Abstract
To understand the functional significance of RNA processing for the expression of plastome-encoded photosynthesis genes, we investigated the nuclear mutation hcf107 of Arabidopsis. The mutation is represented by two alleles, both of which lead to a defective photosystem II (PSII). In vivo protein labeling, in vitro phosphorylation, and immunoblot experiments revealed that the psbB gene product (CP47) and an 8-kD phosphoprotein, the psbH gene product (PsbH), are absent in mutant plants. PsbH and PsbB are essential requirements for PSII assembly in photosynthetic eukaryotes, and their absence in hcf107 is consistent with the PSII-less mutant phenotype. RNA gel blot hybridizations showed that the hcf107 mutation specifically impairs the accumulation of some but not all oligocistronic psbH transcripts that are released from the pentacistronic psbB-psbT-psbH-petB-petD precursor RNA by intergenic endonucleolytic cleavage. In contrast, neither the levels nor the sizes of psbB-containing RNAs are affected. S1 nuclease protection analyses revealed that psbH RNAs are lacking only where psbH is the leading cistron and that they are processed at position −45 in the 5′ leader segment of psbH. These data and additional experiments with the cytochrome b6f complex mutant hcf152, which is defective in 3′ psbH processing, suggest that only those psbH-containing transcripts that are processed at their −45 5′ ends can be translated. Secondary structure analysis of the 5′ psbH leader predicted the formation of stable stem loops in the nonprocessed transcripts, which are unfolded by processing at the −45 site. We propose that this unfolding of the psbH leader segment as a result of RNA processing is essential for the translation of the psbH reading frame. We suggest further that HCF107 has dual functions: it is involved in intercistronic processing of the psbH 5′ untranslated region or the stabilization of 5′ processed psbH RNAs, and concomitantly, it is required for the synthesis of CP47.
INTRODUCTION
Plastidial genes of land plants typically are organized in polycistronic transcription units (Herrmann et al., 1992; Sugiura, 1992). These transcription units often are composed heterogeneously and reflect their postendosymbiotic assembly from different cyanobacterial progenitor genes or operons (Douglas, 1994). The evolutionary origin also may explain the highly complex transcript patterns of plastid transcription units and the many auxiliary and regulatory factors that are required for the expression of these operons (reviewed by Barkan and Goldschmidt-Clermont, 2000).
The complex transcript patterns are caused in part by multiple transcriptional initiation. However, the great majority of the transcripts are generated by RNA processing. Processing involves the splicing of introns, endonucleolytic cleavage in intercistronic regions, exonucleolytic and/or endonucleolytic trimming of RNA 5′ and 3′ ends, polyadenylation, and RNA editing (reviewed by Monde et al., 2000). The functional significance of these processing events is only beginning to be understood, but a detailed explanation of the in vivo function of plastidial RNA processing is still lacking.
Both the 5′ and 3′ ends of plastid transcripts carry determinants for regulating RNA turnover. The 3′ ends often are folded into stem and loop structures that bind specific proteins and that appear to act as barriers against 3′ exonucleolytic degradation (reviewed by Monde et al., 2000). There is evidence that ribonucleoprotein complexes also are assembled at the 5′ RNA segments and that these structures serve to determine the stability of the corresponding RNA (Salvador et al., 1993; Nickelsen et al., 1994). Besides their function in RNA stability, the 5′ untranslated regions play an important role in translation (reviewed by Bruick and Mayfield, 1999; Zerges, 2000). Transregulatory factors have been identified that bind to cis-active elements within these segments and regulate the translational activity of the RNA (Danon and Mayfield, 1991; Zerges et al., 1997). Differences in the size of the 5′ untranslated region attributable to multiple processing sites may influence the translational activities of the RNAs. This notion is supported by the analysis of the high-chlorophyll fluorescence mutant crp1 (for chloroplast RNA processing) of maize, which revealed that the fully processed petB and petD RNAs are better templates for the translational machinery than are their polycistronic precursors (Barkan et al., 1994). Findings in bacteria suggest that depending on their length, the 5′ untranslated regions may fold into different secondary structures, which affects their translational activity (reviewed by Lindahl and Hinnebusch, 1992).
We are interested in understanding the functional significance of RNA processing for the expression of plastid genes and are pursuing a genetic approach with Arabidopsis as the model plant. Our focus is on the psbB-psbT-psbH-petB-petD operon, which serves as a paradigm for a typical plastidial, polycistronic transcription unit (Barkan, 1988; Westhoff and Herrmann, 1988). The genes of this unit encode five thylakoid membrane polypeptides that belong to two different protein complexes, photosystem II (PSII) (psbB, psbT, and psbH) and the cytochrome b6f complex (petB and petD). psbB, psbT, and psbH encode the CP47 chlorophyll apoprotein and the T subunit and H subunit of PSII, the latter of which has been called the 10-kD phosphoprotein. petB and petD encode cytochrome b6 and subunit IV of the cytochrome b6/f complex. The peculiar feature of this transcription unit is the presence of another PSII gene, psbN, that is located in the intercistronic segment of psbT and psbH but is transcribed in the opposite direction and thus is not a component gene of the psbB-psbT-psbH-petB-petD operon (Kohchi et al., 1988).
Here we report a recessive nuclear mutation in Arabidopsis, named hcf107 (Meurer et al., 1996b), that is represented by two alleles (hcf107-1 and hcf107-2). The mutants lack PSII as a result of a deficiency in the synthesis of subunits CP47 (PsbB) and PsbH. At the RNA level, hcf107 mutant plants are affected in the accumulation of processed psbH-containing RNAs. We show that only psbH transcripts with a −45 5′ end are absent, whereas processing at the psbH 3′ site proceeds normally. The deficiency in psbH transcripts with the −45 5′ end is correlated with the inability to synthesize the PsbH protein. We discuss the presence of secondary structures in the unprocessed 5′ psbH leader region and propose that unfolding of the psbH leader segment attributable to RNA processing is essential for the translation of the psbH reading frame.
RESULTS
Two Allelic, Recessive High-Chlorophyll Fluorescence (hcf) Mutants with a Defective PSII
By standardized screening of ethyl methanesulfonate– and T-DNA–mutagenized lines of Arabidopsis for hcf phenotypes (Meurer et al., 1996b), two pale green mutants, hcf107 (Meurer et al., 1996b) and DEI117 (this study), were identified that were defective in the RNA pattern of the psbB-psbT-psbH-petB-petD transcription unit (see below). Homozygous mutant plants were found to be seedling lethal when grown on soil but could be maintained on sucrose-supplemented agar medium, on which they grew like wild-type plants. Segregational analysis of the mutant phenotype in backcrosses of heterozygous mutant plants to wild type revealed that both mutations were recessive. Crosses of heterozygous hcf107 to heterozygous DEI117 mutant plants resulted in a 3:1 segregation of wild-type to mutant phenotypes (data not shown). This finding demonstrated that the two mutations were allelic; consequently, the mutants were named hcf107-1 and hcf107-2.
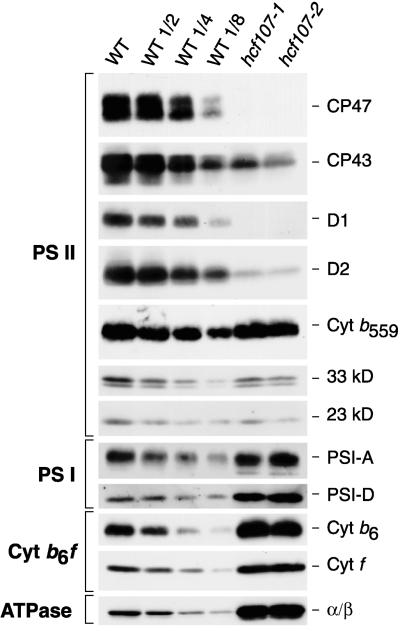
Chlorophyll fluorescence induction experiments showed that both mutants lacked variable fluorescence, which is diagnostic for a defective PSII (Meurer et al., 1998). The P700 redox kinetics revealed that the electron flow to PSI is inhibited but that the photosystem itself is functional (hcf107-1, Meurer et al., 1996b; hcf107-2, data not shown). The deficiency in PSII function was reflected at the protein level. Immunoblot analyses (Figure 1) with a representative set of antisera specific for the major subunits of PSII indicated that in both mutants, the relative amounts of the PSII reaction center core subunits CP47 and D1 were below the level of detection and that CP43 and D2 were reduced drastically. In contrast, the relative levels of the 33- and 23-kD proteins of the water-splitting complex and of cytochrome b559 were not altered significantly.
