Abstract
The organization of microtubule arrays in the plant cell cortex involves interactions with the plasma membrane, presumably through protein bridges. We have used immunochemistry and monoclonal antibody 6G5 against a candidate bridge protein, a 90-kD tubulin binding protein (p90) from tobacco BY-2 membranes, to characterize the protein and isolate the corresponding gene. Screening an Arabidopsis cDNA expression library with the antibody 6G5 produced a partial clone encoding phospholipase D (PLD), and a full-length gene was obtained by sequencing a corresponding expressed sequence tag clone. The predicted protein of 857 amino acids contains the active sites of a phospholipid-metabolizing enzyme and a Ca2+-dependent lipid binding domain and is identical to Arabidopsis PLDδ. Two amino acid sequences obtained by Edman degradation of the tobacco p90 are identical to corresponding segments of a PLD sequence from tobacco. Moreover, immunoprecipitation using the antibody 6G5 and tobacco BY-2 protein extracts gave significant PLD activity, and PLD activity of tobacco BY-2 membrane proteins was enriched 6.7-fold by tubulin-affinity chromatography. In a cosedimentation assay, p90 bound and decorated microtubules. In immunofluorescence microscopy of intact tobacco BY-2 cells or lysed protoplasts, p90 colocalized with cortical microtubules, and taxol-induced microtubule bundling was accompanied by corresponding reorganization of p90. Labeling of p90 remained along the plasma membrane when microtubules were depolymerized, although detergent extraction abolished the labeling. Therefore, p90 is a specialized PLD that associates with membranes and microtubules, possibly conveying hormonal and environmental signals to the microtubule cytoskeleton.
INTRODUCTION
Microtubules (MTs), a major component of the plant cytoskeleton, are involved in important cellular functions during growth and development (Lloyd, 1991; Cyr, 1994; Cyr and Palevitz, 1995; Kost et al., 1999; Nick, 1999). Before mitosis, MTs form a preprophase band, which predicts the plane of future cell division. Subsequently, they rearrange into the mitotic spindle and eventually the phragmoplast during cytokinesis. During interphase, they form parallel ordered arrays on the internal face of the plasma membrane. The parallel organization of newly deposited cellulose microfibrils in the cell wall mirrors the orientation of the interphase MT array (Williamson, 1991). Conversely, the MT network also is known to respond to changes in cell wall organization (Fisher and Cyr, 1998). Through their interaction with cellulose microfibril deposition, MTs play an important role in directional cell expansion (Gertel and Green, 1977; Giddings and Staehelin, 1991).
Interphase MT arrays in the cell cortex respond to diverse developmental and extracellular stimuli. Cortical MT arrays typically change their orientation from transverse to longitudinal to the growth axis as cells mature (Laskowski, 1990; Cyr and Palevitz, 1995; Wymer and Lloyd, 1996; Granger and Cyr, 2001). Wounding causes MTs in neighboring cells to reorient parallel to the edges of the wound (Hush et al., 1990). MTs orient perpendicular to the direction of an applied mechanical pressure (Hush and Overall, 1991; Zandomeni and Schopfer, 1994) or electric field (Hush and Overall, 1991; Blackman and Overall, 1995) and orient parallel to centrifugal force (Wymer et al., 1996). In flax leaves attacked by a noncompatible strain of rust fungus, the mesophyll cells focus their MT arrays toward the point of attack (Kobayashi et al., 1994). Plant hormones, including abscisic acid, cytokinins, auxin, ethylene, and gibberellic acid, cause interphase MT arrays to reorient in many tissues (reviewed by Shibaoka, 1994), as can exposure to blue or red light (Nick et al., 1990).
Cortical MTs are connected to the plasma membrane by protein links. Electron microscopy has shown cross-bridges connecting cortical MTs to the inner leaflet of the plasma membrane (Gunning and Hardham, 1982; Vesk et al., 1996). Incubating protoplast ghosts with Na2CO3 or 0.6 M KCl disrupts the binding of cortical MTs, consistent with the presence of MT–membrane protein linkage (Sonobe and Takahashi, 1994). Digestion of protoplasts with trypsin and chymotrypsin causes detachment of cortical MTs from the plasma membrane, suggesting that a membrane-spanning protein is involved in MT anchoring (Akashi et al., 1990). There also are indications that transmembrane MT connections extend to the cell wall. When the cell wall is removed during the preparation of protoplasts, MT arrays typically become disorganized (Lloyd et al., 1980; Galway and Hardham, 1986; Simmonds, 1992). Similarly, MTs in protoplasts are cold sensitive but become resistant to cold when the cell wall regenerates, suggesting that the cell wall stabilizes MTs through transmembrane proteins (Akashi et al., 1990). Furthermore, the addition of exogenous extensin to the cold-treated protoplasts rendered MTs stable, indicating that cell wall proteins may modulate the action of these putative transmembrane proteins (Akashi et al., 1990).
Interestingly, the petunia mutant trapu (Dubois et al., 1996) and the Arabidopsis mutants fass and tonneau (Torres-Ruiz and Jurgens, 1994; Traas et al., 1995) lack a preprophase band and have disorganized interphase MT arrays, although these are still located in the cell cortex. Therefore, the mechanism of attachment of MTs to the plasma membrane and their organization into ordered arrays likely involve more than one linkage protein.
Little is known about the proteins responsible for MT–plasma membrane interactions. Tubulin itself may be involved, possibly through a hydrophobic domain on the tubulin molecule or indirectly through interaction with an integral membrane protein (Sonesson et al., 1997). A MT-nucleating protein, γ-tubulin, also is located in the cell cortex (Liu et al., 1994), and a large amount of γ-tubulin associates with a purified plasma membrane fraction (Stoppin-Mellet et al., 2000). Putative MT-associated proteins of varying sizes have been isolated from plant cytosolic fractions, although only a few have been characterized extensively. Elongation factor-1α is known to associate with MTs in a calmodulin-sensitive manner (Durso and Cyr, 1994; Durso et al., 1996), and an isozyme of the p86 subunit of eukaryotic initiation factor-(iso)4F is capable of regulating end-to-end annealing of MTs in plant cells (Hugdahl et al., 1995). A chaperonin (Nick et al., 2000), a heat-shock protein (Petrasek et al., 1998), and importin-α (Smith and Raikhel, 1998) also have been localized to MTs in plant cells. A 65-kD protein from carrot bundles MTs in vitro, maintaining an intermicrotubule spacing similar to that seen in intact cells (Chan et al., 1999). A group of corresponding genes, named NtMAP65-1, recently isolated from tobacco encode a protein that localizes to areas of overlapping MTs (Smertenko et al., 2000). The Tangled1 gene of maize, which is involved in the spatial control of cytokinesis, encodes a highly basic MT binding protein (Smith et al., 2001), and Arabidopsis AtKTN1 encodes a katanin-like protein (Burk et al., 2001). MOR1, a conserved MT-associated protein, appears to be essential for the organization of cortical MTs in Arabidopsis (Whittington et al., 2001). Various MT motor proteins have been isolated from plants, with a number of kinesin homologs cloned (Asada and Collings, 1997; Krishnakumar and Oppenheimer, 1999; Barroso et al., 2000; Cai et al., 2000). However, there is no evidence that any of these proteins interact with the plasma membrane.
Marc et al. (1996) isolated several tubulin binding proteins from tobacco BY-2 cells. A 90-kD polypeptide (p90) was found mainly in the detergent-solubilized microsomal fraction and colocalized with cortical MTs, suggesting that it is a potential candidate for MT–plasma membrane linkage. Here we show that p90 binds and decorates MTs in vitro. In protoplasts, it follows taxol-induced reorganization of cortical MTs and remains associated with the plasma membrane even when the MTs are depolymerized, although it can be removed by subsequent detergent extraction. Screening of an Arabidopsis cDNA expression library with monoclonal antibody 6G5 against the tobacco p90 produced a clone encoding phospholipase D (PLD), consistent with PLD activity that we found in immunoprecipitates of tobacco BY-2 proteins with the antibody 6G5 and with an enrichment in PLD activity after tubulin-affinity chromatography of membrane proteins. We propose that the membrane-associated PLD is involved in the transduction of hormonal and environmental signals to the MT cytoskeleton.
RESULTS
p90 Reacts with Monoclonal Antibodies 6G5 and 4B5
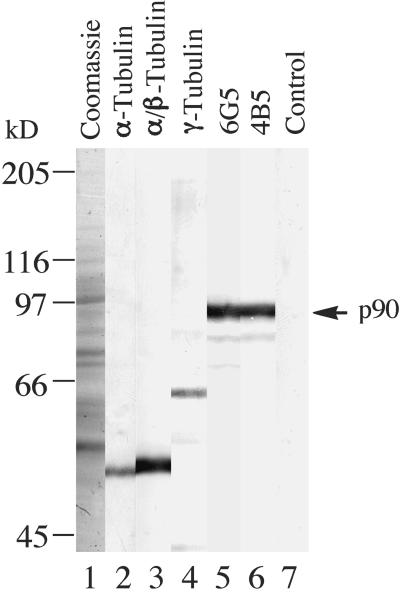
Our initial immunochemical characterization of the tobacco p90 (Marc et al., 1996) was based on the monoclonal antibody 6G5 (class IgM) raised against the purified 90-kD polypeptide. To safeguard against the possibility that the antibody could react with a target epitope located on an otherwise unrelated protein, we prepared another monoclonal antibody, 4B5 (class IgG), against p90. An immunoblot of tobacco membrane proteins in Figure 1 (lanes 5 and 6) shows that both antibodies recognized a 90-kD band. Additional, fainter bands of lower molecular mass probably are proteolytic fragments. Controls, using heat-inactivated antibody 6G5 (lane 7) or omitting the primary antibody (data not shown), were negative. The two antibodies did not cross-react with any proteins from the tubulin family, in contrast to distinct immunoreactive bands obtained with antibodies against α-tubulin, a polyclonal serum against soybean α/β-tubulin, and a peptide serum against a conserved region of γ-tubulin (Figure 1, lanes 2 to 4). In immunofluorescence microscopy of tobacco protoplast ghosts, the antibody 4B5 again colocalized in a punctate manner along MTs (Figure 2), as in the images obtained previously with the antibody 6G5 (Marc et al., 1996; see also Figure 7 below). Therefore, we conclude that both antibodies specifically recognize p90 on immunoblots and in immunofluorescence microscopy.
