and shows that homozygous mutant embryos fail to reach term and have a number of abnormalities in neural and haematopoietic development. [DOI] [PubMed] [Google Scholar]
- Fan G, Ma X, Kren BT, Steer CJ. The retinoblastoma gene product inhibits TGF-beta1 induced apoptosis in primary rat hepatocytes and human HuH-7 hepatoma cells. Oncogene. 1996;12:1909–1919. This article and [68] describe the role of the retinoblastoma protein in apoptosis. [PubMed] [Google Scholar]
- Berry DE, Lu Y, Schmidt B, Fallon PG, O'Connell C, Hu SX, Xu HJ, Blanck G. Retinoblastoma protein inhibits IFN-gamma induced apoptosis. Oncogene. 1996;12:1809–1819. See [67]. [PubMed] [Google Scholar]
- Zacksenhaus E, Jiang Z, Chung D, Marth JD, Phillips RA, Gallie BL. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 1996;10:3051–3064. doi: 10.1101/gad.10.23.3051. This paper places pRb at a nodal point in controlling cellular proliferation, differentiation, and death during terminal differentiation of skeletal muscles in vivo. [DOI] [PubMed] [Google Scholar]
- Lee MH, Williams BO, Mulligan G, Mukai S, Bronson RT, Dyson N, Harlow E, Jacks T. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. This article provides the first in vivo evidence that p107 and pRb have overlapping functions in some tissues of the developing and adult mouse. [DOI] [PubMed] [Google Scholar]
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. This article and [72,73] are general reviews on angiogenesis of tumors. [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. See [71]. [DOI] [PubMed] [Google Scholar]
- Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. See [71]. [DOI] [PubMed] [Google Scholar]
- Rak J, Kerbel RS. Ras regulation of vascular endothelial growth factor and angiogenesis. Methods Enzymol. 2001;333:267–283. doi: 10.1016/s0076-6879(01)33062-8. This article describes the Ras regulation of vascular endothelial growth factor. In this paper the authors explore the possibility that Ras antagonists and signal transduction inhibitors may synergize with a number of other antiangiogenic modalities such as direct-acting antiangiogenic agents (for example, endostatin) or antivascular regimens involving low-dose continuous chemotherapy as a vasculature-targeting strategy. [DOI] [PubMed] [Google Scholar]
- Holmgren L, Jackson G, Arbiser J. p53 induces angiogenesis-restricted dormancy in a mouse fibrosarcoma. Oncogene. 1998;17:819–824. doi: 10.1038/sj.onc.1201993. The first evidence that p53 regulates angiogenesis. [DOI] [PubMed] [Google Scholar]
- Claudio PP, Stiegler P, Howard CM, Bellan C, Minimo C, Tosi GM, Rak J, Kovatich A, De Fazio P, Micheli P, et al. RB2/p130 gene-enhanced expression down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in vivo. Cancer Res. 2001;61:462–468. The first evidence that pRb2 inhibits angiogenesis. [PubMed] [Google Scholar]


