Abstract
Chloroplasts are a major destination of protein traffic within leaf cells. Protein import into chloroplasts is mediated by a set of translocon complexes at the chloroplast envelope. Current data indicate that the expression of translocon genes is regulated in a tissue-specific manner, possibly to accommodate the higher import demand of chloroplasts in leaves and the lower demand of plastids in other tissues. We have designed a transgene-based positive screen to isolate mutants disrupted in protein import into plastids. The first locus we isolated, CIA2, encodes a protein containing a motif conserved within the CCT family of transcription factors. Biochemical analysis indicates that CIA2 is responsible for specific upregulation of the translocon genes atToc33 and atToc75 in leaves. Identification of CIA2 provides new insights into the tissue-specific regulation of translocon gene expression.
INTRODUCTION
Most proteins in chloroplasts are encoded by the nucleus and imported post-translationally into chloroplasts. Except for some outer envelope membrane proteins, nucleus-encoded chloroplast proteins are synthesized as higher molecular weight precursors with N-terminal extensions called transit peptides. Transit peptides are necessary and sufficient for the import of precursor proteins into chloroplasts. Transport across the double membrane envelope is mediated by a set of translocon components located in the envelope. Several translocon components have been identified from pea chloroplasts by cross-linking or coimmunoprecipitating with importing precursor proteins (reviewed by Schleiff and Soll, 2000). They are collectively named Toc (for translocon at the outer envelope membrane of chloroplasts) and Tic (for translocon at the inner envelope membrane of chloroplasts) proteins (Schnell et al., 1997).
Among the Toc components identified, Toc159 is proposed to function as the transit peptide receptor (Perry and Keegstra, 1994; Ma et al., 1996). A considerable amount of evidence indicates that Toc75 is the major component of the protein-translocating channel in the outer membrane (Hinnah et al., 1997; Reumann et al., 1999). The function of Toc34 is not clear. It has been shown to be tightly associated with Toc75 (Seedorf et al., 1995). Arabidopsis has two Toc34 orthologs, atToc34 and atToc33. These two proteins seem to have distinct but overlapping functions (Gutensohn et al., 2000).
Techniques such as coimmunoprecipitation and cross-linking, when used to identify translocon components, usually identify abundant and stably associated components. Regulatory components that are present in minute amounts or only transiently, and upstream regulators present in different locations, usually are missed by these techniques.
More recently, genetics has been used to study protein import into chloroplasts. Arabidopsis mutants have been found for two translocon components, atToc159 (Bauer et al., 2000) and atToc33 (Jarvis et al., 1998). These mutants confirmed that the translocon components identified by cell biology techniques function in chloroplast import. They also provided valuable information on the physiology and regulation of chloroplast protein import. Furthermore, maize and Arabidopsis mutants defective in protein transport to the thylakoid also have been isolated (Voelker and Barkan, 1995; Voelker et al., 1997; Roy and Barkan, 1998; Amin et al., 1999; Moore et al., 2000). These mutants helped to further define the various pathways of transport to the thylakoid and even helped to uncover a new export pathway in Escherichia coli. However, all of the mutants mentioned above were isolated either accidentally in screens for other mutants or by reverse genetics.
We have designed a transgene-based screening strategy to systematically isolate mutants in the chloroplast protein import pathway. We report here the cloning and characterization of the mutant locus cia2. CIA2 encodes a nuclear protein that positively regulates the transcript abundance of atToc33 and atToc75 in leaves.
RESULTS
Screening Strategy and Mutant Isolation
For mutant isolation, we designed and transformed into wild-type Arabidopsis a reporter construct, TP30, to use as a selection marker. As shown in Figure 1A, TP30 encoded two fusion proteins. The first fusion protein was the transit peptide of the small subunit of ribulose bisphosphate carboxylase (RBCS) fused to hygromycin phosphotransferase (HPT). The second fusion protein contained the maize waxy transit peptide fused to the E. coli β-glucuronidase (GUS) (Klösgen et al., 1989). Both fusion constructs were placed under the control of the 35S promoter of cauliflower mosaic virus. Our screening was based on the following rationale. In wild-type plants containing the TP30 reporter, plants should remain hygromycin sensitive as a result of the sequestering of HPT into chloroplasts by the RBCS transit peptide (Figure 1B). However, in mutants defective in chloroplast protein import, some HPT may remain in the cytosol, and the mutants should be more hygromycin resistant. The original purpose of the second marker, waxy-GUS, was to eliminate possible cis mutations that affect the expression of the transgene. However, because of technical difficulties of observing GUS staining in chloroplasts, this marker was not used in the actual screens.