Figure 1.
Immunoblot Detection of p90 in Tobacco BY-2 Membrane Proteins.
Membrane proteins, prepared by extracting microsomal fractions of tobacco BY-2 cells with the detergent 3-([3-cholamidopropyl]dimethylamino)-1-propanesulfonate (Chaps), were separated by electrophoresis on 8% (w/v) SDS–polyacrylamide gels, transferred onto a nitrocellulose membrane, and probed with antibodies. Lane 1, Coomassie blue–stained gel; lane 2, monoclonal antibody against α-tubulin; lane 3, polyclonal serum against soybean α/β-tubulin; lane 4, peptide serum against a conserved region of γ-tubulin; lane 5, monoclonal antibody 6G5; lane 6, monoclonal antibody 4B5; lane 7, control using a preheated antibody 6G5. Immunoreactions were detected with alkaline phosphatase. The arrow at right indicates the position of the immunoreactive 90-kD polypeptide (p90). Bars at left indicate the positions of molecular mass standards.
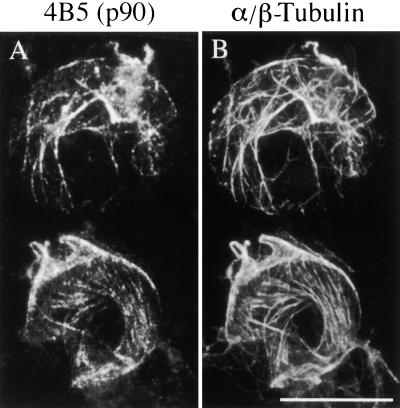
Figure 2.
p90 Colocalizes with Cortical MTs in Protoplasts.
Tobacco BY-2 protoplasts were processed for immunofluorescence microscopy by using mouse monoclonal antibody 4B5 together with rabbit polyclonal serum against soybean α/β-tubulin, followed by a mixture of Texas Red–conjugated anti-mouse and fluorescein isothiocyanate (FITC)-conjugated anti-rabbit secondary antibodies.
(A) p90 visualized with the antibody 4B5 and Texas Red–conjugated secondary antibody.
(B) α/β-Tubulin visualized with serum against soybean tubulin and FITC-conjugated secondary antibody.
Bar in (B) = 10 μm for (A) and (B).
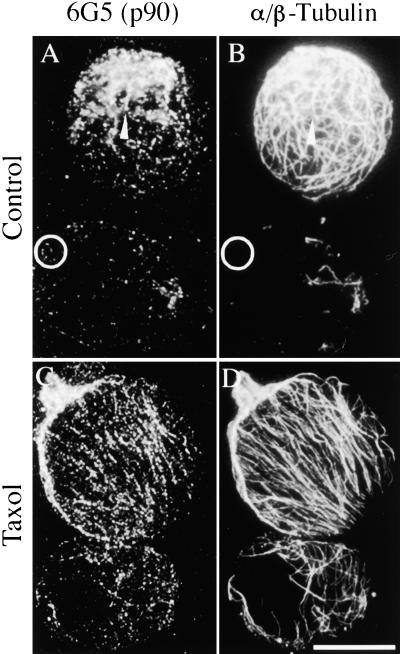
Figure 7.
Colocalization of p90 with Cortical MTs in Protoplasts Accompanies Taxol-Induced Reorganization.
Protoplasts were prepared from tobacco BY-2 cells with or without preincubation in 10 μM taxol and then processed for immunofluorescence microscopy. The preparations were double labeled using antibody 6G5 together with rabbit serum against soybean α/β-tubulin, followed by a mixture of Cy3-conjugated anti-mouse ([A] and [C]) and FITC-conjugated anti-rabbit ([B] and [D]) secondary antibodies.
(A) and (B) Control protoplasts prepared without taxol. p90 associates with MTs in a punctate manner, although some spots appear in MT-free areas of the protoplast ghost (circles).
(C) and (D) Protoplasts with taxol-induced parallel MT arrays and matching punctate arrays of p90.
Bar in (D) = 20 μm for (A) to (D).
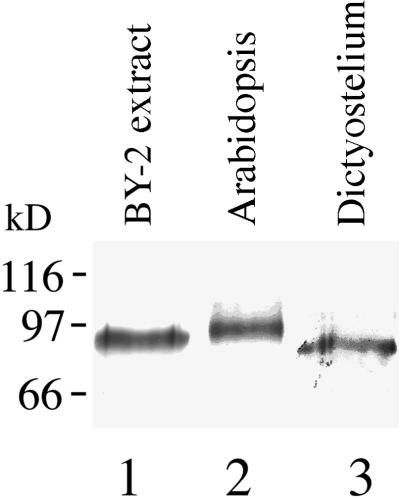
Immunoblotting with Antibody 6G5 Reveals p90-Related Protein Epitopes in Other Plants and Dictyostelium
To determine how widespread p90 might be, we used the antibody 6G5 to probe nitrocellulose blots of protein extracts from other species. The resulting immunoblots showed that the antibody 6G5 recognized a 97-kD immunoreactive band in Arabidopsis and an 88-kD band in Dictyostelium (Figure 3). Immunoreactions similar to that in Arabidopsis were detected also in another dicotyledon, soybean, and the monocotyledons wheat and onion (data not shown). p90 or immunologically related proteins, therefore, appear to be ubiquitous in plants and slime molds. Immunoblotting of HeLa cell protein extract with the antibody 6G5 was inconclusive.
Figure 3.
p90 Is Present in Plants and Lower Eukaryotes.
Protein extracts from plant tissue and Dictyostelium were separated on 10% (w/v) SDS–polyacrylamide gels (10 to 20 μg protein/lane), transferred onto a nitrocellulose membrane, and probed with the 6G5 antibody followed by alkaline phosphatase detection. Lane 1, tobacco BY-2 Chaps extract; lane 2, Arabidopsis seedlings; lane 3, Dictyostelium. Bars at left indicate the positions of molecular mass standards.
Antibody 6G5 Detects a PLD from an Arabidopsis cDNA Expression Library
p90 is of interest as a potential mediator of plasma membrane–cytoskeleton interactions, prompting us to isolate the gene. We used the antibody 6G5 to screen an Arabidopsis cDNA expression library for clones producing the corresponding recombinant protein by using a monoclonal antibody against α-tubulin as a control. Initial screening produced five positive clones, and a single clone remained positive during subsequent rescreening under high antibody dilution. Sequencing of the clone gave a partial cDNA of 2084 nucleotides encoding a polypeptide of 656 amino acids, with 111 nucleotides of 3′ untranslated sequence (GenBank accession number AF274239). We obtained a full-length cDNA clone by identifying the corresponding expressed sequence tag (clone H6C4T7; Arabidopsis Biological Resource Center) (Newman et al., 1994) and sequencing the clone completely. The full-length clone (GenBank accession number AF306345) encodes a polypeptide of 857 amino acids with a molecular mass of 97,779 D and a pI of 6.70 (Figure 4, AtPLD98), with six nucleotides of 5′ untranslated sequence and 190 nucleotides of 3′ untranslated sequence. The deduced amino acid sequence is identical to that of Arabidopsis PLDδ (GenBank accession number AF322228) (Figure 4, AtPLDδ).
Figure 4.
Alignment of Amino Acid Sequences Encoded by PLD cDNA Clones from Arabidopsis and Tobacco.
Shown is an alignment of Arabidopsis PLD98 (AtPLD98) with Arabidopsis PLDδ (AtPLDδ) and a partial cDNA clone of tobacco PLD (NtPLD) prepared using the CLUSTAL W algorithm (Thompson et al., 1994). Shaded boxes show sequence identity, and dashes indicate gaps introduced to optimize the sequence alignment. Putative functional domains are indicated by lines above the sequences as follows: C2, a Ca2+-dependent lipid binding domain; pas, phospholipid-metabolizing enzyme active sites. An amino acid microsequence from tobacco p90, BY2seq1, is highly homologous with AtPLD98 residues 314 to 328 and identical to the corresponding region in the tobacco PLD.
The predicted protein AtPLD98 contains the double HxKxxxD motif (HQKCVLVD and HAKGMIVD) at residues 362 to 369 and 707 to 714 (Figure 4), which is thought to be the active site of phospholipid-metabolizing enzymes in all PLDs (Ponting and Kerr, 1996; Wang 2000). Residues 75 to 107 resemble the Ca2+-dependent lipid binding domain (Figure 4, C2 domain) present in all plant PLDs (Wang, 1997, 2000).
To confirm that we have identified the correct gene, we obtained two amino acid microsequences by Edman degradation of the original tobacco p90. Sequence SEGIMGTSDEETRRY is highly homologous with amino acid residues 314 to 328 in AtPLD98 (Figure 4) as well as other PLDs in the database. Moreover, the sequence is identical to the corresponding site from a partial PLD clone from tobacco (GenBank accession number AAF05818) (Figure 4, NtPLD). Sequence IIGTFTGVQENLTI is partially homologous with residues 843 to 857 in AtPLD98 (Figure 4) as well as other PLDs in the database; this sequence also is identical to the corresponding site from a partial PLD clone from tobacco (K. Chapman, personal communication). Therefore, the Arabidopsis cDNA clone that we isolated most likely is the correct gene. Indeed, the antibody 6G5 also recognizes a recombinant protein expressed by the Arabidopsis cDNA clone (data not shown).