Figure 1.
Immunoblot Analysis of Thylakoid Membrane Proteins from hcf107 and Wild Type.
The lanes were loaded with 10 μg (wild type [WT], hcf107-1, and hcf107-2), 5 μg (WT 1/2), 2.5 μg (WT 1/4), or 1.25 μg (WT 1/8) of total membrane proteins from ∼3-week-old plants grown on a sucrose-supplemented Gelrite medium (see Methods). Proteins were detected with the indicated antiserum to subunits of PSII, PSI, the cytochrome (Cyt) b6f complex, and the chloroplast ATP synthase (ATPase) as described (Meurer et al., 1996b).
When equal amounts of thylakoid membrane proteins are analyzed and PSII levels are reduced drastically, a relative increase in the level of the other thylakoid membrane complexes is to be expected. Figure 1 shows that such relative increases in the levels of PSI (subunits PSI-A and PSI-D), the cytochrome b6f complex (Cyt f and Cyt b6), and the ATP synthase (α and β subunits) were observed in both allelic mutants. The joint results of the spectroscopic and immunoblot analyses indicate that the mutations in hcf107-1 and hcf107-2 primarily affect PSII and that these mutants are to be classified as PSII mutants.
Hcf107 Mutant Seedlings Are Deficient in the Synthesis and/or Stability of CP47 and an 8-kD Plastome-Encoded Protein
Diminished amounts of proteins may be attributable to either impaired translation or accelerated degradation of proteins. To distinguish between these two possibilities, the synthesis of thylakoid membrane proteins was investigated in intact mutant seedlings by pulse-labeling experiments with 35S-methionine. To obtain an easily interpretable labeling pattern, the synthesis of the nucleus-encoded chloroplast proteins was blocked with cycloheximide, and only the plastome-encoded proteins were investigated.
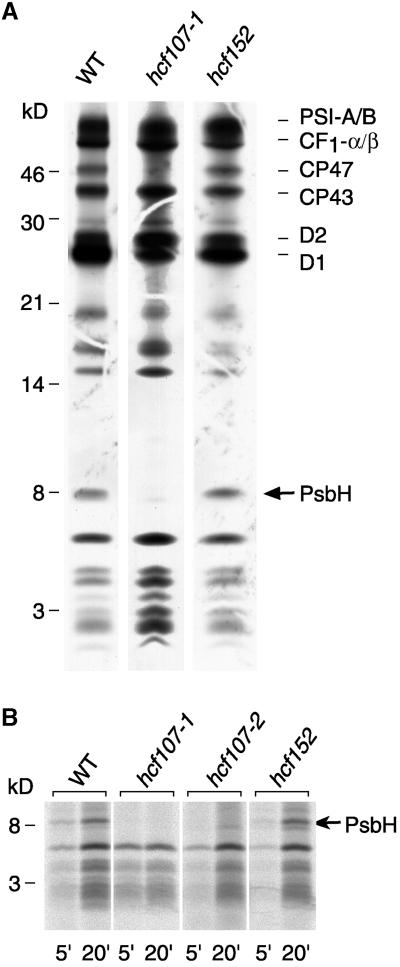
Figure 2A shows that the protein-labeling patterns of mutant hcf107-1 and the wild type were qualitatively similar after a 15-min labeling period. Subunits CP43 and D2 of PSII, the A and B subunits of PSI (PSI-A/B), and the α and β subunits of the chloroplast ATP synthase (CF1-α/β) were synthesized at approximately wild-type levels. In contrast, the incorporation of 35S label into the D1 polypeptide was reduced significantly. Moreover, the labeling of CP47 and a small polypeptide of ∼8 kD was not detectable in hcf107 (Figure 2A), regardless of whether short (5 min) or longer (20 min) labeling periods were used (Figure 2B). We conclude from these findings that hcf107 is defective in the synthesis of both CP47 and the 8-kD protein or that the lack of labeling of these proteins is attributable to a very rapid turnover.
Figure 2.
In Vivo Protein Synthesis of Plastome-Encoded Membrane Proteins of hcf107-1, hcf107-2, hcf152, and Wild-Type (WT) Plants.
(A) Pulse labeling for 15 min. Wild-type and mutant proteins with equivalent amounts of radioactivity (100,000 cpm) were separated electrophoretically on a 12.5% polyacrylamide-SDS/urea gel (Schägger and von Jagow, 1987), blotted onto a nitrocellulose membrane, and analyzed by fluorography. The position of the PsbH subunit of PSII is indicated. CF1-α/β, α and β subunits of the chloroplast ATP synthase; WT, wild type.
(B) Kinetics of 35S incorporation into the 8-kD protein. The labeling periods were 5 min (5′) and 20 min (20′). Only the lower part of the polyacrylamide-SDS/urea gel is shown. The gel was loaded with the following amounts of protein and radioactivity: WT, 5 μg of protein (5′, 20,000 cpm; 20′, 80,000 cpm); hcf107-1, 10 μg of protein (5′, 25,000 cpm; 20′, 40,000 cpm); hcf107-2, 15 μg of protein (5′, 20,000 cpm; 20′, 60,000 cpm); and hcf152, 15 μg of protein (5′, 20,000 cpm; 20′, 90,000 cpm). Radiolabeled proteins were detected by phosphorimaging.
The 8-kD Protein Exhibits the Typical Features of the PsbH Protein
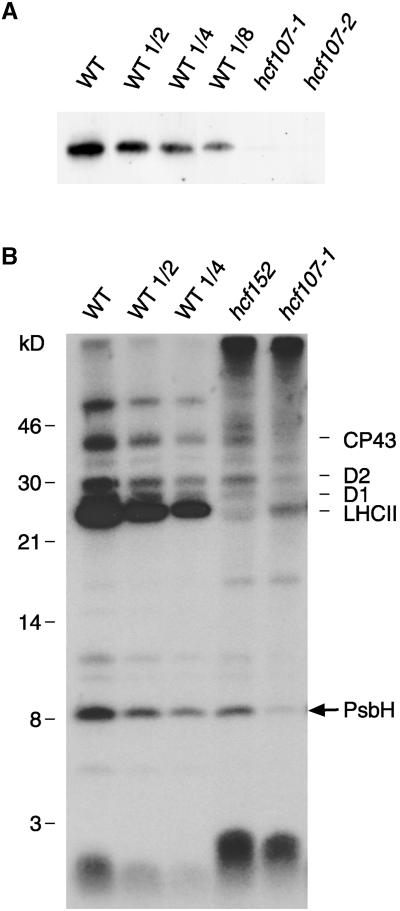
The apparent molecular mass of the 8-kD protein is almost identical to the deduced size of the PsbH subunit of PSII (8.1 kD), whose gene is part of the psbB-psbT-psbH-petB-petD transcription unit. The PsbH protein is a constituent subunit of the PSII reaction center and, in green plants, an essential requirement for the assembly of this photosystem (Summer et al., 1997; O'Connor et al., 1998). To confirm the identity of the 8-kD protein as the psbH gene product, two experimental lines were followed. First, an antiserum was raised against a glutathione S-transferase fusion of the PsbH protein and used to quantitate PsbH levels in the two allelic mutants (Figure 3A). Second, because the PsbH subunit of PSII is a phosphoprotein (Farchaus and Dilley, 1986; Michel and Bennett, 1987), in vitro phosphorylation experiments were performed (Figure 3B).
Figure 3.