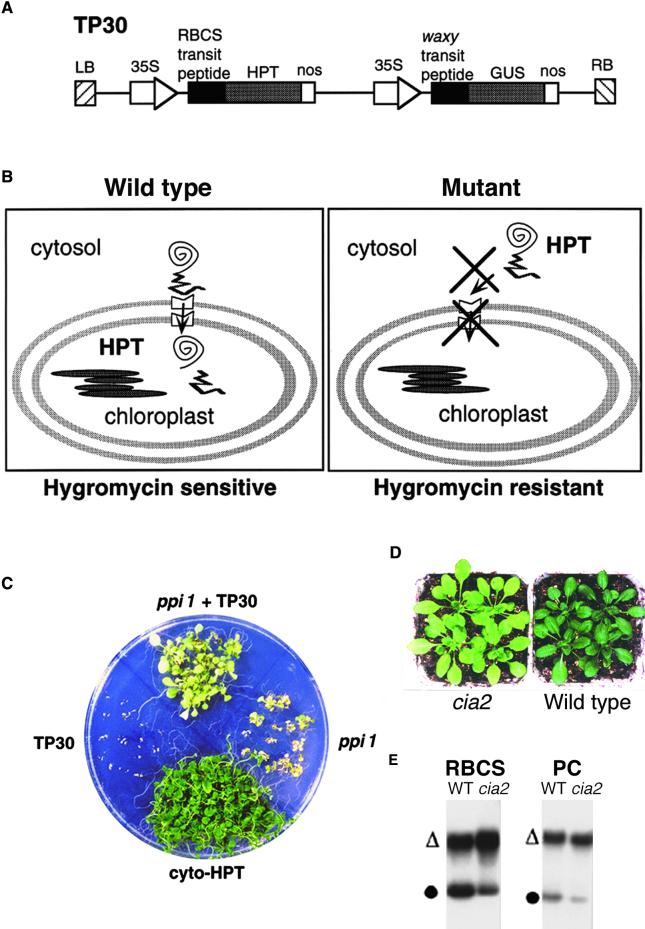
Figure 1.
Screening of cia Mutants.
(A) Scheme of the TP30 construct. LB and RB, left and right borders of the T-DNA, respectively; 35S, the 35S promoter of cauliflower mosaic virus; nos, nopaline synthase terminator.
(B) The screening strategy. Wild-type plants remain hygromycin sensitive as a result of the sequestering of HPT into chloroplasts by the RBCS transit peptide. Mutants are more hygromycin resistant because of the detention of HPT in the cytosol caused by defective chloroplast protein import.
(C) Positive demonstration of the screen. ppi1 + TP30, a ppi1 mutant line containing the TP30 marker. Plants were grown on medium containing 60 μg/mL hygromycin for 26 days.
(D) The visible phenotype of cia2. Plants were grown on soil for 26 days.
(E) The cia2 mutant is defective in chloroplast protein import. Chloroplasts were isolated from 28-day-old plants and used for in vitro protein import experiments with precursor proteins of RBCS and plastocyanin (PC). Open triangles indicate the precursor proteins, and closed circles indicate the imported mature proteins. WT, wild type.
To demonstrate the feasibility of our screen, we crossed the TP30 construct into the known chloroplast protein import mutant ppi1, which is a null mutant of atToc33 (Jarvis et al., 1998). The ppi1 mutant containing the TP30 reporter was indeed more hygromycin resistant than were the wild-type TP30 plants (Figure 1C). A control transgenic line containing only the HPT gene without the RBCS transit peptide also was generated. This line was very hygromycin resistant because of the cytosolic localization of HPT (Figure 1C, cyto-HPT).
We first isolated six potential mutants from 26,000 ethyl methanesulfonate–mutagenized TP30 M2 seed. We named these mutants cia (for chloroplast import apparatus). Cloning and characterizations of the cia2 mutant are described in this article.
Characterization of the cia2 Mutant
The cia2 mutant has a pale phenotype (Figure 1D). To determine the nature of the mutation, cia2 was backcrossed to the unmutagenized parental TP30 line. F1 progeny were green and hygromycin sensitive (data not shown). F2 progeny segregated in a wild type/mutant ratio of 3:1 (263:87), indicating that cia2 was a single-gene recessive mutation.
To confirm that cia2 was in fact defective in chloroplast protein import, chloroplasts were isolated from cia2 and used to perform import experiments. As shown in Figure 1E, cia2 chloroplasts had reduced import efficiency compared with that of the wild type. The import of both the stromal protein RBCS and the thylakoid lumenal protein plastocyanin was reduced, indicating that cia2 was defective in the general protein import pathway.