Immunoprecipitation of Tobacco BY-2 Proteins with Antibody 6G5 Yields PLD Activity
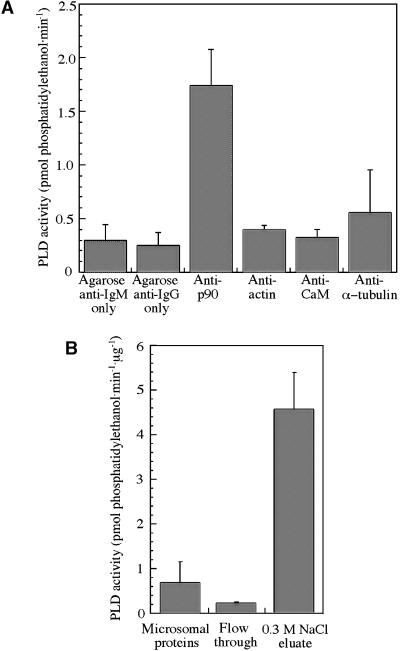
To confirm that tobacco p90 is a PLD, we incubated tobacco BY-2 protein extract with the antibody 6G5 followed by precipitation with agarose-conjugated secondary antibody and assayed the immunoprecipitate for PLD activity (production of phosphatidylethanol). Figure 5A shows that PLD activity was approximately fivefold higher in immunoprecipitates with the antibody 6G5 compared with controls using the secondary antibody–agarose conjugate alone (P < 0.01, t test) or using primary antibodies against actin or calmodulin. Immunoprecipitation with antibodies against tubulin did not produce significant PLD activity above control values (P > 0.05, t test) (by potential coimmunoprecipitation of p90) under our experimental conditions.
Figure 5.
PLD Activity in Tobacco BY-2 Proteins.
Protein samples were incubated with a reaction mixture containing fluorescent phosphatidylcholine and phosphatidylethanol, the products were separated by thin-layer chromatography, and the fluorescence of phosphatidylethanol was measured with a spectrophotometer.
(A) PLD activity in immunoprecipitated pellets. A significant (P < 0.01) PLD activity was measured in pellets immunoprecipitated with antibody 6G5 against p90 and agarose-conjugated secondary antibody (anti-IgM). Only background levels of PLD activity were detected in pellets obtained with antibodies against actin, calmodulin (CaM), and α-tubulin, followed by agarose-conjugated secondary antibody (anti-IgG). The data show the mean values ±sem from at least three independent experiments for each point.
(B) PLD activity in tobacco BY-2 protein fractions from tubulin-affinity chromatography. The PLD activity in detergent (Chaps) extract of tobacco BY-2 microsomes was depleted by ∼67% in the column flow-through fraction and enriched 6.7-fold in tubulin binding proteins eluted with 0.3 M NaCl. The data show the mean values ±sem obtained from one set of the three protein fractions, each analyzed by four independent PLD assays.
PLD Activity in Tobacco BY-2 Extracts Is Enriched by Tubulin-Affinity Chromatography
In our original isolation of p90, we used detergent extract of tobacco BY-2 microsomes and tubulin-affinity chromatography to isolate tubulin binding proteins (Marc et al., 1996). Densitometry of the immunoreactive 90-kD band in the 0.3 M NaCl eluate from the tubulin column, relative to that in the membrane fraction (Figure 6, lanes 3 and 4 in Marc et al., 1996), showed that the tubulin-affinity chromatography had enriched the p90 signal ∼4.3-fold. When we assayed the corresponding protein samples for PLD activity, the chromatography had enriched PLD activity 6.7-fold, with ∼67% depletion in PLD activity in the column flow-through fraction (Figure 5B), consistent with the hypothesis that p90 is a PLD that binds to tubulin. Interestingly, PLD activity was reduced approximately fivefold when the reaction mixture lacked phosphatidylinositol 4,5-bisphosphate but contained 1 mM rather than 100 μM CaCl2 (data not shown), conditions that favor PLDα activity but not the activity of other isoforms.
Figure 6.
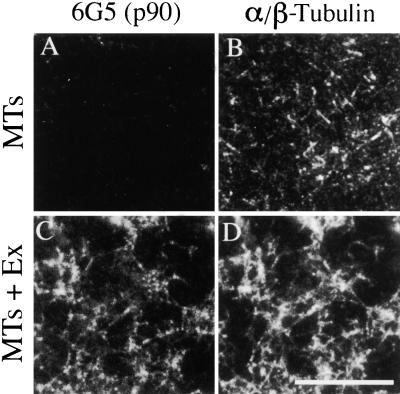
p90 Decorates MTs in Vitro.
Taxol-stabilized neuronal MTs were incubated with a Chaps extract (Ex) of tobacco BY-2 membrane proteins and sedimented by centrifugation. The pelleted protein complexes were spread on microscope slides, fixed, and double labeled using mouse monoclonal antibody 6G5 together with rabbit serum against soybean α/β-tubulin, followed by a mixture of Cy3-conjugated anti-mouse ([A] and [C]) and FITC-conjugated anti-rabbit ([B] and [D]) secondary antibodies.
(A) and (B) Control MTs incubated in buffer alone, showing the absence of labeling with 6G5 antibody.
(C) and (D) MTs decorated with p90 after incubation with membrane proteins.
Bar in (D) = 10 μm for (A) to (D).
p90 from a Detergent Extract of Tobacco Membranes Binds and Decorates Taxol-Stabilized MTs
The p90 protein was isolated initially using tubulin-affinity chromatography followed by cosedimentation with taxol-stabilized MTs and gel electrophoresis (Marc et al., 1996). To confirm that p90 does in fact bind to MTs rather than precipitating spontaneously during the sedimentation step, we analyzed the pelleted proteins by immunofluorescence microscopy by using double labeling with 6G5 and anti-α/β-tubulin antibodies. Immunofluorescent images of taxol-stabilized neuronal MTs incubated in buffer alone showed numerous short, thin, and freely scattered MTs labeled with anti-α/β-tubulin but not with the antibody 6G5 (Figures 6A and 6B). In contrast, after incubation with membrane proteins, the MTs were arranged in thicker, longer, and variably interconnected bundles that were labeled with both the 6G5 and anti-α/β-tubulin antibodies (Figures 6C and 6D). Thus, the bound p90 appears as MT decorations, indicating a close association with MTs, although other proteins from the membrane extract may be involved in MT bundling.
p90 Localizes to Cortical MTs, and Its Distribution Is Modified by Taxol
Because p90 was isolated initially from the microsomal fraction, including endomembranes (Marc et al., 1996), it is necessary to confirm its association with the cell cortex. In control protoplasts prepared in the absence of taxol, the 6G5 antibody gave punctate labeling that aligned with the anti-α/β-tubulin labeling of the MTs (Figures 7A and 7B) as well as randomly dispersed dots in MT-free areas (Figure 7A, circle), indicative of an association with the plasma membrane. In protoplasts prepared from cells that were preincubated with taxol, the MTs formed ordered arrays of parallel bundles (Figure 7D) similar to those reported previously (Kuss-Wymer and Cyr, 1992). Nonetheless, the association of p90 with MTs persisted during this reorganization as a punctate label along the MTs (Figures 7C and 7D). Therefore, the linear colocalization of p90 with cortical MTs is determined by the MTs and not the other way around.
p90 Remains Attached in the Cell Cortex after Removal of MTs but Can Be Extracted with a Detergent
If p90 is attached to the plasma membrane, either directly or indirectly by a linker protein, solubilizing the membrane with a detergent should release it. To test this hypothesis, we first removed the cortical MTs by cold treatment in combination with 5 mM CaCl2 before labeling with antibodies. Although this treatment removed most MTs or reduced them to small rods and specks (Figures 8B and 8D), it left behind numerous dots labeled with the antibody 6G5 (Figure 8A). Similar results were obtained by removing MTs using the anti-MT drug propyzamide (data not shown). In contrast, additional extraction of the protoplast ghosts with 0.05% (v/v) Triton X-100 greatly reduced the frequency and intensity of the immunoreactive dots (Figure 8C). p90, therefore, is anchored in the cortex by attachment to the plasma membrane independently of MTs.
Figure 8.
p90 Associates with Protoplast Plasma Membrane in the Absence of MTs but Can Be Extracted with a Detergent.
Tobacco BY-2 protoplasts, prepared after preincubation with 10 μM taxol, were treated with calcium and detergent and then processed for immunofluorescence microscopy using double labeling with antibodies against p90 and α/β-tubulin as in Figure 7.
(A) and (B) Cortical MTs were removed from the protoplast ghosts by cold treatment in the presence of 5 mM CaCl2 for 30 min. Nonetheless, numerous randomly dispersed dots labeled with antibody 6G5 remained attached to the plasma membrane.
(C) and (D) Protoplast ghosts were additionally extracted with 0.05% (w/v) Triton X-100 (TX-100) for 10 min. This treatment removed most of the p90 labeling.