Identification of the 8-kD Protein by Immunoblotting and in Vitro Phosphorylation Experiments.
(A) Immunoblot analysis with an antiserum to the PsbH subunit of PSII. The analysis was performed and labels are as described in the legend to Figure 1.
(B) In vitro phosphorylation with thylakoids of hcf107 (hcf107-1), hcf152, and wild-type (WT) seedlings. For experimental details, see Methods. Wild-type and mutant proteins with equivalent amounts of radioactivity (80,000 cpm) were separated electrophoretically on a 12.5% polyacrylamide-SDS/urea gel (Schägger and von Jagow, 1987) and analyzed by fluorography.
Figure 3A shows that the levels of immunologically detectable PsbH protein were depleted drastically in the two allelic hcf107 mutants. Although traces of PsbH protein were found in hcf107-1, the protein appeared to be completely absent in hcf107-2.
In vitro phosphorylation assays provided additional proof that the PsbH protein is depleted in hcf107 plants (Figure 3B). Wild-type thylakoids showed the expected phosphorylation pattern, with CP43, D2, D1, the light-harvesting chlorophyll a/b binding proteins of PSII (LHCII), and the PsbH protein being the major phosphoproteins of the thylakoid membrane (Rintamäki et al., 1997). The phosphorylation pattern of hcf107-1 thylakoids was strikingly different. Almost no 32P label was detected in the PsbH protein, and the label in the other PSII proteins (i.e., CP43, D2, D1, and LHCII) was reduced drastically. The lack of label in D1 and PsbH was to be expected, because hcf107 was depleted in these proteins. However, CP43, LHCII, and, to a lesser extent, D2 were present in hcf107 (see Figure 1 for CP43 and D2; data not shown for LHCII), and the lack of phosphorylation of these proteins may be a secondary effect of the mutation. It is known that the phosphorylation of the PSII proteins CP43, D2, D1, and LHCII is redox controlled via reduction of plastoquinone and the cytochrome b6f complex (Allen et al., 1981; Bennett et al., 1988), and it is plausible that a PSII-less mutant is defective in this type of control.
Two major conclusions can be drawn from these in vivo protein synthesis, immunoblotting, and in vitro phosphorylation experiments: (1) the PsbH protein is not detectable (hcf107-2) or is only barely detectable (hcf107-1) in the mutant background, and (2) this depletion is caused by a drastic decrease in its rate of synthesis or by a very rapid turnover.
The hcf107 Mutation Affects the Accumulation of psbH-Containing RNAs
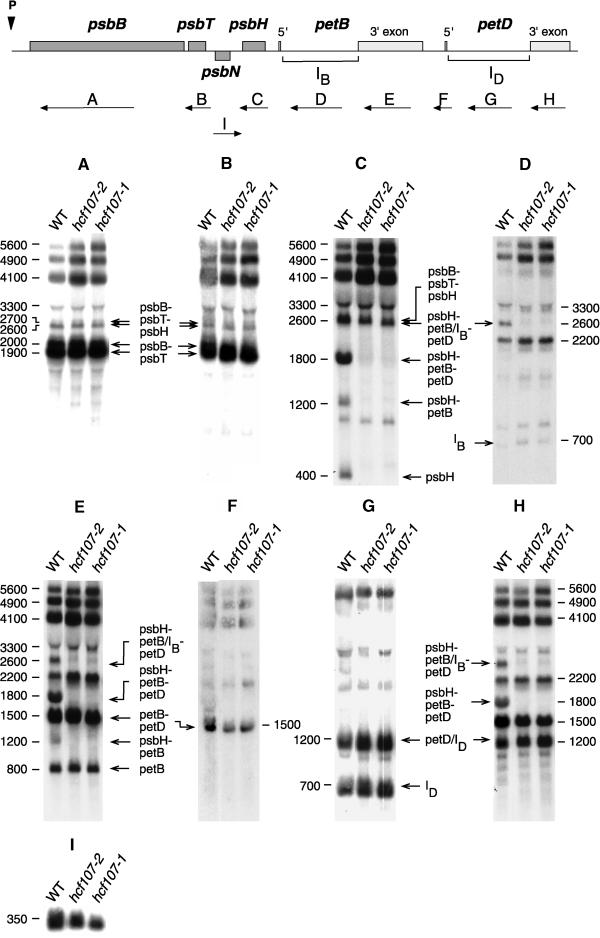
Preliminary RNA gel blot analyses indicated that the hcf107 mutation affects only the RNA pattern of the psbB-psbT-psbH-petB-petD transcription unit, because no significant changes in the RNA levels or patterns of other plastid- and nucleus-encoded genes for photosynthetic proteins were detected (Meurer et al., 1996b; data not shown). To precisely identify the defect in the RNA pattern of the psbB-psbT-psbH-petB-petD transcription unit, all genic and extragenic regions of this transcription unit of Arabidopsis were cloned (Felder, 1999) and used as probes in RNA gel blot hybridization experiments. The psbN gene, which is located between psbT and psbH but is transcribed from the opposite strand, was included in this analysis.
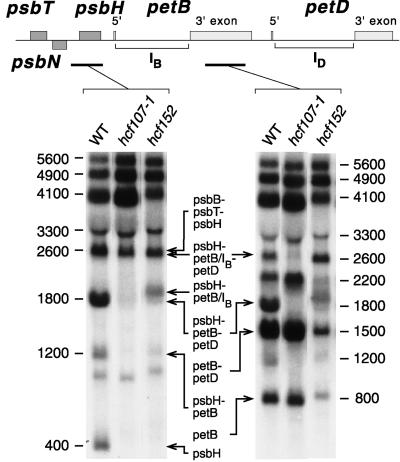
Figure 4 shows that the defects in both hcf107-1 and hcf107-2 affected only the psbH part of the psbB-psbT-psbH-petB-petD transcription unit. Oligocistronic psbB, psbT, petB, and petD transcripts accumulated in hcf107 mutant plants as in the wild type. Specifically, neither the amounts nor the apparent sizes of the two dicistronic psbB-psbT transcripts of 1900 and 2000 nucleotides and of the two tricistronic psbB-psbT-psbH transcripts of 2600 and 2700 nucleotides differed between the wild type and mutant. These doublets of psbB-psbT and psbB-psbT-psbH transcripts are conserved in monocotyledonous and dicotyledonous plants (Barkan, 1988; Westhoff and Herrmann, 1988; Hird et al., 1991). It reflects two different psbB RNA 5′ ends that arise by transcriptional initiation and an additional processing step (Westhoff, 1985; Westhoff and Herrmann, 1988). The accumulation of the psbN transcript also was not disturbed by the mutation. In both mutants, the 350-nucleotide single psbN RNA was detected, although the abundance of this RNA was somewhat lower in hcf107-1 and hcf107-2 than in wild-type plants.
Figure 4.
Transcript Pattern of the psbB-psbT-psbH-petB-petD Transcription Unit and psbN in the Mutants hcf107-1 and hcf107-2.
Eight micrograms of total leaf RNA from 3-week-old mutant and wild-type (WT) seedlings was analyzed in each RNA gel blot hybridization ([A] to [H]). The hybridization probes and their locations are indicated with arrows and letters (A to H) at the top. The introns of petB and petD are marked as IB and ID. Sizes of the transcripts (in nucleotides) referred to in the text and their genic compositions are indicated.