Molecular Cloning of the CIA2 Locus
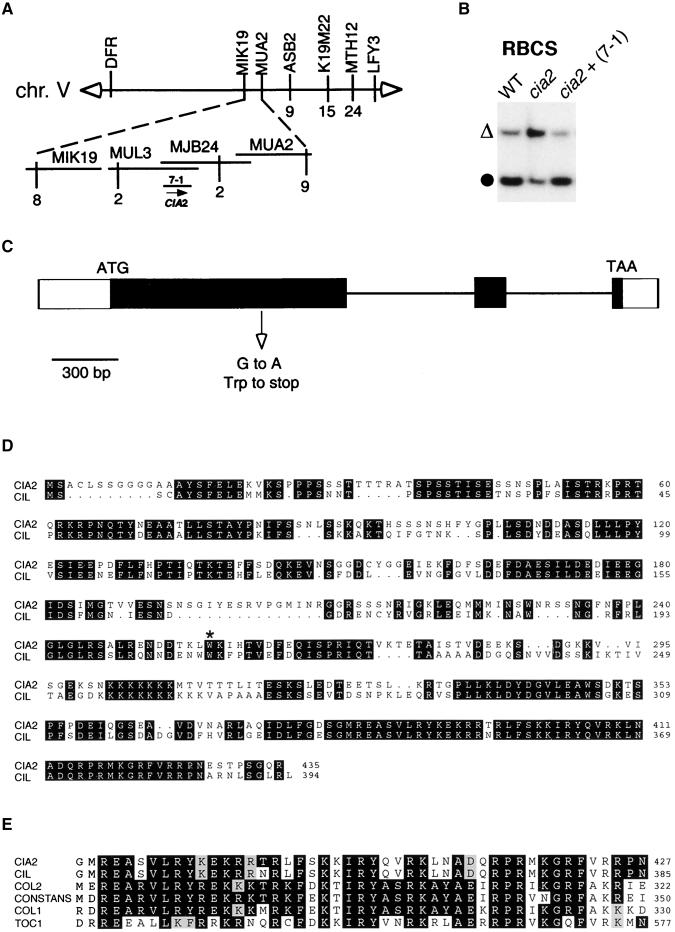
We isolated the CIA2 locus by positional cloning. As shown in Figure 2A, the cia2 mutation was mapped initially to the bottom arm of chromosome V between the cleaved amplified polymorphic sequence (CAPS) markers DFR and LFY (Konieczny and Ausubel, 1993). Additional CAPS markers were identified using sequences available at the time of cloning. Data from four recombinant plants delimited the CIA2 gene to the region contained by P1 artificial chromosomes (PACs) MJB24 and MUL3. These two PACs were shotgun cloned into a transformation vector and introduced into cia2. One of the subclones, 7-1, rescued the pale phenotype of cia2 (data not shown). Chloroplast import experiments further confirmed that the 7-1 genomic fragment also rescued the import defect of cia2 (Figure 2B).
Figure 2.
Molecular Cloning of CIA2.
(A) Summary of positional cloning of CIA2. Vertical lines indicate the positions of PCR-based markers. Values beneath the lines indicate the number of recombinant plants. The direction of transcription of the CIA2 ORF is indicated (arrow). chr., chromosome.
(B) The cia2 import defect was rescued by the 7-1 genomic fragment. Experimental conditions and symbols are as in Figure 1E except that chloroplasts were isolated from 21-day-old plants. cia2+ (7-1), cia2 mutant transformed with the 7-1 genomic fragment; WT, wild type.
(C) Gene structure of CIA2 and the position of the cia2 mutation. Closed boxes indicate exons.
(D) Deduced amino acid sequences encoded by CIA2 and CIL. The asterisk marks the mutated tryptophan residue in CIA2.
(E) Comparison of CCT motifs of CIA2, CIL, Arabidopsis CONSTANS, COL1, COL2, and TOC1.
Only one open reading frame (ORF) was predicted to be fully contained on clone 7-1. Sequencing of this ORF in the cia2 mutant identified a G-to-A mutation at nucleotide 770 that converted a tryptophan residue to a stop codon (Figures 2C and 2D). A wild-type copy of the cDNA was transformed into cia2, and this cDNA also rescued the cia2 phenotypes (data not shown). Therefore, we concluded that this ORF encoded the CIA2 protein. Homology searches in databases identified a CIA2-like (CIL) gene on chromosome IV encoding a protein with 65% identity to CIA2 (Figure 2D). CIL most likely encodes an isoform of CIA2.
CIA2 encodes a novel 435-residue polypeptide containing a C-terminal CCT motif. The CCT motif is a basic stretch of ∼45 amino acids highly conserved within a group of plant-specific transcription factors (Riechmann et al., 2000), including CONSTANS, CONSTANS-like (COL), and TIMING OF CAB EXPRESSION 1 (TOC1) (Figure 2E). CONSTANS controls photoperiodic flowering response (Putterill et al., 1995). TOC1 is involved in controlling circadian rhythms in Arabidopsis (Strayer et al., 2000).