Bar in (D) = 20 μm for (A) to (D).
p90 Colocalizes with MT Arrays throughout the Cell Cycle
The consistent association of p90 with taxol-stabilized MTs in vitro and with cortical MTs in the protoplasts in situ raises the possibility that it persists through cell division. To test this, we have processed whole tobacco BY-2 cells for immunofluorescence microscopy by using double labeling with the monoclonal antibody 6G5 and polyclonal serum against soybean α/β-tubulin. During interphase, the antibody 6G5 again colocalized with MTs in the cell cortex, showing a punctate distribution (Figures 9A and 9B) similar to the images obtained with the protoplast ghosts (Figure 7). As the MT arrays reorganized through the cell cycle, the 6G5 labeling of p90 followed the MTs to the preprophase band, the mitotic spindle, and the phragmoplast (Figures 9C to 9H). Although the background labeling was high compared with that in the protoplast ghosts, likely because of limited access of the large IgM molecule of the antibody 6G5 into whole cells, the colocalization with MTs throughout the cell cycle was apparent nonetheless. Because the cells were fixed with aldehyde as the first step in the processing protocol, the images obtained were not affected by artifactual redistribution (Melan and Sluder, 1992) of soluble p90.
Figure 9.
p90 Colocalizes with MT Arrays throughout the Cell Cycle.
Tobacco BY-2 cells were fixed and processed for immunofluorescence microscopy by using double labeling with antibodies against p90 and α/β-tubulin as in Figure 7. At all stages of the cell cycle, the MT arrays were matched with punctate arrays of p90.
(A) and (B) Interphase cortical array.
(C) and (D) Preprophase band.
(E) and (F) Metaphase spindle.
(G) and (H) Cytokinetic phragmoplast.
Bar in (H) = 20 μm for (A) to (H).
DISCUSSION
Tobacco p90 Is a PLD with Immunological and Sequence Homology with an Arabidopsis PLD
Three lines of experimental evidence support the notion that the tobacco p90 protein is a PLD. First, immunoprecipitation of tobacco BY-2 proteins with antibody 6G5 yields PLD activity. Moreover, PLD activity in tobacco BY-2 proteins is enriched by tubulin-affinity chromatography, in parallel with an increased amount of p90 detected on immunoblots of protein fractions with antibody 6G5. Second, two amino acid sequences obtained by peptide microsequencing match exactly regions of the polypeptide encoded by the partial cDNA clone of tobacco PLD (K. Chapman, personal communication). Third, the antibody 6G5 has picked out a PLD clone in the screening of the Arabidopsis cDNA library, and it also reacts with the recombinant protein on immunoblots. The size of the predicted protein AtPLD98 corresponds to that of the protein recognized by antibody 6G5 on blots of Arabidopsis protein extract. Immunoblots of protein extracts from another dicotyledon, two monocotyledons, and Dictyostelium also showed similar immunoreactive polypeptide bands, suggesting that related proteins are widespread in the plant kingdom and slime molds. Phospholipases A2, C, and D regulate diverse processes in higher plants, including embryo maturation, germination, cell elongation, senescence, wounding responses, osmotic stress, and pathogen responses (reviewed by Chapman, 1998). PLD, which is present as numerous isoforms in plants, catalyzes the hydrolysis of the terminal phosphodiester bond of glycerophospholipids to generate phosphatidic acid and a hydrophilic head group, such as choline (Wang, 1997, 2000). It is thought to play a major role in cellular processes, including the generation of second messengers (Wang, 1999) and membrane degradation (Paliyath and Droillard, 1992).
Tobacco p90 Binds to MTs
Immunofluorescence microscopy with antibody 6G5 showed that tobacco p90 colocalizes in situ with cortical MT arrays in both BY-2 tobacco protoplast ghosts and whole-cell preparations. This colocalization is not an artifact of the polyethyleneimine coating of the slides, because in whole-cell preparations, p90 still associated with the spindle, phragmoplast, preprophase band, and interphase MTs, all of which are separated from the surface of the glass slide by cell wall material. The decoration of MTs seen in immunofluorescence microscopy further demonstrates its association with MTs. Also, we found that binding of p90 to MTs was inhibited by heating the Chaps extract to 60 to 80°C (data not shown), indicating that the binding reaction requires a native protein and not just an opportunistic electrostatic attraction. Although the pI of the tobacco p90 is not known yet (this clone is being sequenced; K. Chapman, personal communication), we note that the corresponding AtPLD98 is neutral and therefore unlikely to bind to the acidic C terminus of tubulin (Fosket and Morejohn, 1992) by electrostatic forces. The nature of the MT binding site remains to be determined. Sequence similarity searches have not detected a region of significant homology between known MT binding sites and either Arabidopsis PLD or the partially sequenced tobacco PLD, although this is not surprising because MT binding sites generally are divergent.
Tobacco p90 Associates with the Plasma Membrane during Interphase, Most Likely through a Calcium-Dependent Lipid Binding Domain
Although the microsomal fraction of BY-2 tobacco from which p90 was isolated originally (Marc et al., 1996) also contains vesicles and endomembranes, immunolocalization of p90 to cortical MTs in protoplast ghosts and whole cells indicates that it does associate with the plasma membrane. Some of the immunoreactive dots in the control protoplast ghosts appeared to be unattached to MTs, and many dots remained associated with the membrane after the MTs were depolymerized by the Ca2+ and cold treatment. In contrast, detergent extraction removed the immunoreactive dots, consistent with their proposed attachment to the lipid bilayer. It also appears that p90 can move laterally within the plasma membrane, as indicated by their redistribution after taxol treatment. Presumably, taxol-induced bundling and reorientation of MTs (Kuss-Wymer and Cyr, 1992) are accompanied by a corresponding redistribution of p90.
Considering that p90 associates with not only cortical MTs but also the preprophase band, spindle, and phragmoplast, it seems likely that p90 is able to dissociate from the plasma membrane at the onset of mitosis and reattach at the end of cell division. The transient association of p90 with membranes may be through the C2 domain (Wang, 2000). The C2 domain, which is present in the AtPLD98 and possibly also encoded in the corresponding tobacco PLD clone that remains to be sequenced, probably plays a role in regulating the differing responses of PLD isoforms to Ca2+ (Pappan et al., 1997). The C2 domain also is found in many proteins that are involved in intracellular and intercellular signaling, including cytosolic phospholipase A2, phospholipase C, UNC-13, synaptotagmin ras-GTPase, rabphilin-3A, GAP proteins, protein kinase C, and perforin (Ponting and Parker, 1996; Kiyosue and Ryan, 1997). Some C2 proteins, including phospholipase A2 and protein kinase C, are indeed activated by contact with membranes, suggesting that C2 domains may facilitate protein translocation between the cytosol and membranes (Exton, 1994; Wang, 1997).
Possible Role of Plasma Membrane–PLD–MT Association
There is mounting evidence that PLDs play an important role in the regulation of the actin cytoskeleton in yeast and metazoans. PLD mediates the formation of actin stress fibers (Cross et al., 1996), and PLD-mediated increases in phosphatidic acid activate the polymerization of actin in fibroblast cells (Ha and Exton, 1993). The PLD–protein kinase C pathway regulates the assembly of integrin and F-actin focal point contacts during the spreading and proliferation of tumor cells (Ghiso et al., 1997). PLD also is known to interact with actin binding proteins, including gelsolin, which may amplify PLD activity (Steed et al., 1996), and nonerythroid spectrin, which inhibits it (Lukowski et al., 1996). Interestingly, the MT-depolymerizing agent colchicine stimulates the formation of actin stress fibers in fibroblasts (Jung et al., 1997). However, this did not activate PLD, possibly because the signaling pathways for MT depolymerization and stress fiber formation are separate (Jung et al., 1997) or the PLD activation step may be located upstream of the MT depolymerization step.
Although various PLD isoforms have been investigated in plants (Wang, 1997, 1999, 2000), there are no reports of association with MTs, suggesting that tobacco p90 and its Arabidopsis homolog are specialized PLDs. Interestingly, a mouse PLD2 associates with the plasma membrane and reorganizes the cytoskeleton in rat fibroblast cells (Colley et al., 1997). In plants, MT organization and PLD also may be connected, especially given that stimuli that affect PLD activity also cause MT reorganization. For example, wounding induces a transient, systemic Ca2+-dependent increase in membrane-associated PLD (Ryu and Wang, 1996) as well as an increase in phosphatidic acid (Lee et al., 1997), and wounding also leads to a rapid realignment of MTs (Hush et al., 1990). Likewise, bacterial infection increases PLD levels and localizes it to the plasma membrane (Young et al., 1996), and pathogen attack also causes MTs to cluster at the infection site (Kobayashi et al., 1994). Another signaling connection may involve plant hormones, which are known to affect MT organization (Shibaoka, 1994). Abscisic acid signaling in stomatal guard cells, which leads to an efflux of K+ that facilitates stomatal closure, is mediated by PLD (Jacob et al., 1999). Abscisic acid also causes the depolymerization of MTs in stomatal guard cells (Jiang et al., 1996), and MTs, in turn, are involved in guard cell opening (Zhou et al., 1999; Huang et al., 2000; Marcus et al., 2001). Although the precise role of MTs in guard cell opening is unclear (Assmann and Baskin, 1998), it has been suggested that this process may involve MT-associated proteins (Marcus et al., 2001). Future work should reveal if the tobacco p90 and its Arabidopsis homolog serve this role. Interestingly, abscisic acid induces the expression of another enzyme in phospholipid metabolism, phosphatidylinositol-4-phosphate 5-kinase (Mikami et al., 1998), and a phosphatidylinositol 4-kinase activity associates with the cytoskeleton in carrot protoplasts (Xu et al., 1992).