The primary (5600 nucleotides), partially spliced (4900 nucleotides), and fully spliced (4100 nucleotides) pentacistronic psbB-psbT-psbH-petB-petD transcripts were present as in the wild type. However, the amounts of these RNAs were higher in both allelic mutants than in the wild type, suggesting that the processing of the polycistronic transcripts into oligocistronic psbH RNAs was slowed down. This conclusion was confirmed by further inspection of the RNA pattern of the psbH region. Although the tricistronic psbB-psbT-psbH RNAs (2600 and 2700 nucleotides) accumulated to wild-type levels, other processed psbH-containing transcripts (i.e., those of 2600, 1800, 1200, and 400 nucleotides) were reduced drastically or below the level of detection (Figure 4). The 2600- and 1800-nucleotide RNAs are tricistronic psbH-petB-petD transcripts, with petB containing its intron [psbH-petB(IB)-petD; 2600 nucleotides] or not [psbH-petB-petD; 1800 nucleotides]. Note that the psbH-petB(IB)-petD RNA is of the same size as the tricistronic psbB-psbT-psbH transcript. The 1200-nucleotide transcript is a dicistronic psbH-petB RNA, and the 400-nucleotide transcript represents a monocistronic psbH RNA. All of these depleted RNAs have in common that psbH is their leading cistron. This finding suggests that the hcf107 mutation causes a defect in intercistronic processing between psbT and psbH.
hcf107 Mutant Plants Do Not Accumulate psbH RNAs with the −45 5′ End
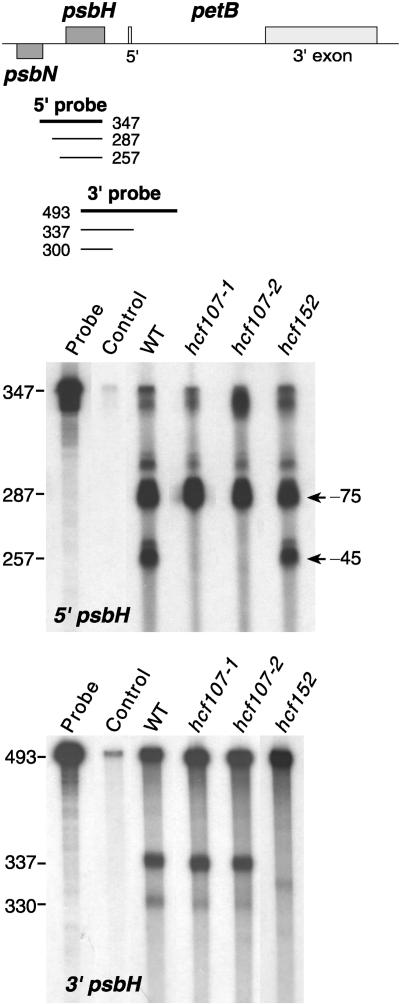
To substantiate these findings and to take into account the fact that the intercistronic processing between psbT and psbH was reported to result in psbH RNAs with two different 5′ ends (Westhoff and Herrmann, 1988), S1 nuclease mapping experiments were performed with RNA from wild-type and hcf107-1/hcf107-2 plants.
With wild-type RNA and a single-stranded psbH 5′ DNA probe, the S1 nuclease assays resulted in two protected fragments of 287 and 257 nucleotides (Figure 5). This would correspond to psbH RNA 5′ ends located at −45 and −75 with respect to the translation initiation codon of psbH (Figure 6). The 287-nucleotide protected fragment was significantly stronger than the 257-nucleotide fragment, suggesting that transcripts with the −75 5′ untranslated psbH leader segment accumulate to higher levels than do those with the −45 end. Only relatively small amounts of fully protected probe were obtained with wild-type RNA (Figure 5). This was not in accord with our expectation, because the amounts of psbH transcripts that are not processed between psbT and psbH, that is, the pentacistronic psbB-psbT-psbH-petB-petD RNAs of 5600, 4900, and 4100 nucleotides and the tricistronic 2600/2700-nucleotide psbB-psbT-psbH RNAs (Figure 4), were significantly larger than the combined levels of the 2600-, 1800-, 1200-, and 400-nucleotide processed psbH RNAs. Consequently, S1 nuclease treatment of the hybridization reaction should have resulted in correspondingly large amounts of fully protected 5′ psbH probe.
Figure 5.
S1 Nuclease Protection Mapping of the 5′ and 3′ Termini of psbH Transcripts in Wild-Type, hcf107-1, hcf107-2, and hcf152 Mutant Plants.
Total leaf RNA was annealed with probes spanning the 5′ and 3′ ends of psbH. The control reaction contained no RNA. Sizes of protected fragments (in bases) were determined by coelectrophoresis with a DNA sequence ladder. WT, wild type.
Figure 6.
Nucleotide Sequence of the Intergenic Region between psbN and psbH in Arabidopsis with Mapped RNA 5′ Ends.
Start codons are printed in boldface italic letters. The directions of translation are indicated by horizontal arrows, and the directions of mapped 5′ sites are indicated by vertical arrows.
S1 protection analysis with the 3′ psbH probe resulted essentially in only one partially protected fragment (Figure 5), indicating that there is a single major processing site between psbH and the 5′ exon of petB. In addition, large amounts of fully protected probe were obtained (Figure 5), reflecting the corresponding level of the unprocessed psbH-petB RNA segment (Figure 4).
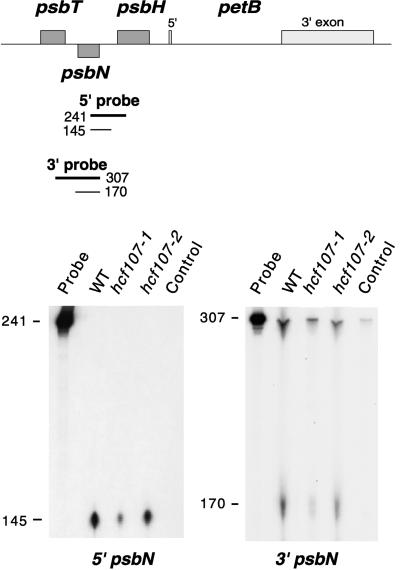
S1 nuclease protection mapping with psbN 5′ and 3′ probes yielded a single RNA 5′ and 3′ end, respectively (Figure 7). The 5′ end of the psbN RNA overlaps approximately six nucleotides with the putative −75 psbH 5′ end (Figure 6).
Figure 7.
S1 Nuclease Protection Mapping of the 5′ and 3′ Termini of psbN Transcripts in Wild-Type, hcf107-1, and hcf107-2 Mutant Plants.
The positions and sizes of the 5′ and 3′ probes (thick lines) and the S1 protected fragments (thin lines) are depicted in the map at the top of the figure. WT, wild type.
This close proximity of the psbN 5′ end and the −75 psbH 5′ end raises doubts about whether the −75 psbH 5′ end could be an artifact that is produced by the concurrent hybridization of complementary RNAs and a single-stranded DNA probe. During hybridization of the 5′ psbH DNA probe to unprocessed psbT-psbH RNA segments, the psbN RNA present also hybridizes to these transcripts. Because RNA-RNA hybrids are more stable than DNA-RNA hybrids under the chosen hybridization conditions (Sugimoto et al., 1995), this concurrent reaction results in psbT-psbH nonprocessed RNAs that have hybridized both the psbN RNA and the 5′ psbH single-stranded DNA probe. S1 nuclease digests the overlapping DNA probe; therefore, the 287-nucleotide protected fragment does not necessarily indicate the existence of the −75 RNA but may have to be considered as an artifact. The finding that full-size protection of the psbH 5′ probe is drastically lower than full-size protection of the psbH 3′ probe supports this conclusion (Figure 5). We conclude, therefore, that processing between psbT and psbH most likely yields only one major RNA 5′ end and that this 5′ end is located at position −45 in front of the psbH start codon.
Hybridization of the psbH 5′ probe with RNA from both hcf107-1 and hcf107-2 yielded only the putative −75 5′ end; that is, psbH RNAs with the −45 5′ end were not detectable (Figure 5). No differences between hcf107 and the wild type were observed when S1 nuclease protection analysis was performed with a psbH 3′ probe (Figure 5) or with psbN 5′ and 3′ probes (Figure 7). It follows that the hcf107 mutation prevents processing at the −45 processing site between psbT and psbH or, alternatively, the stabilization of psbH RNAs with the 5′ end. Because this defect is associated with a lack of PsbH accumulation, a free psbH 5′ end appears to be essential for PsbH synthesis, and psbH transcripts with a nonprocessed psbT-psbH intercistronic segment are translationally inactive.