Subcellular Localization of CIA2
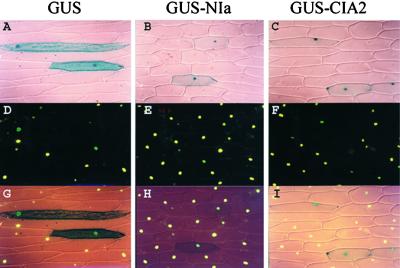
The CCT motif is predicted to be a nuclear localization signal (Robert et al., 1998), and all of the CCT motif–containing proteins are shown to be transcription factors. Therefore, we wondered if CIA2 was a nuclear protein. CIA2 was fused to the C terminus of GUS and transiently expressed in onion epidermal cells. As shown in Figure 3A, cells transformed with GUS alone had uniform staining throughout the cells. Cells transformed with GUS fused either to a known nuclear protein, potyvirus nuclear inclusion protein (Carrington et al., 1991), or to CIA2 showed nuclear localization of the fusion proteins (Figures 3B and 3C). These data indicated that CIA2 is a nuclear protein.
Figure 3.
Subcellular Localization of GUS-CIA2.
Plasmids encoding various proteins, as labeled at top, were transformed into onion epidermal cells by using particle bombardment. Tissues were stained simultaneously with X-gluc and the nucleus-specific dye Cytox.
(A) to (C) Visualization of X-gluc staining.
(D) to (F) Visualization of Cytox staining.
(G) to (I) Superimposition of (A) + (D), (B) + (E), and (C) + (F), respectively.
Expression of CIA2 and CIL
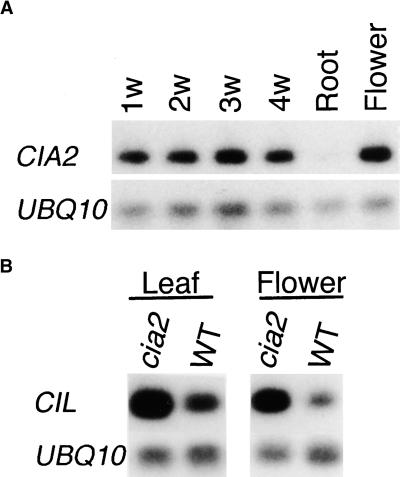
As shown in Figure 4A, the CIA2 gene was expressed in leaves and young flower buds. Almost no expression was detected in roots. The expression pattern of CIL was similar to that of CIA2 (data not shown). Interestingly, the amount of CIL transcript was increased in the cia2 mutant (Figure 4B). These data suggested that there might be a regulation loop between CIA2 and CIL. When CIA2 was not functioning, as in the cia2 mutant, the CIL transcript level was upregulated to compensate for the loss.
Figure 4.
Expression of CIA2 and CIL.
The amount of transcript for each gene from various tissues and developmental stages was analyzed by reverse transcription–PCR with gene-specific primers and visualized by DNA gel blots probed with radiolabeled probes.
(A) Expression patterns of CIA2 in different tissues and various leaf developmental stages. 1w to 4w, 1- to 4-week-old leaf tissues; UBQ10, the Arabidopsis ubiquitin gene (Sun and Callis, 1997) used as a loading control.
(B) The amount of CIL transcript was upregulated in cia2. WT, wild type.
Regulation of atToc75 and atToc33 by CIA2
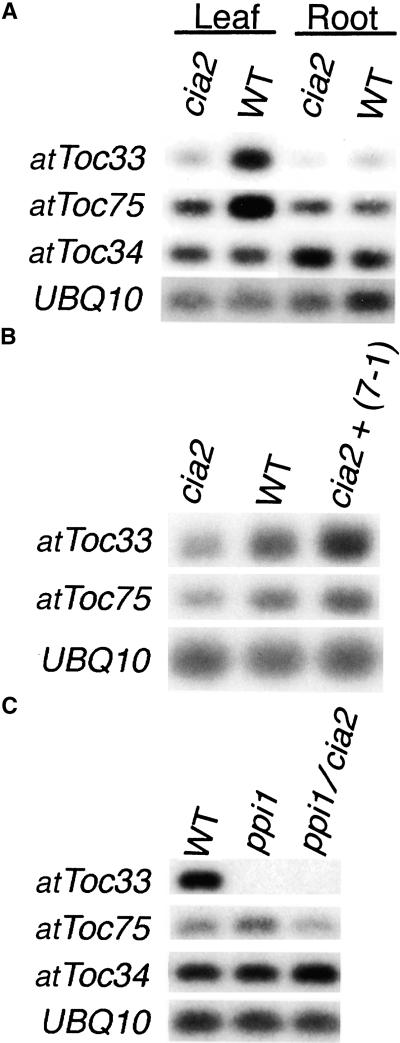
The CCT motif and the nuclear localization of CIA2 suggested that the role of CIA2 in chloroplast protein import lies in regulating the translocon components at the transcription level. Therefore, we checked the transcript abundance of all of the known Tic/Toc components. Expression of most Tic/Toc genes was not affected (data not shown). However, as shown in Figure 5A, in wild type, atToc33 and atToc75 had a twofold to threefold higher expression level in leaves than in roots. In contrast, this upregulation in leaves was abolished in cia2, and the transcript levels were reduced to a basal level similar to that in roots. In comparison, the expression of atToc34, which had a similar expression level in leaves and roots, was not affected by the cia2 mutation. These results suggest that CIA2 is responsible for the leaf-specific upregulation of atToc75 and atToc33. The reduction of atToc75 and atToc33 transcript levels in cia2 was restored by complementing the mutant with the 7-1 genomic fragment containing the wild-type CIA2 gene (Figure 5B), confirming that the reduction was caused by the cia2 mutation. This result also correlated with the result that the 7-1–complemented plant had restored chloroplast protein import efficiency (Figure 2B). The expression of atToc75 and atToc33 in roots was not affected by the cia2 mutation (Figure 5A), which correlated with the absence of expression of CIA2 in roots (Figure 4A).