Interactions of the plasma membrane/MT-associated PLD and its products with other proteins also remain to be explored. Elongation factor-1α (EF-1α), which is involved in protein translation (Browning, 1996), binds MTs in the presence of weak lipophilic acids (Moore and Cyr, 2000) and undergoes Ca2+-mediated proteolysis in the presence of PLDα from castor bean (Ransom-Hodgkins et al., 2000). EF-1α can bind, bundle, stabilize, and promote the assembly of MTs in vitro (Moore and Cyr, 2000, and references cited therein), and it also binds and bundles actin (Liu et al., 1996). Thus, EF-1α has the potential to be an effector of signals to both the MT and actin cytoskeletons. PLD itself is activated by various components of signaling pathways, including G-proteins, calcium, phosphatidylinositol 4,5-bisphosphate, phospholipase C–activated protein kinase C, and tyrosine kinases (reviewed by Wang, 1999). The interaction of PLD with EF-1α is of particular interest because it links a major generator of second messengers with a protein that induces numerous cellular responses (Browning, 1996).
Colocalization of the PLD with cortical MTs also may be involved in targeting the second messengers it generates into specific domains of the plasma membrane (Kondo and Kakiuchi, 1994), thus modulating their function. The membrane-associated PLD is an obvious candidate as an anchor protein for MTs, although transmembrane proteins also are implicated (Akashi and Shibaoka, 1991; Williamson, 1991; Sonesson et al., 1997). The cell cycle–dependent association of the PLD with the plasma membrane may play a role in releasing MTs from the plasma membrane before mitosis. Interestingly, the yeast PLD homolog SPO14 is necessary for meiosis, possibly through its ability to generate membranes (Rudge et al., 1998). PLD activity may be similarly important in plant cell division.
In summary, the discovery of the unique membrane/MT-associated PLD opens an exciting area that connects signaling pathways with the cytoskeleton. Experiments are in progress to evaluate the effects of PLD inhibitors on the organization of MT arrays in plant cells.
METHODS
Plant Material
Tobacco BY-2 cells (Nicotiana tabacum Bright Yellow 2) were grown in suspension using Murashige and Skoog (1962) plant salt mixture (ICN Biochemicals, Costa Mesa, CA) supplemented with 2.72 mM KH2PO4, 1 mg/L thiamine, 0.2 mg/L 2,4-D, 100 mg/L inositol, and 3% (w/v) sucrose, pH 5.7. The cultures were maintained on a gyratory shaker at 130 rpm and 25°C (Nagata et al., 1981). Cells were subcultured by transferring 1.5 mL of 7-day-old suspension into 100 mL of fresh medium.
Solutions
Plasma membrane (PM) buffer: 50 mM Pipes, 1 mM MgSO4, and 1 mM EGTA, pH 6.8. Lysis buffer: 50 mM Pipes, 2 mM MgSO4, 2.5 mM EGTA, 300 mM sucrose, 5 mM dl-DTT, 5 μg/mL each of antipain, leupeptin, chymostatin, and pepstatin, and 25 μg/mL each of phenylmethylsulfonyl fluoride, Na-benzoyl-l-arginine methyl ester, and Na-p-tosyl-l-arginine methyl ester, pH 6.8. Microtubule (MT)-stabilizing buffer: 50 mM Pipes, 5 mM EGTA, and 1 mM MgSO4, pH 6.9. Chemicals were supplied by Sigma-Aldrich (Castle Hill, New South Wales, Australia) unless specified otherwise.
Isolation of Membrane Proteins
Membrane protein extracts were prepared as described (Marc et al., 1996). Briefly, tobacco BY-2 cells were washed and resuspended in lysis buffer and disrupted by explosive decompression in a French press (American Instruments, Silver Spring, MD). The lysate was separated by differential centrifugation into microsomal and cytosolic fractions. Proteins were extracted from the microsomal fraction by resuspending the pellet in PM buffer supplemented with 10 mM 3-([3- cholamidopropyl]dimethylamino)-1-propanesulfonate (Chaps), 2 mM DTT, and 2 and 10 μg/mL protease inhibitors as described above, and incubated on ice for 50 min. Detergent-insoluble material was sedimented by centrifugation at 48,000g for 1 to 2 hr. Bulk quantities of detergent-solubilized membrane proteins were frozen at −80°C.
Preparation of Monoclonal Antibodies
Tubulin binding proteins from the membrane Chaps extract, isolated by tubulin-affinity chromatography (Marc et al., 1996), were separated by SDS-PAGE and lightly stained with aqueous Coomassie Brilliant Blue R = 250. The 90-kD band was carved out, and the protein was electroeluted and dialyzed to remove the SDS. BALB/c mice were immunized, and their spleen cells were fused with SP2/0-Ag-14 myeloma cells (Harlow and Lane, 1989). Hybridomas were screened by immunofluorescence microscopy and by immunoblotting, and selected lines were cloned by limiting dilution before being grown to large volumes. The antibodies chosen included two designated 4B5 and 6G5. The culture supernatant with the 6G5 antibody was concentrated 10-fold by ultrafiltration and transfer into PBS (3 mM KH2PO4, 7 mM K2HPO4, and 150 mM NaCl, pH 7.2) containing 50% (w/v) glycerol.
Cosedimentation Assays
Bovine brain MTs were assembled by mixing taxol (National Cancer Institute, Bethesda, MD) and pure bovine brain tubulin at equimolar concentrations at 37°C in PM buffer containing 0.5 mM GTP. Alternatively, carrot MTs were prepared by adding taxol to purified carrot tubulin (Cyr and Palevitz, 1989) in PM buffer and incubating at room temperature. Assembled MTs were sedimented at 14,000g for 15 min and resuspended in PM buffer supplemented with 10 μM taxol.
Taxol was added to 200 μL of tobacco BY-2 Chaps extract (0.7 to 1.0 mg protein/mL; clarified by centrifugation at 14,000g for 15 min) to a final concentration of 10 μM before the addition of 40 μL of 3 mg/mL MT preparation. The mixture was incubated at room temperature for 10 to 15 min, and the MT-protein complexes were then sedimented at 14,000g for 10 min. Supernatants and pellets were analyzed by SDS-PAGE and immunoblotting with the antibody 6G5. For immunofluorescence microscopy, the pellet was resuspended in PM buffer, and the MTs were settled for 20 min onto slides that had been precoated with 0.1% (w/v) polyethyleneimine (PEI) and fixed with 4% (w/v) paraformaldehyde in PM buffer. After washing with PBS, the preparations were processed for double labeling with antibody 6G5 and polyclonal serum against soybean α/β-tubulin as for protoplast ghosts (see below).
To test the heat stability of the 90-kD protein, 200-μL volumes of the tobacco BY-2 Chaps extract were treated for 10 min at temperatures of 25, 45, 60, 70, 80, 90, and 100°C and then centrifuged at 14,000g for 15 min. Taxol was added to the clarified heat-treated Chaps extracts to 10 μM final concentration, followed by the addition of 40 μL of MT preparation, as in the cosedimentation assays described above. Pellets were analyzed by SDS-PAGE and immunoblotting with the antibody 6G5.
Preparation of Protein Extracts for Electrophoresis
Arabidopsis seedlings were grown between two sheets of moist filter paper in a Petri dish standing on its side. Whole 7-day-old seedlings were separated from the seed coat, washed in cold distilled water, and homogenized in a glass homogenizer with Laemmli (1970) sample buffer containing protease inhibitors as described above but without glycerol or bromphenol blue. The homogenate was spun at 14,000g for 30 min to remove particulate material. Protein concentration was estimated with Bradford protein assay (Bio-Rad) by using BSA as a standard. Protein samples for electrophoresis were completed by adding glycerol to 10% (w/v) and bromphenol blue. Dictyostelium discoideum cells (from Marcus Fechheimer, University of Georgia, Athens) were washed, disrupted in a glass homogenizer with Laemmli (1970) sample buffer containing protease inhibitors as described above, boiled for 2 min, and clarified in a centrifuge at 14,000g for 5 min.
Electrophoresis and Immunoblotting
Protein samples were separated by SDS-PAGE according to Laemmli (1970) by using 8 or 10% resolving gels. For immunoblotting, the separated proteins were transferred electrophoretically onto 0.45-μm nitrocellulose sheets (Bio-Rad) by the method of Towbin et al. (1979) and air dried. The sheets were then rehydrated for 15 min with PBS containing 0.05% (v/v) Tween 20 and blocked for 15 min with PBS containing 1% (w/v) BSA (fraction V; Sigma), 10% (v/v) lamb serum (Gibco BRL, Helena Laboratories, Glen Waverley, Victoria, Australia), and 0.05% (v/v) Tween 20. Blocked sheets were incubated for 1 hr with the 6G5 monoclonal antibody diluted 1:50 or 1:100 in dilution buffer (PBS containing 1% [w/v] BSA, 1% [v/v] lamb serum, and 0.05% [v/v] Tween 20). Alkaline phosphatase–conjugated sheep anti-mouse IgG (diluted 1:1000 in dilution buffer) was used as a reporter antibody. Blots were developed with 20 mM nitroblue tetrazolium and 75 mM 5-bromo-4-chloro-3-indolyl phosphate in carbonate buffer (100 mM NaHCO3, 50 mM Na2CO3, and 4 mM MgCl2, pH 9.5).
For Figure 1, proteins from the tobacco BY-2 Chaps extract were separated on a curtain gel and transferred to nitrocellulose as described above. Strips of the nitrocellulose were incubated with antibodies (all in dilution buffer) against the following: α-tubulin (1:100; Amersham), γ-tubulin (1:1000; Dibbayawan et al., 1995), soybean α/β-tubulin (1:400), and antibody 6G5 (1:100). For control, the antibody 6G5 was heated at 80°C for 10 min before use.
Protein samples for microsequencing by Edman degradation were obtained from tubulin binding proteins from the membrane Chaps extract isolated by tubulin-affinity chromatography (Marc et al., 1996). The proteins were separated by SDS-PAGE and transferred onto a sequencing-grade polyvinylidene difluoride membrane (Millipore, Bedford, MA) using 3-[cyclohexylamino]-1-propanesulfonic acid transfer buffer. Proteins on the membrane were stained with Coomassie Brilliant Blue R = 250 and the 90-kD band was carved out for analysis in an Applied Biosystems (Hershey, PA) 473A Protein Sequencer.