Defects in psbH 3′ End Processing Do Not Affect psbH Translation
To confirm these conclusions, the Arabidopsis mutant hcf152 was included in the analysis. hcf152 is defective in the cytochrome b6f complex but contains a functional PSII (K. Meierhoff and S. Felder, unpublished data). Accordingly, PsbH was synthesized (Figure 2) and phosphorylated (Figure 3) in hcf152 as in the wild type. More specifically, hcf152 was affected in the processing of RNAs from the psbH-petB part of the psbB-psbT-psbH-petB-petD transcription unit (K. Meierhoff and S. Felder, unpublished data). This is illustrated by the RNA gel blots shown in Figure 8. As in hcf107, the 1800-nucleotide tricistronic psbH-petB-petD transcript, the 1200-nucleotide dicistronic psbH-petB transcript, and the 400-nucleotide monocistronic psbH RNA were lacking or at least depleted drastically. On the other hand, the 2600-nucleotide psbH-petB(IB)-petD RNA and a psbH-petB(IB) RNA of ∼1900 nucleotides were present in hcf152. Thus, hcf152 was depleted in some but not all processed psbH-containing transcripts with a free psbH 5′ end. S1 nuclease protection analysis (Figure 5) revealed that hcf152 was impaired in 3′ psbH processing, whereas the 5′ psbH processing was normal and the −45 RNA 5′ end was generated. We conclude from these findings that defective 3′ psbH processing does not affect PsbH synthesis provided that psbH RNAs with the −45 5′ end are available.
Figure 8.
Comparative RNA Gel Blot Hybridization Analysis of the psbH-petB Segment of Mutants hcf152 and hcf107-1.
The probes used are indicated in the map at the top of the figure. Experimental conditions were the same as those described in the legend to Figure 4. WT, wild type.
DISCUSSION
The expression of plastid genes depends on the activities of a large number of auxiliary and regulatory factors, the majority of which are encoded by nuclear genes (Barkan and Goldschmidt-Clermont, 2000). The identification of these factors and the elucidation of their function within the network of plastidial gene regulation are a prerequisite if one wishes to understand how plant cells accumulate photosynthetically active chloroplasts. Here we discuss the nuclear mutation hcf107 of Arabidopsis, which causes a defect in the synthesis and/or turnover of the PSII reaction center proteins CP47 (PsbB) and PsbH. We show that hcf107 mutants specifically lack processed psbH-containing transcripts, whereas they are not affected in the accumulation of processed psbB RNAs. Our analysis of this mutation reveals novel information about the mechanisms that control chloroplast translation.
Chlorophyll fluorescence measurements (Meurer et al., 1996b) indicated a functional defect of PSII that was reflected at the level of PSII proteins. The PSII reaction center core proteins CP47, D1, and PsbH were not or were barely detectable in mutant plants. Subunits CP43 and D2 were present but reduced significantly, whereas the accumulation of cytochrome b559 was not impaired. Both spectroscopic and immunological data indicated that the other thylakoid membrane protein complexes (i.e., PSI, the cytochrome b6f complex, and the ATP synthase) were not affected primarily by the hcf107 mutation.
The lack of intact PSII complexes correlated with a deficiency in the accumulation of radioactively labeled CP47 (PsbB) and PsbH. Because the two proteins were not labeled even when short pulses were applied, we conclude that most likely their synthesis and not their stability is impaired in hcf107. CP47 and PsbH are the only detectable plastome-encoded PSII proteins that are not synthesized in hcf107 chloroplasts, suggesting that this defect is primarily responsible for the accumulation of truncated, nonfunctional PSII reaction centers.
Mutational analyses and/or directed gene inactivation experiments with the cyanobacterium Synechocystis PCC 6803, the unicellular green alga Chlamydomonas reinhardtii, and the higher plant Arabidopsis support this conclusion. In both the cyanobacterium (Vermaas et al., 1988) and the two photosynthetic eukaryotes (Monod et al., 1992; Meurer et al., 1996a), the presence of CP47 is absolutely required for the assembly and/or stability of PSII reaction center complexes. In contrast, the requirement of PsbH for PSII assembly appears to differ between photosynthetic prokaryotes and eukaryotes. The protein is necessary for the assembly and/or stability of PSII reaction centers in C. reinhardtii (Summer et al., 1997; O'Connor et al., 1998), although it is not essential for PSII biogenesis in the cyanobacterium at low light intensities. PsbH, however, is needed for PSII assembly/stability in the cyanobacterium under high light conditions (Mayers et al., 1993). The exact functions of these proteins in the assembly of PSII are not understood. Kinetic analysis of PSII assembly in hcf107 by pulse labeling and subsequent separation of PSII assembly intermediates by two-dimensional gel electrophoresis revealed that the formation of the first detectable assembly intermediate (i.e., the D1–D2–cytochrome b559 complex) was not disturbed (H. Plücken, B. Müller, P. Westhoff, and L. Eichacker, unpublished data). This finding indicates that in Arabidopsis, CP47 and PsbH are required at later stages in the PSII assembly process.
At the RNA level, the deficiency in CP47 and PsbH synthesis was correlated only with defects in the psbH part of the psbB-psbT-psbH-petB-petD transcript profile but not with defects in the psbB segment. Neither the levels nor the sizes of the oligocistronic psbB-containing RNAs (i.e., the two dicistronic psbB-psbT transcripts and the tricistronic psbB-psbT-psbH RNAs) were altered in the hcf107 mutant background (Figure 4). This finding suggests that the transcriptional initiation in front of psbB and the 5′ processing of the transcript produced is normal in hcf107 mutants. In contrast, all processed psbH RNAs with psbH as the leading cistron did not accumulate in hcf107.
It may be surprising that PsbH synthesis and accumulation were impaired dramatically in hcf107 although pentacistronic psbH-psbT-psbH-petB-petD RNAs and the tricistronic psbB-psbT-psbH RNA accumulated. However, these observations are similar to findings in the crp1 mutant of maize (Barkan et al., 1994; Fisk et al., 1999). crp1 cannot generate a monocistronic petD RNA and is disturbed concomitantly in the biogenesis of the cytochrome b6f complex. In both mutants, the processing of the polycistronic precursor transcript to a monocistronic RNA appears to be necessary to generate translatable RNAs. However, our comparison of hcf107 with hcf152 demonstrated that the translation of psbH does not depend on the presence of a monocistronic psbH transcript per se. hcf152, like hcf107, is depleted in the monocistronic psbH transcript because the mutant cannot cleave the psbH-petB intergenic RNA segment. Nevertheless, hcf152 contains an active PSII. The fundamental difference is the formation of the psbH 5′ leader. Both mutants, like the wild type, accumulate psbH RNAs with the putative −75 end, provided that this RNA 5′ end actually exists (see Results for more details). However, only hcf152 is capable of generating psbH RNAs with the −45 5′ end. It follows that translation of psbH is possible only when a psbH 5′ leader with the −45 site has been formed and that processing of the 3′ trailer is not necessary for translation to occur.
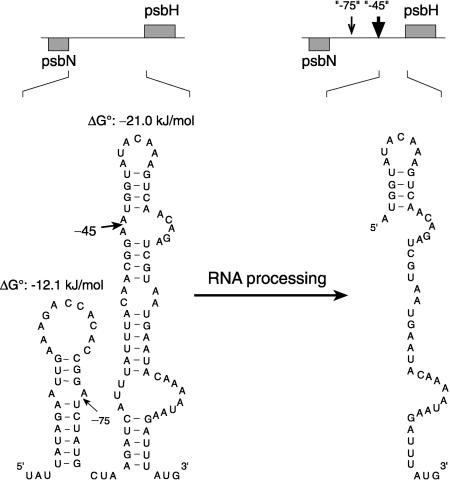
The requirement of the −45 psbH leader for translation raises the question of the inhibiting mechanism for the translation of those psbH transcripts that do not start at the −45 site. A likely answer to this question emerges when the 5′ upstream sequences of psbH are compared for their potential to form secondary structures (Figure 9). The unprocessed psbH leader, like the −75 leader, can fold into stable stem and loop structures in which the −75 and −45 processing sites are located in single-stranded bulges. However, no thermodynamically stable secondary structures can be predicted if the psbH leader is processed at −45. It is tempting to assume, therefore, that processing at −45 is required to unfold the psbH leader, which enables ribosome binding and subsequently the initiation of translation.