Figure 5.
Regulation of atToc75 and atToc33 by CIA2.
The amount of transcript for each gene from various tissues was analyzed by reverse transcription–PCR with gene-specific primers and visualized by DNA gel blots probed with radiolabeled probes. UBQ10, the Arabidopsis ubiquitin gene (Sun and Callis, 1997) used as a loading control; WT, wild type.
(A) The amounts atToc33 and atToc75 transcripts were reduced in cia2 to levels similar to those in roots.
(B) The amounts atToc33 and atToc75 transcripts were restored by complementing cia2 with the 7-1 genomic fragment.
(C) The amount of atToc75 transcript was upregulated by the ppi1 mutation and downregulated again by the cia2 mutation.
In pea chloroplasts, Toc75 and Toc34 have been shown to be tightly associated (Seedorf et al., 1995). In vitro imported atToc33 and atToc34 also were shown to be associated with Toc75 in spinach chloroplasts (Gutensohn et al., 2000). This close physical proximity may indicate a functional interaction. Interestingly, in the atToc33 null mutant ppi1, the transcript level of atToc75 was increased (Figure 5C), suggesting that there might be a feedback regulation loop between atToc33 and atToc75. The increase in atToc75 transcript level was reduced by the cia2 mutation in the ppi1 cia2 double mutant, confirming that atToc75 was a target of CIA2.
DISCUSSION
We have designed a screening strategy to systematically isolate mutants disturbed in plastid protein import. The first mutant we characterized, cia2, defined a component that regulates the transcript abundance of atToc75 and atToc33 in leaves.
Among various plastids, leaf chloroplasts import the largest amount of proteins, which account for >50% of total soluble proteins in leaves (Mullet, 1988). Pea Toc75 has been shown to have a higher expression level in leaves than in roots (Tranel et al., 1995). Arabidopsis atToc159 also has a similar expression pattern (Yu and Li, 2001). The higher expression levels are most likely required to accommodate the higher import demand of chloroplasts in the leaf tissue. Indeed, chloroplasts have been shown to import proteins more efficiently than leucoplasts (Wan et al., 1996). Our data indicated that for atToc75 and atToc33, this specific upregulation in leaves is controlled by a mechanism involving CIA2.
Both the hygromycin-resistant phenotype of the cia2 mutant and the isolated chloroplasts from cia2 showed that chloroplasts of cia2 were deficient in protein import. However, when we checked the steady state protein level of isolated chloroplasts, we did not observe a consistent reduction in atToc75 or atToc33. The same results also have been observed for chloroplasts isolated from plants of different ages: although chloroplasts from younger tissue import proteins more efficiently than chloroplasts from older tissue (Dahlin and Cline, 1991), we did not observe a change in the protein levels of various translocon components (T.-S. Yu and H.-m. Li, unpublished results). It is possible that only newly synthesized atToc75 and atToc33 are functional in protein import and that the steady state protein level does not reflect the import capacity of chloroplasts. It is also possible that CIA2 regulates other yet-unidentified translocon components in addition to atToc75 and atToc33. Another possibility is that the direct target of CIA2 is another upstream component that regulates the activity of certain translocon components. In this case, reduction in atToc75 and atToc33 transcripts would be an indirect effect caused by the reduced activity of the translocon.
CIL encodes a protein that shares 65% identity with CIA2. It is also specifically upregulated in the cia2 mutant. Therefore, CIL most likely functions as an isoform of CIA2. This redundancy could explain the mild phenotype of the cia2 mutant. However, CIL obviously could not compensate completely for the loss of CIA2 because the cia2 mutant is still defective in chloroplast protein import. CIL and CIA2, therefore, may have distinct but overlapping functions. It will be interesting to study the phenotypes of cil mutants and the double mutant cia2/cil.
The CIA2 protein shares 27 identical residues in a 46-residue region with CONSTANS within the CCT motif (Figure 2E). Other CCT motif–containing proteins, such as TOC1, TOC1-like (Strayer et al., 2000), CONSTANS, COL, and the ABSCISIC ACID–INSENSITIVE 3–interacting protein AIP1 (Kurup et al., 2000), all share a very high degree of identity within this motif. It has been suggested that a high degree of amino acid conservation in a particular region may indicate that this region is located on the surface of the protein and is responsible for interacting with other proteins in a multiprotein complex (Kisters-Woike et al., 2000). Indeed, the CCT motif in AIP1 and CONSTANS has been shown to be responsible for interacting with other proteins (Kurup et al., 2000). It is possible that the CCT motif within these proteins and CIA2 is responsible for their interactions with some common transcription machinery. Other regions in the proteins may provide the specificity for regulations within each pathway. Experiments are under way to investigate CIA2-interacting proteins.