Preparation of Protoplasts, Membrane Ghosts, and Whole Cells
Protoplasts were prepared from 5- or 6-day-old tobacco BY-2 cells by digesting the cell walls with 1% (w/v) cellulase Y-C and 0.1% (w/v) pectolyase Y-23 (Seishin Corporation, Tokyo, Japan) in 450 mM mannitol supplemented with 0.5% (w/v) BSA, pH 5.4. Some protoplasts were made from cells that were pretreated for 30 min with 10 μM taxol added to the culture medium before the digestion step. After enzyme digestion, protoplasts were washed twice in a wash buffer (10 mM Pipes, 285 mM mannitol, and 100 mM KCl, pH 6.8) and settled onto PEI-coated 12-well slides (ICN Biochemicals, Aurora, OH) for 15 min. The protoplasts were then lysed by flicking the slide to produce protoplast ghosts and fixed with 4% (w/v) paraformaldehyde in MT-stabilizing buffer for 30 min. Fixed ghosts were washed with PBS, air dried for 15 min, and rehydrated with PBS.
Whole tobacco BY-2 cells (5 or 6 days old) were settled onto PEI-coated slides for 30 min and fixed for 30 min with 4% (w/v) paraformaldehyde in MT-stabilizing buffer supplemented with 1% (v/v) DMSO and 0.05% (v/v) Triton X-100. The preparations were then washed with PBS, digested for 2 min with the enzyme mixture as for the protoplasts, washed again with PBS, air dried, and rehydrated with PBS.
Immunofluorescence Microscopy
Fixed protoplast ghosts and whole cells, attached to multiwell slides, were permeabilized with methanol at −20°C for 5 min, rehydrated with PBS, and incubated with antibodies diluted in PBS containing 1% (w/v) BSA and 0.02% (w/v) Triton X-100 for 1 hr at room temperature. For double labeling, we used monoclonal antibody 6G5 (diluted 1:50) or monoclonal antibody 4B5 (culture supernatant, neat) together with polyclonal serum against soybean α/β-tubulin (diluted 1:50 for protoplast ghosts and 1:200 for whole cells). The material was washed with PBS and incubated for 1 hr in Cy3-conjugated anti-mouse IgG (Sigma) together with fluorescein isothiocyanate (FITC)–conjugated sheep anti-rabbit IgG (Silenus, Amrad Operations, Boronia, Victoria, Australia) secondary antibodies (diluted 1:500 and 1:30, respectively). Controls without one or both of the primary antibodies were negative. After washing with PBS, the preparations were stained with 1 μg/mL 4′,6-diamidine-2-phenylindole dihydrochloride in PBS and mounted in Citifluor AF2 medium (Alltech Associates, Baulkham Hills, New South Wales, Australia). Controls with single fluorochromes showed no significant spectral overlap (bleed-through) between FITC and Cy3 by using our equipment.
Material was viewed using a Zeiss Axiophot microscope equipped with standard epifluorescence optics (Carl Zeiss, Oberkochen, Germany) and photographed using Kodak T-Max film at 400/27 ISO. Alternatively, material was viewed with an Olympus Provis AX70 microscope (Olympus Optical Co. Ltd., Tokyo, Japan) equipped with epifluorescence optics and an acquisition/deconvolution system (Scanalytics, Fairfax, VA). Images were captured using a cooled charge-coupled device camera (Photometrics Sensys, Tucson, AZ) and printed with an Epson 800 printer (Epson Pty. Ltd., Sydney, New South Wales, Australia) or dye-sublimation printer NP-1600M (Codonics, Middleburg Heights, OH).
Screening of the Arabidopsis cDNA Library
Escherichia coli XL-1 Blue MRF′ cells (Stratagene) were added to 3000 to 4000 plaque-forming units of an Arabidopsis λZap II cDNA library (CD4-16; Arabidopsis Biological Resource Center, Columbus, OH), mixed with top agar and spread evenly across each 150-mm plate with Luria-Bertani agar medium, and incubated at 42°C for 4 hr (Sambrook et al., 1989). Hybond-C nitrocellulose filters (Amersham), presoaked in 10 mM isopropylthio-β-galactoside, were placed onto bacteriophage colonies on each plate and incubated at 37°C for 4 hr. The filters were then washed with 20 mM Tris-HCl, pH 7.5, containing 150 mM NaCl (Tris-buffered saline [TBS]) and 0.05% (v/v) Tween 20 (TBST), incubated for 1 hr with either antibody 6G5 diluted 1:100 or monoclonal anti-α-tubulin (B512, Sigma) diluted 1:2000, washed with TBST, and incubated with alkaline phosphatase–conjugated monoclonal sheep anti-mouse antibody (Silenus) diluted 1:1000. After washing with TBST and then TBS, the filters were developed with 20 μM nitroblue tetrazolium and 75 μM 5-bromo-4-chloro-3-indolyl phosphate in substrate buffer (100 mM Tris-HCl, pH 9.5, containing 100 mM NaCl and 5 mM MgCl2). Positive bacteriophage plaques were excised and titrated before secondary and tertiary screens at higher antibody dilutions, and a colony was selected from each plate to make glycerol stocks (Sambrook et al., 1989).
In vivo excision of inserts was performed according to the ExAssist Interference-Resistant Helper Phage instruction manual with SOLR Strain (Stratagene). Briefly, stocks of chosen bacteriophage plaques were mixed with XL1-Blue MRF′ cells and ExAssist helper phage (Stratagene), grown in Luria-Bertani broth, and briefly heated at 65 to 70°C, and the debris was centrifuged. The supernatant was incubated with the SOLR cells, and phagemid-containing cells were grown overnight on Luria-Bertani plates under ampicillin (50 μg/mL) selection. An expressed sequence tag clone containing the full phospholipase D (PLD) cDNA (GenBank accession number W43825; Newman et al., 1994) was obtained from the Arabidopsis Biological Resource Center.
Plasmid Isolation, Sequencing, and Sequence Analysis
Standard plasmid isolation (Sambrook et al., 1989) was slightly modified as follows: 40 μg of RNase was added before the extraction step, which was performed successively with phenol, phenol/chloroform, and chloroform/isoamyl alcohol (24:1). The pelleted plasmids were then dissolved in 20 μL of water. T3 and T7 primers were used initially for sequencing, followed by other primers designed to enable sequencing of entire clones. Dye-terminator chemistry sequencing using an ABI 373A Sequencer (Applied Biosystems, La Jolla, CA) was performed at SUPAMAC (Sydney University, New South Wales, Australia). Consensus sequence was obtained from these sequencing runs using SEQUENCHER software (Gene Codes Corporation, Ann Arbor, MI). Sequence similarities were analyzed using the BLAST search engine (Altschul et al., 1990) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST) and multiple alignments using CLUSTAL W (Thompson et al., 1994) at Pole Bioinformatique Lyonnais (http://pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalwan.html).
Immunoprecipitation
Protein extracts were prepared from 3- to 5-day-old tobacco BY-2 cells. The cells were lysed using a French press as described previously (Marc et al., 1996), and the lysate was centrifuged for 10 min at 4000g and 4°C. The supernatant was then incubated with 10 mM Chaps in lysis buffer on ice for 30 to 50 min to extract membrane proteins and clarified at 200,000g at 4°C for 20 min. Immunoprecipitation was performed according to Harlow and Lane (1989) with modifications as follows. Protein samples (1 mg/mL) were mixed with immunoprecipitation buffer (20 mM Tris-HCl, 1 mM EGTA, 1 mM MgCl2, 10 mM Chaps, 1 mM DTT, and protease inhibitor mix [Boehringer Mannheim, Roche Molecular Biochemicals, Indianapolis, IN], pH 7.5) to give a reaction volume of 100 μL. Primary antibodies were added, and the mixture was incubated on ice for 1 hr, followed by the addition of a 10-μL suspension of secondary antibody–agarose conjugates and further incubation for 30 to 60 min. For primary antibodies, we used antibody 6G5 (IgM; 10 μL of hybridoma supernatant), mouse monoclonal anti-α-tubulin clone DM1A (IgG1; Sigma; 1 μL of ascites), mouse monoclonal anti-actin clone 3H11 (IgG; 10 μL of hybridoma supernatant), and mouse monoclonal anti-calmodulin clone 1D10 (IgG; 10 μL of hybridoma supernatant). For secondary antibodies, we used goat immunoglobulin against mouse IgM conjugated to agarose (Sigma) or goat immunoglobulin against mouse IgG conjugated to agarose (Sigma).
After incubation with antibodies, the reaction mixture was underlaid with an ∼100-μL cushion of 50% sucrose in immunoprecipitation buffer, and the agarose-immune complex was sedimented in a microcentrifuge at 400g for 1 min. The supernatant was aspirated, the sucrose cushion was carefully removed, and the pelleted agarose-immune complexes were resuspended in 13 μL of immunoprecipitation buffer.
PLD Assays
PLD assays were performed using immunoprecipitated samples or protein fractions from tubulin-affinity chromatography (Marc et al., 1996). Protein sample (2 to 20 μL) was added to a total reaction volume of 80 μL containing 50 mM Mes, pH 6.5, 5 mM MgCl2, 100 μM CaCl2, 1.5% (v/v) ethanol, and a phospholipid mixture made up at two times strength giving final concentrations of 140 μM 7-nitro-benz-2-oxa-1,3-diazole-conjugated phosphatidylcholine, 200 μM phosphatidylethanolamine (Avanti Polar Lipids, Alabaster, AL), and 30 μM phosphatidylinositol bisphosphate (Sigma). Reactions were performed for 20 min at room temperature with shaking at 100 rpm. Assay reactions were stopped by the addition of 300 μL of chloroform:methanol (1:2), after which 80 μL of chloroform and 80 μL of 2 M KCl were added. Lipids were extracted and separated by thin-layer chromatography, and phosphatidylethanol fluorescence was quantified as described previously (Ritchie and Gilroy, 1998).