Figure 9.
Secondary Structure Model of the Unprocessed and Processed psbT-psbH Intergenic RNA.
Structures were determined at 35°C and 1 M NaCl as an extrapolation to cellular conditions. The −45 processing site is labeled by a thick arrow, and the probably artifactual processing site at −75 is indicated by a thin arrow. The start codon of the psbH reading frame is printed in boldface. ΔG°, standard free energy change.
Masking of translational initiation sites by intramolecular RNA folding is well documented for prokaryotes (McCarthy and Gualerzi, 1990; Lindahl and Hinnebusch, 1992), and there is increasing evidence that the formation of intramolecular or intermolecular RNA/RNA duplexes functions as a translational controlling mechanism in plastids as well. In barley, methyl jasmonate treatment leads to a decline in in vitro–translatable rbcL RNA, although the total levels of rbcL transcripts do not change significantly. The decline in translational activity is correlated with a shift in the length of the 5′ leader of rbcL from −59 to −94. It has been suggested that intermolecular base pairing of a 35-base motif in the distal part of the 5′ leader with the extreme terminal part of 16S rRNA is the reason why the translation of the −94 rbcL transcripts is impaired (Reinbothe et al., 1993). The crp1 mutant of maize mentioned above may represent one example of translational blockage by intramolecular base pairing. Cleavage of the petD message from the polycistronic precursor transcript could release the translation initiation region from inhibitory interactions with upstream RNA sequences (Barkan et al., 1994). Similarly, the intercistronic cleavage of the dicistronic ndhD-psaC transcript appears to abolish the intramolecular interaction between an eight-nucleotide sequence in the psaC coding region and its complementary eight-nucleotide sequence in the 5′ untranslated leader of ndhD. As a consequence, the blockage in ndhD translation is removed (Hirose and Sugiura, 1997). In summary, we propose that the HCF107 gene product is necessary, directly or indirectly, for the unfolding of secondary structures within the psbH 5′ leader. This releases the inhibition of psbH translation in transcripts with the unprocessed or the −75 psbH leader.
The hcf107 mutants exhibit deficiencies similar to those of the Mbb1 mutant of C. reinhardtii. Like hcf107, Mbb1 does not synthesize CP47—the synthesis of PsbH has not been analyzed yet—and is defective in the assembly of PSII complexes (Monod et al., 1992). Mbb1 accumulates neither dicistronic psbB-psbT transcripts nor monocistronic psbH RNAs (Vaistij et al., 2000b). In contrast, the hcf107 mutation of Arabidopsis affects only the accumulation of psbH RNAs with the −45 leader and leaves all other psbH RNAs as well as all psbB transcripts unaffected. Hence, with respect to their effects on RNA accumulation, the two mutations are clearly different.
What mechanistic functions can be imagined for HCF107 that would explain the effects on both CP47 and PsbH synthesis? HCF107 might act as a site-specific endonuclease that cleaves the psbH untranslated region at position −45 or as an accessory component of a nonspecific endonuclease, thereby conferring site specificity to that endonuclease. Alternatively, the HCF107 gene product may be primarily a stabilizer of psbH-containing transcripts once they are processed at −45. However, these suggestions for HCF107 function do not take into account the fact that HCF107 also is required for the synthesis of CP47 without an apparent effect on psbB transcripts. The effects of the hcf107 mutations on both targets can be reconciled more easily if one assumes that HCF107 is an essential part of a protein complex that is multifunctional. This complex would be necessary for the synthesis of CP47 and perhaps also PsbH by serving as a translational activator. The HCF107 complex also provides RNA processing and/or stabilization functions that are needed for psbH, but not for psbB, transcripts.
The recent cloning of the Mbb1 gene of Chlamydomonas (Vaistij et al., 2000a) and of HCF107 from Arabidopsis (Figure 10A; A. Sane, B. Stein, and P. Westhoff, unpublished data) is in line with this suggestion. HCF107 is identical to the tetratricopeptide repeat protein BAA9482 of Arabidopsis that Vaistij et al. (2000a) have identified as a homolog of Mbb1. BLAST searches followed by a phylogenetic analysis, moreover, indicate that the two proteins are true evolutionary orthologs (Figure 10B). The tetratricopeptide repeat motif is well known for mediating protein–protein interactions (Blatch and Lässle, 1999), and for Mbb1 it has already been shown that this protein is part of a large complex (Vaistij et al., 2000a). Future experiments will need to elucidate the composition of these complexes to explain the mechanisms of function of Mbb1/HCF107.
Figure 10.
Sequence Comparison and Evolutionary Analysis of HCF107 and Mbb1.
(A) Sequence alignment of the predicted HCF107 and Mbb1 precursor proteins. The putative plastid transit peptides of the two proteins were determined by ChloroP search (http://www.cbs.dtu.dk/services/ChloroP/) and are indicated in boldface. The tetratricopeptide-like repeat of HCF107 is labeled by a thick line above the sequence.
(B) Phylogenetic analysis of HCF107 and Mbb1. HCF107 was used as the query sequence for a BLASTP search (http://www.ncbi.nlm.nih.gov/BLAST/). Protein sequences with alignment scores >50 were subjected to phylogenetic analysis using parsimony (PAUP 4.0b8; Sinauer Associates, Sunderland, MA). Only the relevant part of the tree is shown. Bootstrap values and protein identifiers are indicated.
METHODS
Growth Conditions
Seed of Arabidopsis thaliana were surface-sterilized and sown on sucrose-supplemented medium containing 0.3% (w/v) Gelrite (Carl Roth GmbH, Karlsruhe, Germany). Seedlings were grown with a 16-hr photoperiod at a photon flux density of 20 to 50 μmol·m−2·sec−1 and a temperature of 23°C.
The mutant hcf107-1 was selected from a collection of mutants induced by ethyl methanesulfonate (Meurer et al., 1996b), whereas hcf107-2 (originally named DEI117) was selected from a collection of T-DNA lines (Bechtold et al., 1993; Bouchez et al., 1993). Mutant plants that exhibited the phenotype with high-chlorophyll fluorescence were selected in the dark under UV light as described (Meurer et al., 1996b).
Antiserum Production and Immunoblot Analyses
The nucleotide sequence encoding the N-terminal 46 amino acids of the PsbH protein of Arabidopsis was amplified by polymerase chain reaction (PCR). The 5′ and 3′ primers contained a BamHI or a XhoI site, respectively, for in-frame fusion with the glutathione S-transferase (GST) sequence of the pGEX-4T3 expression vector (Amersham Pharmacia Biotech, Uppsala, Sweden). The construction of the recombinant plasmid and the expression in Escherichia coli were as described (Meurer et al., 1998). The GST-PsbH fusion protein produced was found to be soluble and was purified by affinity chromatography on glutathione–Sepharose 4B (Amersham Pharmacia Biotech) as recommended by the manufacturer. A polyclonal antiserum was raised in rabbits by BioGenes (Berlin, Germany). The specificity of the antiserum was tested by immunoblotting (Meurer et al., 1996b).
In Vivo Labeling of Proteins
Primary leaves from five to six 12-day-old mutant or wild-type seedlings were incubated for 15 min in 50 μL of double distilled water containing 40 μg/μL cycloheximide. The preincubation medium was then replaced by 50 μL of water containing 250 μCi of 35S-methionine (specific activity >1000 Ci/mmol; Amersham Buchler, Braunschweig, Germany) and 20 μg/μL cycloheximide. Radioactive methionine was allowed to incorporate for varying periods at room temperature in ambient light. After labeling, leaves were frozen immediately at −70°C to block further incorporation. The frozen leaves were processed as described (Meurer et al., 1996a).