The expression of translocon genes should be regulated developmentally and spatially. The characterization of CIA2 provided insights into the tissue-specific regulation of atToc75 and atToc33. However, not all translocon genes with a higher expression level in leaves (e.g., atToc159) are regulated by CIA2. Furthermore, the pattern of higher expression levels in younger leaves than in older leaves is not affected by the cia2 mutation (data not shown). These regulations most likely are achieved by other transcription factors. More mutants need to be characterized to understand the complex network of transcription regulators that control translocon gene expression.
METHODS
Reporter Line Construction, Mutagenesis, and Screening
The TP30 reporter construct was made first by inserting the Escherichia coli hygromycin phosphotransferase (HPT) gene into the SphI and EagI sites of pGEM22/3-SEE (Li et al., 1992), which contains the coding sequence of the soybean prRBCS (for ribulose bisphosphate carboxylase) transit peptide upstream of the SphI site. The prRBCS-HPT fragment was excised with HindIII and EcoRI, blunt ended with the Klenow fragment of DNA polymerase I, and inserted into the SmaI site of pMDI, which has a 35S promoter followed by multiple cloning sites and then a NOS (for nopaline synthase) terminator. The 35S promoter-prRBCS-HPT-NOS fragment then was excised with HindIII and EcoRI and inserted into the HindIII-EcoRI site of pBI121 (Clontech, Palo Alto, CA).
The TP30 transgene was transformed into the Columbia ecotype of Arabidopsis thaliana by vacuum infiltration. One of the transgenic lines, TP30-11 was homozygous for a single insertion of TP30 on the bottom arm of chromosome III and was used for further studies. Seed of TP30-11 plants were mutagenized with 20 mM ethyl methanesulfonate, sowed on soil, and allowed to self-pollinate. M2 seed (26,000 seed) from 13 pools were grown on 1 × Murashige and Skoog (1962) medium with Gamborg's vitamins, 2% sucrose, and 60 μg/mL hygromycin at 22°C under a 16-hr-light/8-hr-dark cycle for 14 days. Unmutagenized TP30-11 plants usually had only two cotyledons with occasionally two very small true leaves. Plants with more than four true leaves were selected as hygromycin resistant.
Molecular Cloning of CIA2 and Quantification of Transcripts
All molecular characterizations of the cia2 mutant were performed with lines that had been backcrossed at least twice. The cia2 mutant was crossed to the Landsberg erecta ecotype. DNA was isolated from 1597 F2 mutant seedlings for mapping of the cia2 locus.
P1 artificial chromosomes (PACs) MUL3 and MJB24 were partially digested with various enzymes and subcloned into the transformation vector pPZP221 (Hajdukiewicz et al., 1994). These subclones were transformed into Agrobacterium tumefaciens and introduced into the cia2 mutant by using the floral dip method (Clough and Bent, 1998). Full-length cDNA of CIA2 was amplified using a C-terminal primer designed according to the expressed sequence tag (42C7T7) that contained the C-terminal half of CIA2 and an N-terminal primer designed according to the annotation in the database. 5′ and 3′ rapid amplification of cDNA ends experiments confirmed that our cDNA contained the entire protein-coding region of CIA2.
Amounts of transcripts for various genes were analyzed by reverse transcription–polymerase chain reaction (PCR). First-strand cDNAs were synthesized using the Superscript Preamplification System (Gibco BRL, Rockville, MD) with RNA isolated from various tissues and developmental stages. Primers specific for each gene were used to amplify each transcript with 20 cycles of PCR reactions by using the first-strand cDNAs as templates. Sequences of the specific primers are as follows: CIA2 (forward, 5′-ggaagattcataccgttgatttc-3′; reverse, 5′-aatctcaagagctctgtttcttc-3′), CIL (forward, 5′-caaagagctctgaagtta-cgg-3′; reverse, 5′-gatttataccttcatacgcggg-3′), atToc33 (forward, 5′-tcttatcggcgaacaagtcgtccgt-3′; reverse, 5′-gtttgttgctacatcagttatcgcc-3′), atToc34 (forward, 5′-ctaccttggtctctcgcacaagatc-3′; reverse, 5′-tgtcaacatgaatcgccttgttgcc-3′), atToc75 (forward, 5′-agggtatgcttg-tgctcaagttgtg-3′; reverse, 5′-acagaaccacctggctggaatgaag-3′), and UBQ10 (forward, 5′-ggatctcactcgcgaccg-3′; reverse, 5′-cttcttaagcataacagagacgag-3′). Amounts of PCR products were quantified using DNA gel blots hybridized with radiolabeled probes and quantified with a phosphorimager.