Acknowledgments
We thank Janet Elliott (Research School of Biological Sciences, Australian National University, Canberra) for her expert help in producing the monoclonal antibodies, Nicola Young (University of Sydney) for preparing the cosedimentation assays and immunoblots, Dr. Seiichiro Hasezawa (University of Tokyo) for kindly providing the tobacco BY-2 cell culture, Dr. Marcus Fechheimer (University of Georgia, Athens) for providing a sample of Dictyostelium, and Dr. Kent Chapman (University of North Texas, Denton) for sharing with us the unpublished tobacco PLD sequence. Taxol was a generous gift from the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute (Bethesda, MD). This work was supported by competitive research Grants A19230505 and A19700148 from the Australian Research Council to J.M. and an Australian Postgraduate Award to J.C.G.
References
- Akashi, T., and Shibaoka, H. (1991). Involvement of transmembrane proteins in the association of cortical microtubules with the plasma membrane in tobacco BY-2 cells. J. Cell Sci. 98 169–174. [Google Scholar]
- Akashi, T., Kawasaki, S., and Shibaoka, H. (1990). Stabilization of cortical microtubules by the cell wall in cultured tobacco cells: Effects of extensin on the cold-stability of cortical microtubules. Planta 182 363–369. [DOI] [PubMed] [Google Scholar]
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. [DOI] [PubMed] [Google Scholar]
- Asada, T., and Collings, D. (1997). Molecular motors in higher plants. Trends Plant Sci. 2 29–37. [Google Scholar]
- Assmann, S.M., and Baskin, T.I. (1998). The function of guard cells does not require an intact array of cortical microtubules. J. Exp. Bot. 49 163–170. [Google Scholar]
- Barroso, C., Chan, J., Allan, V., Doonan, J., Hussey, P., and Lloyd, S. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: Characterization of a novel kinesin, DcKRP120-2. Plant J. 24 859–868. [DOI] [PubMed] [Google Scholar]
- Blackman, L.M., and Overall, R.L. (1995). Electric fields affect the orientation of cortical microtubules and cell expansion in pea callus. Protoplasma 189 256–266. [Google Scholar]
- Browning, K.S. (1996). The plant translational apparatus. Plant Mol. Biol. 32 107–144. [DOI] [PubMed] [Google Scholar]
- Burk, D.H., Liu, B., Zhong, R., Morrison, W.H., and Ye, Z.-H. (2001). A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13 807–827. [PMC free article] [PubMed] [Google Scholar]
- Cai, G., Romagnoli, S., Moscatelli, A., Ovidi, E., Bambellini, G., Tiezzi, A., and Cresti, M. (2000). Identification and characterization of a novel microtubule-based motor associated with membranous organelles in tobacco pollen tubes. Plant Cell 12 1719–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J., Jensen, C.G., Jensen, L.C.W., Bush, M., and Lloyd, C.W. (1999). The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc. Natl. Acad. Sci. USA 96 14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, K.D. (1998). Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 3 419–426. [Google Scholar]
- Colley, W.C., Sung, T.-C., Roll, R., Jenco, J., Hamond, S.M., Altshuller, Y., Bar-Sagi, D., Morris, A.J., and Frohman, M.A. (1997). Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7 191–201. [DOI] [PubMed] [Google Scholar]
- Cross, M.J., Roberts, S., Ridley, A.J., Hodgkin, M.N., Stewart, A., Claesson-Welsh, L., and Wakelam, M.J.O. (1996). Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr. Biol. 6 588–597. [DOI] [PubMed] [Google Scholar]
- Cyr, R.J. (1994). Microtubules in plant morphogenesis: Role of the cortical array. Annu. Rev. Cell Biol. 10 153–180. [DOI] [PubMed] [Google Scholar]
- Cyr, R.J., and Palevitz, B.A. (1989). Microtubule-binding proteins from carrot. I. Initial characterization and microtubule bundling. Planta 177 254–260. [DOI] [PubMed] [Google Scholar]
- Cyr, R.J., and Palevitz, B.A. (1995). Organization of cortical microtubules in plant cells. Curr. Opin. Cell Biol. 7 65–71. [DOI] [PubMed] [Google Scholar]
- Dibbayawan, T.P., Harper, J.D.I., Elliott, J.E., Gunning, B.E.S., and Marc, J. (1995). A γ-tubulin that associates specifically with centrioles in HeLa cells and the basal body complex in Chlamydomonas. Cell Biol. Int. 19 559–567. [DOI] [PubMed] [Google Scholar]
- Dubois, F., Ha, D.B.D., Sangwan, R.S., and Durand, J. (1996). The petunia tra1 gene controls cell elongation and plant development, and mediates responses to cytokinins. Plant J. 10 47–59. [Google Scholar]
- Durso, N.A., and Cyr, R.J. (1994). A calmodulin-sensitive interaction between microtubules and a higher plant homolog of elongation factor-1α. Plant Cell 6 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durso, N.A., Leslie, J.D., and Cyr, R.J. (1996). In situ immunocytochemical evidence that a homolog of protein translation elongation factor EF-1α is associated with microtubules in carrot cells. Protoplasma 190 141–150. [Google Scholar]
- Exton, J.H. (1994). Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1212 26–42. [DOI] [PubMed] [Google Scholar]
- Fisher, D.D., and Cyr, R.J. (1998). Extending the microtubule/microfibril paradigm. Plant Physiol. 116 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket, D.E., and Morejohn, L.C. (1992). Structural and functional organization of tubulin. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43 49–92. [Google Scholar]
- Galway, M.E., and Hardham, A.R. (1986). Microtubule reorganization, cell wall synthesis and establishment of the axis of elongation in regenerating protoplasts of the alga Mougeotia. Protoplasma 135 130–143. [Google Scholar]
- Gertel, E.T., and Green, P.B. (1977). Cell growth pattern and wall microfibrillar arrangement. Plant Physiol. 60 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiso, J.A.A., Farias, E.F., Alonso, D.F., Arregui, C., and Joffe, E.B.D. (1997). A phospholipase D and protein kinase C inhibitor blocks the spreading of murine mammary adenocarcinoma cells altering F-actin and β1-integrin point contact distribution. Int. J. Cancer 71 881–890. [DOI] [PubMed] [Google Scholar]
- Giddings, T.H., Jr., and Staehelin, L.A. (1991). Microtubule-mediated control of microfibril deposition: A re-examination of the hypothesis. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (London: Academic Press), pp. 85–99.
- Granger, C.L., and Cyr, R.J. (2001). Spatiotemporal relationships between growth and microtubule orientation as revealed in living root cells of Arabidopsis thaliana transformed with green-fluorescent-protein gene construct (GFP-MBD). Protoplasma 216 201–214. [DOI] [PubMed] [Google Scholar]
- Gunning, B.E.S., and Hardham, A.R. (1982). Microtubules. Annu. Rev. Plant Physiol. 33 651–698. [Google Scholar]
- Ha, K.-S., and Exton, J.H. (1993). Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J. Cell Biol. 123 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1989). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Huang, R.F., Wang, X.C., and Lou, C.H. (2000). Cytoskeletal inhibitors suppress the stomatal opening of Vicia faba L. induced by fusicoccin and IAA. Plant Sci. 156 65–71. [DOI] [PubMed] [Google Scholar]
- Hugdahl, J.D., Bokros, C.L., and Morejohn, L.C. (1995). End-to-end annealing of plant microtubules by the p86 subunit of eukaryotic initiation factor-(iso)4F. Plant Cell 7 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hush, J.M., and Overall, R.L. (1991). Electrical and mechanical fields orient cortical microtubules in higher plant tissues. Cell Biol. Int. Rep. 15 551–560. [Google Scholar]
- Hush, J.M., Hawes, C.R., and Overall, R.L. (1990). Interphase microtubule re-orientation predicts a new cell polarity in wounded pea roots. J. Cell Sci. 96 47–61. [Google Scholar]
- Jacob, T., Ritchie, S., Assmann, S.M., and Gilroy, S. (1999). Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc. Natl. Acad. Sci. USA 96 12192–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, C.-J., Nakajima, N., and Kondo, N. (1996). Disruption of microtubules by abscisic acid in guard cells of Vicia faba L. Plant Cell Physiol. 37 697–701. [Google Scholar]
- Jung, H.I., Shin, I., Park, Y.M., Kang, K.W., and Ha, K.S. (1997). Colchicine activates actin polymerization by microtubule depolymerization. Mol. Cells 7 431–437. [PubMed] [Google Scholar]
- Kiyosue, T., and Ryan, C.A. (1997). A novel gene of tomato preferentially expressed in fruit encodes a protein with a Ca2+-dependent lipid-binding domain. Plant Mol. Biol. 35 969–972. [DOI] [PubMed] [Google Scholar]
- Kobayashi, I., Kobayashi, Y., and Hardham, A.R. (1994). Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195 237–247. [Google Scholar]
- Kondo, T., and Kakiuchi, T. (1994). Fluorescence microscopic imaging of hydrolysis of phospholipid monolayers by phospholipase D at the air–water interface. Thin Solid Films 244 887–889. [Google Scholar]
- Kost, B., Mathur, J., and Chua, N.-H. (1999). Cytoskeleton in plant development. Curr. Opin. Plant Biol. 2 462–470. [DOI] [PubMed] [Google Scholar]
- Krishnakumar, S., and Oppenheimer, D.G. (1999). Extragenic suppressors of the Arabidopsis zwi-3 mutation identify new genes that function in trichome branch formation and pollen tube growth. Development 126 3079–3088. [DOI] [PubMed] [Google Scholar]
- Kuss-Wymer, C.L., and Cyr, R.J. (1992). Tobacco protoplasts differentiate into elongate cells without net microtubule depolymerization. Protoplasma 168 64–72. [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Laskowski, M.J. (1990). Microtubule orientation in pea stem cells: A change in orientation follows the initiation of growth rate decline. Planta 181 44–52. [DOI] [PubMed] [Google Scholar]
- Lee, S., Suh, S., Kim, S., Crain, R.C., Kwak, J.M., Nam, H.-G., and Lee, Y. (1997). Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 12 547–556. [Google Scholar]
- Liu, B., Joshi, H.C., Wilson, T.J., Silflow, C.D., Palevitz, B.A., and Snustad, D.P. (1994). γ-Tubulin in Arabidopsis: Gene sequence, immunoblot, and immunofluorescent studies. Plant Cell 6 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G., Tang, J., Edmonds, B.T., Murray, J., Levin, S., and Condeelis, J. (1996). F-actin sequesters elongation factor 1-α from interaction with aminoacyl-tRNA in a pH-dependent reaction. J. Cell Biol. 135 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, C.W., ed (1991). The Cytoskeletal Basis of Plant Growth and Form. (London: Academic Press).