In Vitro Phosphorylation of Thylakoid Proteins
In vitro phosphorylation of thylakoid membrane proteins was performed essentially as described (Koivuniemi et al., 1995). Chloroplasts isolated by differential centrifugation (Meurer et al., 1996a) were resuspended in 50 mM Hepes-KOH, pH 7.6, 100 mM sorbitol, 5 mM MgCl2, and 5 mM NaCl at 0.4 mg chlorophyll/mL. Ten microcuries of α-32P-ATP (Amersham-Pharmacia) and NaF (10 mM final concentration) were added to 100 μL of thylakoid membrane suspension, and the assay was incubated for 30 min at 23°C at a photon flux density of 50 μmol photons·m−2·sec−1. The reaction was terminated by centrifugation, and the membranes were washed twice in incubation medium (without NaF). The pelleted membranes were resuspended in SDS sample buffer, electrophoresed on 12.5% polyacrylamide-SDS/urea gels (Schägger and von Jagow, 1987), and finally analyzed by phosphorimaging.
RNA Gel Blot Hybridization Analysis
RNA gel blot analysis of total leaf RNA was performed as described (Meurer et al., 1996a). Hybridization probes were made by cloning either PCR fragments or restriction fragments as described by Felder (1999). The probes were labeled by random priming.
S1 Nuclease Protection Mapping
DNA templates for the synthesis of single-stranded DNA probes for S1 mapping of psbH and psbN RNA 5′ and 3′ ends were prepared by conventional PCR (Dieffenbach and Dveksler, 1995) using the following pairs of primers (5′/3′): psbH 5′, GCGGATCCAAGATGGCGA-CTAGGGAC/TCTAGACAACAGTTTGTGTAGC; psbH 3′, GGAAGG-CCTGTTCTAGATCTGGTC/GGCGGATCCTAAGTATGAATCATAA; psbN 5′, GCGGATCCGGATCTCTTAGTTGTTG/GCTCTAGAACT-ATCTTCAACAGT; psbN 3′, GCGGATCCACGATCAAATTTATGGA/AC-TCTAGAACAGCAACCCTAGTC. The DNA fragments were purified by agarose gel electrophoresis and extracted from the gel using the QIAEX II Gel Extraction Kit (Qiagen, Hilden, Germany) according to the suggestions of the manufacturer. Uniformly radioactively labeled probes were then prepared by linear PCR using only the 3′ primer. The 20-μL reactions contained 20 to 50 ng of double-stranded DNA fragment, 20 ng of 3′ primer, 200 μM deoxynucleotide triphosphates, 15 to 20 μCi of α-32P-dATP, and 1.5 units of Taq polymerase (Appligene-Oncor, Heidelberg, Germany) in the appropriate buffer. Standard cycling conditions (35 times) were 94°C for 5 min, 50°C for 1 min, 72°C for 1.50 min, and 72°C for 7 min. The labeled probes were then mixed with loading buffer (0.3% bromphenol blue, 0.3% xylene cyanole FF, 10 mM EDTA, pH 7.5, and 97.5% deionized formamide) and electrophoresed on a 4% polyacrylamide/urea sequencing gel for 1.5 hr. After the gel was exposed to an x-ray film for 5 min, the labeled fragments were excised, crushed, and incubated in 300 μL of elution buffer (0.3 M sodium acetate, pH 6.0, 10 mM EDTA, and 0.2% SDS) at 37°C for 2 hr. The gel slurry was filtered through cotton wool, and the eluted DNA was precipitated with 20 μg of tRNA and 700 μL of ethanol at −20°C for 2 hr. The dried pellet was dissolved in 10 to 20 μL of deionized formamide at −20°C overnight. Twenty micrograms of total leaf RNA was precipitated with sodium acetate and ethanol and dissolved in 14 μL of formamide.
The hybridization reaction was set up by mixing 14 μL of RNA, labeled probe equivalent to 100,000 cpm, and deionized formamide to a final volume of 24 μL. Nucleic acids were denatured at 90°C for 90 sec and then transferred immediately to 48°C. Six microliters of hybridization buffer (2 M NaCl, 5 mM EDTA, and 0.2 M Pipes/NaOH, pH 6.4) was added, and hybridization was allowed to proceed for 7 hr, with the temperature decreased linearly from 48 to 40°C. After hybridization, 300 μL of ice-cold buffer (0.28 M NaCl, 4.5 mM ZnSO4, and 50 mM sodium acetate, pH 4.4) with 150 units of S1 nuclease (Roche Diagnostics, Mannheim, Germany) was added and incubated at 20°C for 1 hr. After phenol/chloroform extraction, nucleic acids were precipitated with sodium acetate/ethanol and dissolved in 3.5 μL of loading buffer. S1-protected fragments were analyzed on a 4% polyacrylamide gel containing 7 M urea. A sequencing reaction was used to determine fragment sizes.
RNA Structure Calculations
Predictions of thermodynamically optimal secondary structures of RNA were made with the software package LinAll (Schmitz and Steger, 1992). Calculations were performed for a temperature of 35°C to extrapolate the ionic strength of the calculations (1 M NaCl) to cellular conditions. This difference is based on systematic studies of the denaturation of potato spindle tuber viroid under different conditions (Riesner and Steger, 1990).
Accession Numbers
The EMBL accession numbers for the proteins described in this article are BAA94982 (HCF107) and CAC19558 (Mbb1).
Acknowledgments
We thank Maria Koczor for technical help and Angela Wenzik for performing some of the in vivo protein labeling experiments. We also are indebted to Dr. Uwe Santore for carefully reading the manuscript. This research was supported by grants from the Deutsche Forschungsgemeinschaft to P.W. and K.M. through SFBs 189 and TR1 at the University of Düsseldorf, by a grant from the German–Israeli Foundation for Scientific Research and Development to P.W., and by a fellowship of the Alexander von Humboldt-Stiftung to A.P.S.
References
- Allen, J.F., Bennett, J., Steinback, K.E., and Arntzen, C.J. (1981). Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291 25–29. [Google Scholar]
- Barkan, A. (1988). Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 7 2637–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan, A., Walker, M., Nolasco, M., and Johnson, D. (1994). A nuclear mutation in maize blocks the processing and translation of several chloroplast mRNAs and provides evidence for the differential translation of alternative mRNA forms. EMBO J. 13 3170–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan, A., and Goldschmidt-Clermont, M. (2000). Participation of nuclear genes in chloroplast gene expression. Biochimie 82 559–572. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris Life Sci. 316 1194–1199. [Google Scholar]
- Bennett, J., Shaw, E.K., and Michel, H. (1988). Cytochrome b6f complex is required for phosphorylation of light-harvesting chlorophyll a/b complex II in chloroplast photosynthetic membranes. Eur. J. Biochem. 171 95–100. [DOI] [PubMed] [Google Scholar]
- Blatch, G.L., and Lässle, M. (1999). The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. Bioessays 21 932–939. [DOI] [PubMed] [Google Scholar]
- Bouchez, D., Camilleri, C., and Caboche, M. (1993). A binary vector based on Basta resistance for in planta transformation of Arabidopsis thaliana. C. R. Acad. Sci. Paris Life Sci. 316 1188–1193. [Google Scholar]
- Bruick, R.K., and Mayfield, S.P. (1999). Light-activated translation of chloroplast mRNAs. Trends Plant Sci. 4 190–195. [DOI] [PubMed] [Google Scholar]
- Danon, A., and Mayfield, S.P.Y. (1991). Light-regulated translational activators: Identification of chloroplast gene specific mRNA binding proteins. EMBO J. 10 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieffenbach, C.W., and Dveksler, G.S. (1995). PCR Primer: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Douglas, S.E. (1994). Chloroplast origins and evolution. In The Molecular Biology of Cyanobacteria, D.A. Bryant, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 91–118.