Nuclear Localization and Chloroplast Protein Import
For nuclear localization, a PCR product containing the entire CIA2 coding region was digested with BamHI and XbaI and subcloned into the BglII- and XbaI-digested plasmid pRG/NIa1-76 (Carrington et al., 1991), replacing the NIa1-76 fragment with CIA2. This fusion construct was transiently expressed in onion epidermal cells by using the Helium Biolistic Gene Transformation System (DuPont) as described (Varagona et al., 1992). After particle bombardment, samples were incubated for 24 hr at 25°C in the dark, stained with 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (X-gluc) assay solution (1 mM X-gluc, 50 mM NaPO4, pH 7.0, 0.05 mM sodium ferricyanide and sodium ferrocyanide, 0.02% Triton X-100, and 100 mM EDTA) for 1 hr, washed, and then stained with the nucleus-specific dye Cytox (Molecular Probes, Eugene, OR). Tissues then were mounted on a glass slide. The location of Cytox-stained nuclei was observed using a fluorescence microscope in the fluorescein isothiocyanate channel, and the localization of X-gluc staining was observed using the same microscope in the transmission channel.
Chloroplasts were isolated from Arabidopsis plants that were grown on 1 × Murashige and Skoog (1962) agar medium with Gamborg's vitamins and 2% sucrose at 22°C under a 16-hr-light/8-hr-dark cycle for the amount of time specified in each figure. The chloroplast isolation procedure was the same as the one used for pea seedlings (Perry et al., 1991) except that a different grinding buffer (Rensink et al., 1998) was used, and the first spin after the Miracloth filtration was changed to 3000g for 3 min.
The double mutant between cia2 and ppi1 was constructed first by crossing the two mutants. Plants that looked like the cia2 mutants were selected from the F2 population. F3 progeny from each cia2 F2 plant were screened for new phenotypes that segregated at a 1:4 ratio. DNA from potential double mutants was isolated. Primers were designed to check for the presence of the T-DNA insertion in atToc33. Another pair of primers (primer sequences 5′-ctcagagaaaacgacgacacaaaattct-3′ and 5-ctcctcatcaacggtggaag-3′) was designed as a cleaved amplified polymorphic sequence (derived CAPS) marker (Michaels and Amasino, 1998) to check for the presence of the G-to-A mutation in the CIA2 gene. The mutant allele with the G-to-A mutation will generate an XbaI site in the PCR-amplified fragment.
GenBank Accession Numbers
The GenBank accession numbers for the sequences discussed in this article are as follows: CIA2 (AF359387), CIL (AF359388), Arabidopsis CONSTANS (CAA64407), COL1 (Y10555), COL2 (C81119), and TOC1 (AF272039).
Acknowledgments
We thank Drs. Kathy Archer, Cheng-Ting Chien, and Laura Olsen for critical reading of the manuscript. We thank Dr. Ralf Bernd Klösgen (Botanisches Institut der Ludwig-Maximilians-Universität, Munich, Germany) for the waxy-GUS construct. This work was supported by grants to H.-m.L. from the National Science Council (NSC 89-2321-B001-005) and Academia Sinica of Taiwan.
References
- Amin, P., Sy, D.A.C., Pilgrim, M.L., Parry, D.H., Nussaume, L., and Hoffman, N.E. (1999). Arabidopsis mutants lacking the 43- and 54-kilodalton subunits of the chloroplast signal recognition particle have distinct phenotypes. Plant Physiol. 121 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, J., Chen, K., Hiltbunner, A., Wehrli, E., Eugster, M., Schnell, D., and Kessler, F. (2000). The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403 203–207. [DOI] [PubMed] [Google Scholar]
- Carrington, J.C., Freed, D.D., and Leinicke, A.J. (1991). Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell 3 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dahlin, C., and Cline, K. (1991). Developmental regulation of the plastid protein import apparatus. Plant Cell 3 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutensohn, M., Schulz, B., Nicolay, P., and Flügge, U.-I. (2000). Functional analysis of the two Arabidopsis homologues of Toc34, a component of the chloroplast protein import apparatus. Plant J. 23 771–783. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25 989–994. [DOI] [PubMed] [Google Scholar]
- Hinnah, S.C., Hill, K., Wagner, R., Schlicher, T., and Soll, J. (1997). Reconstitution of a chloroplast protein import channel. EMBO J. 16 7351–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, P., Chen, L.-J., Li, H.-m., Peto, C.A., Fankhauser, C., and Chory, J. (1998). An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282 100–103. [DOI] [PubMed] [Google Scholar]
- Kisters-Woike, B., Vangierdegom, C., and Müller-Hill, B. (2000). On the conservation of protein sequences in evolution. Trends Biochem. Sci. 25 419–421. [DOI] [PubMed] [Google Scholar]
- Klösgen, R.B., Saedler, H., and Weil, J.-H. (1989). The amyloplast-targeting transit peptide of the waxy protein of maize also mediates protein transport in vitro into chloroplasts. Mol. Gen. Genet. 217 155–161. [DOI] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4 403–410. [DOI] [PubMed] [Google Scholar]