- Lloyd, C.W., Slabas, A.R., Powell, A.J., and Lowe, S.B. (1980). Microtubules, protoplasts and plant cell shape. Planta 147 500–506. [DOI] [PubMed] [Google Scholar]
- Lukowski, S., Lecomte, M.-C., Mira, J.-P., Marin, P., Gautero, H., Russo-Marie, F., and Geny, B. (1996). Inhibition of phospholipase D activity by fodrin. J. Biol. Chem. 271 24164–24171. [DOI] [PubMed] [Google Scholar]
- Marc, J., Sharkey, D.E., Durso, N.A., Zhang, M., and Cyr, R.J. (1996). Isolation of a 90-kD microtubule-associated protein from tobacco membranes. Plant Cell 8 2127–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, A.I., Moore, R.C., and Cyr, R.J. (2001). The role of microtubules in guard cell function. Plant Physiol. 125 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melan, M.A., and Sluder, G. (1992). Redistribution and differential extraction of soluble proteins in permeabilized cultured cells: Implications for immunofluorescence microscopy. J. Cell Sci. 101 731–743. [DOI] [PubMed] [Google Scholar]
- Mikami, K., Katagiri, T., Iuchi, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1998). A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant J. 15 563–568. [DOI] [PubMed] [Google Scholar]
- Moore, R.C., and Cyr, R.J. (2000). Association between elongation factor-1α and microtubules in vivo is domain dependent and conditional. Cell Motil. Cytoskeleton 45 279–292. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Nagata, T., Okada, K., Takebe, I., and Matsui, C. (1981). Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol. Gen. Genet. 184 161–165. [Google Scholar]
- Newman, T., deBruijn, F.J., Green, P., Keegstra, K., Kende, H., McIntosh, L., Ohlrogge, J., Raikhel, N., Somerville, S., Thomashow, M., Retzel, E., and Somerville, C. (1994). Genes galore: A summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 106 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick, P. (1999). Signals, motors, morphogenesis: The cytoskeleton in plant development. Plant Biol. 1 169–179. [Google Scholar]
- Nick, P., Bergfeld, R., Schafer, E., and Schopfer, P. (1990). Unilateral reorientation of microtubules at the outer epidermal wall during photo- and gravitropic curvature of maize coleoptiles and sunflower hypocotyls. Planta 181 162–168. [DOI] [PubMed] [Google Scholar]
- Nick, P., Heuing, A., and Ehmann, B. (2000). Plant chaperonins: A role in microtubule-dependent wall formation. Protoplasma 211 234–244. [Google Scholar]
- Paliyath, G., and Droillard, M.J. (1992). The mechanisms of membrane deterioration and disassembly during senescence. Plant Biochem. Physiol. 30 789–812. [Google Scholar]
- Pappan, K., Zheng, S., and Wang, X. (1997). Identification and characterisation of a novel phospholipase D that requires polyphosphoinositide and submicromolar calcium for activity in Arabidopsis. J. Biol. Chem. 272 7048–7054. [DOI] [PubMed] [Google Scholar]
- Petrasek, J., Freudenreich, A., Heuing, A., Opatrny, Z., and Nick, P. (1998). Heat-shock protein 90 is associated with microtubules in tobacco cells. Protoplasma 202 161–174. [Google Scholar]
- Ponting, C.P., and Kerr, I.D. (1996). A novel family of phospholipase D homologues that include phospholipid synthases and putative endonucleases: Identification of duplicated repeats and potential active site residues. Protein Sci. 5 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C.P., and Parker, P.J. (1996). Extending the C2 domain family: C2s in PKCs δ, ɛ, η, θ, phospholipases, GAPs and perforin. Protein Sci. 5 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom-Hodgkins, W.D., Brglez, I., Wang, X., and Boss, W.F. (2000). Calcium-regulated proteolysis of eEF1A. Plant Physiol. 122 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S., and Gilroy, S. (1998). Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc. Natl. Acad. Sci. USA 95 2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge, S.A., Morris, A.J., and Engebrecht, J. (1998). Relocalization of phospholipase D activity mediates membrane formation during meiosis. J. Cell Biol. 140 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, S.B., and Wang, X. (1996). Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim. Biophys. Acta 1303 243–250. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Shibaoka, H. (1994). Plant hormone–induced changes in the orientation of cortical microtubules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45 527–544. [Google Scholar]
- Simmonds, D.H. (1992). Plant cell wall removal: Cause for microtubule instability and division abnormalities in protoplast cultures? Physiol. Plant. 85 387–390. [Google Scholar]
- Smertenko, A., Saleh, N., Igarashi, H., Mori, H., Hauser-Hahn, I., Jiang, C.-J., Sonobe, S., Lloyd, C.W., and Hussey, P.J. (2000). A new class of microtubule associated proteins in plants. Nat. Cell Biol. 2 750–753. [DOI] [PubMed] [Google Scholar]
- Smith, H.M.S., and Raikhel, N.V. (1998). Nuclear localization signal receptor importin α associates with the cytoskeleton. Plant Cell 10 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L.G., Gerttula, S.M., Han, S., and Levy, J. (2001). Tangled1: A microtubule binding protein required for the spatial control of cytokinesis in maize. J. Cell Biol. 152 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson, A., Berglund, M., Staxen, I., and Widell, S. (1997). The characterization of plasma membrane-bound tubulin of cauliflower using Triton X-114 fractionation. Plant Physiol. 115 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe, S., and Takahashi, S. (1994). Association of microtubules with the plasma membrane of tobacco BY-2 cells in vitro. Plant Cell Physiol. 35 451–460. [Google Scholar]
- Steed, P.M., Nagar, S., and Wennogle, L. (1996). Phospholipase D regulation by a physical interaction with the actin-binding protein gelsolin. Biochemistry 35 5229–5237. [DOI] [PubMed] [Google Scholar]
- Stoppin-Mellet, V., Peter, C., and Lambert, A.M. (2000). Distribution of gamma-tubulin in higher plant cells: Cytosolic gamma-tubulin is part of high molecular weight complexes. Plant Biol. 2 290–296. [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz, R.A., and Jurgens, G. (1994). Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 120 2967–2978. [DOI] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedures and some applications. Proc. Natl. Acad. Sci. USA 76 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas, J., Bellini, C., Nacry, P., Kronenberger, J., Bouchez, D., and Caboche, M. (1995). Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature 375 676–677. [Google Scholar]
- Vesk, P.A., Vesk, M., and Gunning, B.E.S. (1996). Field emission scanning electron microscopy of microtubule arrays in higher plant cells. Protoplasma 195 168–182. [Google Scholar]
- Wang, X. (1997). Molecular analysis of phospholipase D. Trends Plant Sci. 2 261–266. [Google Scholar]
- Wang, X. (1999). The role of phospholipase D in signaling cascades. Plant Physiol. 120 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. (2000). Multiple forms of phospholipase D in plants: The gene family, catalytic and regulatory properties, and cellular functions. Prog. Lipid Res. 39 109–149. [DOI] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Jun Wei, K., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411 610–613. [DOI] [PubMed] [Google Scholar]
- Williamson, R.E. (1991). Orientation of cortical microtubules in interphase plant cells. Int. Rev. Cytol. 129 135–206. [Google Scholar]
- Wymer, C.L., and Lloyd, C.W. (1996). Dynamic microtubules: Implications for cell wall patterns. Trends Plant Sci. 1 222–228. [Google Scholar]
- Wymer, C.L., Wymer, S.A., Cosgrove, D.J., and Cyr, R.J. (1996). Plant cell growth responds to external forces and the response requires intact microtubules. Plant Physiol. 110 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, P., Lloyd, C.W., Staiger, C.J., and Drobak, B.K. (1992). Association of phosphatidylinositol 4-kinase with the plant cytoskeleton. Plant Cell 4 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S.A., Wang, X., and Leach, J.E. (1996). Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell 8 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni, K., and Schopfer, P. (1994). Mechanosensory microtubule reorientation in the epidermis of maize coleoptiles subjected to bending stress. Protoplasma 182 96–101. [DOI] [PubMed] [Google Scholar]
- Zhou, X.M., Wu, W.H., Yuan, M., and Wang, X.C. (1999). Regulation of the inward K+ channels in stomatal guard cells by cytoskeletal microtubules. Chin. Sci. Bull. 44 919–923. [Google Scholar]