- Farchaus, J., and Dilley, R.A. (1986). Purification and partial sequence of the Mr 10,000 phosphoprotein from spinach thylakoids. Arch. Biochem. Biophys. 244 94–101. [DOI] [PubMed] [Google Scholar]
- Felder, S. (1999). Genetische und Molekularbiologische Analyse der RNA-Prozessierung des psbB-Operons von Arabidopsis thaliana. Thesis. (Düsseldorf, Germany: University of Düsseldorf).
- Fisk, D.G., Walker, M.B., and Barkan, A. (1999). Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J. 18 2621–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, R.G., Westhoff, P., and Link, G. (1992). Biogenesis of plastids in higher plants. In Plant Gene Research: Cell Organelles, R.G. Herrmann, ed (Vienna: Springer Verlag), pp. 275–349.
- Hird, S.M., Webber, A.N., Wilson, R.J., Dyer, T.A., and Gray, J.C. (1991). Differential expression of the psbB and psbH genes encoding the 47 kDa chlorophyll a-protein and the 10 kDa phosphoprotein of photosystem II during chloroplast development in wheat. Curr. Genet. 19 199–206. [DOI] [PubMed] [Google Scholar]
- Hirose, T., and Sugiura, M. (1997). Both RNA editing and RNA cleavage are required for translation of tobacco chloroplast ndhD mRNA: A possible regulatory mechanism for the expression of a chloroplast operon consisting of functionally unrelated genes. EMBO J. 16 6804–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi, T., Yoshida, T., Komano, T., and Ohyama, K. (1988). Divergent mRNA transcription in the chloroplast psbB operon. EMBO J. 7 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivuniemi, A., Aro, E.-M., and Andersson, B. (1995). Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 34 16022–16029. [DOI] [PubMed] [Google Scholar]
- Lindahl, L., and Hinnebusch, A. (1992). Diversity of mechanisms in the regulation of translation in prokaryotes and lower eukaryotes. Curr. Opin. Genet. Dev. 2 720–726. [DOI] [PubMed] [Google Scholar]
- Mayers, S.R., Dubbs, J.M., Vass, I., Hideg, E., Nagy, L., and Barber, J. (1993). Further characterization of the psbH locus of Synechocystis sp. PCC 6803: Inactivation of psbH impairs QA to QB electron transport in photosystem 2. Biochemistry 32 1454–1465. [DOI] [PubMed] [Google Scholar]
- McCarthy, J.E.G., and Gualerzi, C. (1990). Translational control of prokaryotic gene expression. Trends Genet. 6 78–85. [DOI] [PubMed] [Google Scholar]
- Meurer, J., Berger, A., and Westhoff, P. (1996. a). A nuclear mutant of Arabidopsis with impaired stability on distinct transcripts of the plastid psbB, psbD/C, ndhH, and ndhC operons. Plant Cell 8 1193–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer, J., Meierhoff, K., and Westhoff, P. (1996. b). Isolation of high-chlorophyll-fluorescence mutants of Arabidopsis thaliana and their characterisation by spectroscopy, immunoblotting and Northern hybridisation. Planta 198 385–396. [DOI] [PubMed] [Google Scholar]
- Meurer, J., Plücken, H., Kowallik, K.V., and Westhoff, P. (1998). A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 17 5286–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, H.P., and Bennett, J. (1987). Identification of the phosphorylation site of an 8.3 kDa protein from photosystem II of spinach. FEBS Lett. 212 103–108. [Google Scholar]
- Monde, R.A., Schuster, G., and Stern, D.B. (2000). Processing and degradation of chloroplast mRNA. Biochimie 82 573–582. [DOI] [PubMed] [Google Scholar]
- Monod, C., Goldschmidt-Clermont, M., and Rochaix, J.-D. (1992). Accumulation of chloroplast psbB RNA requires a nuclear factor in Chlamydomonas reinhardtii. Mol. Gen. Genet. 231 449–459. [DOI] [PubMed] [Google Scholar]
- Nickelsen, J., van Dillewijn, J., Rahire, M., and Rochaix, J.-D. (1994). Determinants for stability of the chloroplast psbD RNA are located within its short leader region in Chlamydomonas reinhardtii. EMBO J. 13 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, H.E., Ruffle, S.V., Cain, A.J., Deak, Z., Vass, I., Nugent, J.H.A., and Purton, S. (1998). The 9-kDa phosphoprotein of photosystem II: Generation and characterisation of Chlamydomonas mutants lacking PSII-H and a site-directed mutant lacking the phosphorylation site. Biochim. Biophys. Acta 1364 63–72. [DOI] [PubMed] [Google Scholar]
- Reinbothe, S., Reinbothe, C., Heintzen, C., Seidenbecher, C., and Parthier, B. (1993). A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J. 12 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner, D., and Steger, G. (1990). Viroids and viroid-like RNAs. In Landoldt-Börnstein, Vol. VII/1, New Series in Biophysics: Nucleic Acids, W. Saenger, ed (Berlin: Springer Verlag), pp. 194–243.
- Rintamäki, E., Salonen, M., Suoranta, U.M., Carlberg, I., Andersson, B., and Aro, E.M. (1997). Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo: Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J. Biol. Chem. 272 30476–30482. [DOI] [PubMed] [Google Scholar]
- Salvador, M.L., Klein, U., and Bogorad, L. (1993). 5′ Sequences are important positive and negative determinants of the longevity of Chlamydomonas chloroplast gene transcripts. Proc. Natl. Acad. Sci. USA 90 1556–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger, H., and von Jagow, G. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166 368–379. [DOI] [PubMed] [Google Scholar]
- Schmitz, M., and Steger, G. (1992). Base-pair probability profiles of RNA secondary structure. Comput. Appl. Biosci. 8 389–399. [DOI] [PubMed] [Google Scholar]
- Sugimoto, N., Nakano, S., Katoh, M., Matsumara, A., Nakamuta, H., Ohmichi, T., Yoneyama, M., and Sasaki, M. (1995). Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry 34 11211–11216. [DOI] [PubMed] [Google Scholar]
- Sugiura, M. (1992). The chloroplast genome. Plant Mol. Biol. 19 149–168. [DOI] [PubMed] [Google Scholar]
- Summer, E.J., Schmid, V.H.R., Bruns, B.U., and Schmidt, G.W. (1997). Requirement for the H phosphoprotein in photosystem II of Chlamydomonas reinhardtii. Plant Physiol. 113 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij, F.E., Boudreau, E., Lemaire, S.D., Goldschmidt-Clermont, M., and Rochaix, J.D. (2000. a). Characterization of Mbb1, a nucleus-encoded tetratricopeptide-like repeat protein required for expression of the chloroplast psbB/psbT/psbH gene cluster in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 97 14813– 14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij, F.E., Goldschmidt-Clermont, M., Wostrikoff, K., and Rochaix, J.D. (2000. b). Stability determinants in the chloroplast psbB/T/H mRNAs of Chlamydomonas reinhardtii. Plant J. 21 469–482. [DOI] [PubMed] [Google Scholar]
- Vermaas, W.F.J., Ikeuchi, M., and Inoue, Y. (1988). Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 17 97–113. [DOI] [PubMed] [Google Scholar]
- Westhoff, P. (1985). Transcription of the gene encoding the 51 kd chlorophyll a-apoprotein of the photosystem II reaction centre from spinach. Mol. Gen. Genet. 201 115–123. [Google Scholar]
- Westhoff, P., and Herrmann, R.G. (1988). Complex RNA maturation in chloroplasts: The psbB operon from spinach. Eur. J. Biochem. 171 551–564. [DOI] [PubMed] [Google Scholar]
- Zerges, W. (2000). Translation in chloroplasts. Biochimie 82 583–601. [DOI] [PubMed] [Google Scholar]
- Zerges, W., Girard-Bascou, J., and Rochaix, J.D. (1997). Translation of the chloroplast psbC mRNA is controlled by interactions between its 5′ leader and the nuclear loci TBC1 and TBC3 in Chlamydomonas reinhardtii. Mol. Cell. Biol. 17 3440–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]