- Kurup, S., Jones, H.D., and Holdsworth, M.J. (2000). Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J. 21 143–155. [DOI] [PubMed] [Google Scholar]
- Li, H.-m., Sullivan, T.D., and Keegstra, K. (1992). Information for targeting to the chloroplastic inner envelope membrane is contained in the mature region of the maize Bt1-encoded protein. J. Biol. Chem. 267 18999–19004. [PubMed] [Google Scholar]
- Ma, Y., Kouranov, A., LaSala, S.E., and Schnell, D.J. (1996). Two components of the chloroplast protein import apparatus IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope. J. Cell Biol. 134 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1998). A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J. 14 381–385. [DOI] [PubMed] [Google Scholar]
- Moore, M., Harrison, M.S., Peterson, E.C., and Henry, R. (2000). Chloroplast oxa1p homolog albino3 is required for post-translational integration of the light harvesting chlorophyll-binding protein into thylakoid membranes. J. Biol. Chem. 275 1529–1532. [DOI] [PubMed] [Google Scholar]
- Mullet, J.E. (1988). Chloroplast development and gene expression. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 475–502. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Perry, S.E., and Keegstra, K. (1994). Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell 6 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, S.E., Li, H.-m., and Keegstra, K. (1991). In vitro reconstitution of protein transport into chloroplasts. Methods Cell Biol. 34 327–344. [DOI] [PubMed] [Google Scholar]
- Putterill, J., Robson, F., Lee, K., Simon, R., and Coupland, G. (1995). The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80 847–857. [DOI] [PubMed] [Google Scholar]
- Rensink, W.A., Pilon, M., and Weisbeek, P. (1998). Domains of a transit sequence required for in vivo import in Arabidopsis chloroplasts. Plant Physiol. 118 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann, S., Davila-Aponte, J., and Keegstra, K. (1999). The evolutionary origin of the protein-translocating channel of chloroplastic envelop membranes: Identification of a cyanobacterial homolog. Proc. Natl. Acad. Sci. USA 96 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann, J.L., et al. (2000). Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 290 2105–2109. [DOI] [PubMed] [Google Scholar]
- Robert, L.S., Robson, F., Sharpe, A., Lydiate, D., and Coupland, G. (1998). Conserved structure and function of the Arabidopsis flowering time gene CONSTANS in Brassica napus. Plant Mol. Biol. 37 763–772. [DOI] [PubMed] [Google Scholar]
- Roy, L.M., and Barkan, A. (1998). A SecY homologue is required for the elaboration of the chloroplast thylakoid membrane and for normal chloroplast gene expression. J. Cell Biol. 141 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff, E., and Soll, J. (2000). Travelling of proteins through membranes: Translocation into chloroplasts. Planta 211 449–456. [DOI] [PubMed] [Google Scholar]
- Schnell, D.J., Blobel, G., Keegstra, K., Kessler, F., Ko, K., and Soll, J. (1997). A consensus nomenclature for the protein-import components of the chloroplast envelope. Trends Cell Biol. 7 303–304. [DOI] [PubMed] [Google Scholar]
- Seedorf, M., Waegemann, K., and Soll, J. (1995). A constituent of the chloroplast import complex represents a new type of GTP-binding protein. Plant J. 7 401–411. [DOI] [PubMed] [Google Scholar]
- Strayer, C., Oyama, T., Schultz, T.F., Raman, R., Somers, D.E., Mas, P., Panda, S., Kreps, J.A., and Kay, S.A. (2000). Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289 768–771. [DOI] [PubMed] [Google Scholar]
- Sun, C.-W., and Callis, J. (1997). Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J. 11 1017–1027. [DOI] [PubMed] [Google Scholar]
- Tranel, P.J., Froehlich, J., Goyal, A., and Keegstra, K. (1995). A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J. 14 2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagona, M.J., Schmidt, R.J., and Raikhel, N.V. (1992). Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein opaque-2. Plant Cell 4 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker, R., and Barkan, A. (1995). Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 14 3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker, R., Mendel-Hartvig, J., and Barkan, A. (1997). Transposon-disruption of a maize nuclear gene tha1 encoding a chloroplast secA homologue: In vivo role of cp-secA in thylakoid protein targeting. Genetics 145 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, J., Blakeley, S.D., Dennis, D.T., and Ko, K. (1996). Transit peptides play a major role in the preferential import of proteins into leucoplasts and chloroplasts. J. Biol. Chem. 271 31227–31233. [DOI] [PubMed] [Google Scholar]
- Yu, T.-S., and Li, H.-m. (2001). Chloroplast translocon components atToc159 and atToc33 are not essential for chloroplast biogenesis in guard cells and root cells. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]