Abstract
We have undertaken a systematic reverse genetic approach to understand R2R3-MYB gene function in Arabidopsis. Here, we report the functional characterization of MYB61 based on the phenotype of three independent insertion alleles. Wide-ranging phenotype screens indicated that MYB61 mutants were deficient in seed mucilage extrusion upon imbibition. This phenotype was expressed in the sporophytic tissues of the seed. Deposition and extrusion of the principal component of the mucilage, a relatively unbranched rhamnogalacturonan, were reduced in the MYB61 mutant seed coats. Additional defects in the maturation of the testa epidermal cells suggested a potential deficiency in extracellular secretion in myb61 lines. Consistent with a proposed role in testa development, reverse transcription–polymerase chain reaction analysis showed the highest MYB61 expression in siliques, which was localized to the seed coat by a β-glucuronidase (GUS) reporter gene fusion. Lower levels of GUS expression were detected in developing vascular tissue. Parallel analysis of the ttg1-1 mutant phenotype indicated that this mutant showed more severe developmental defects than myb61 and suggested that MYB61 may function in a genetic pathway distinct from that of TTG1. The transient nature of seed epidermal characteristics in the ttg1-1 mutant suggested that TTG1 was required for maintenance rather than initiation of testa epidermal differentiation. Germination and seedling establishment were compromised in the myb61 and ttg1-1 mutants under conditions of reduced water potential, suggesting a function for Arabidopsis seed mucilage during germination in dry conditions.
INTRODUCTION
In angiosperms, the mature embryo is surrounded by an outer seed coat that participates in many important processes during seed development and germination. These include the transport of nutrients from the funiculus to the developing embryo, the protection of the embryo, and the control of the length of the dormancy period (Debeaujon et al., 2000a). The seed coat has economic significance as the source of cotton fibers, and during processing of some seed-based products such as coffee and cocoa, the seed coat must be removed. Angiosperm seed coat morphology is extremely diverse, reflecting multiple adaptations to seed dispersal and germination in different environments (Fahn, 1990).
In Arabidopsis, the seed coat differentiates from two ovule integuments (Léon-Kloosterziel et al., 1994). The inner integument forms the tegmen, the site of synthesis of tannins, the characteristic brown pigment of Arabidopsis seeds. The outer integument develops into the testa, the outer epidermis of which differentiates in a complex process into mucilage-containing cells with thickened radial cell walls and central elevations known as columellae (Koornneef, 1981; Western et al., 2000; Windsor et al., 2000). These are reinforced by the deposition of a secondary cell wall. In this respect the development of the seed coat epidermis resembles that of tracheary elements.
The most abundant monosaccharide constituents of Arabidopsis seed mucilage are rhamnose and galacturonic acid, suggesting that the principal polysaccharide is rhamnogalacturonan I (RG I; Goto, 1985; Western et al., 2000). In mature seeds, mucilage is present in a dehydrated form within each epidermal cell; upon contact with water, the mucilage expands, rupturing the primary cell wall and extruding from the seed coat. In this hydrated state, the mucilage envelops the whole seed and forms a pectin hydrogel (Zwieniecki et al., 2001). Seed mucilage has been hypothesized to play a role in germination as an oxygen barrier and in seed dispersal (Gutterman and Shemtov, 1996). However, the function of seed mucilage in Arabidopsis remains unclear.
To date, three pleiotropic loci have been implicated in the development of the testa epidermis in Arabidopsis. Plants lacking a functional APETALA2 (AP2) gene are deficient in the development of columellae and the biosynthesis of mucilage, and strong AP2 mutant alleles such as ap2-1 lead to weakened and misshapen seed coats. This suggests that AP2 regulates multiple aspects of seed coat development (Jofuku et al., 1994), including early steps of seed coat specification. In addition to their well-characterized roles in trichome and root atrichoblast specification, the TRANPARENT TESTA GLABRA1 (TTG1) and GLABRA2 (GL2) genes are essential for columella development in the testa epidermis and for mucilage extrusion during imbibition (Koornneef, 1981; Koornneef et al., 1982). TTG1 encodes a WD40 repeat protein, and GL2 encodes a homeodomain transcription factor (Rerie et al., 1994; Walker et al., 1999). These interact genetically with the tissue-specific MYB transcription factors GLABRA1 (GL1) and WEREWOLF (WER) in the initiation of trichome and root atrichoblast development (Oppenheimer et al., 1991; Lee and Schiefelbein, 1999). The ttg1-1 phenotype can be complemented by overexpression of the maize basic helix-loop-helix (bHLH) transcription factor R (Lloyd et al., 1992), suggesting that TTG1 functions to regulate the activity of Arabidopsis bHLH proteins. One of these has recently been found to be encoded by the GL3 locus (Payne et al., 2000). Although for some time it has been known that TTG1 plays a role in the development of the seed coat epidermis, the effects of the ttg1-1 allele on seed coat development have not been described in detail.
MYB-related proteins comprise a large transcription factor family in higher plants of >100 members (Martin and Paz-Ares, 1997; Arabidopsis Genome Initiative, 2000; Riechmann and Ratcliffe, 2000). They are characterized by the number of conserved helix-loop-helix repeats in the MYB domain, and the largest group in plants containing two repeats is known as R2R3-MYB genes. R2R3-MYB genes are specific to plants and appear to regulate a wide variety of cellular functions in Arabidopsis. ASYMMETRIC LEAVES1 downregulates meristem identity genes in lateral organ primordia (Byrne et al., 2000), and the GL1 and WER genes regulate the initiation of trichome and root atrichoblast, respectively (Oppenheimer et al., 1991; Lee and Schiefelbein, 1999). In addition to their function in the control of organ and cell morphology, MYB-related transcription factors also regulate metabolism. The ATR1 gene controls tryptophan biosynthesis (Bender and Fink, 1998), whereas several MYB genes have been shown to regu-late phenylpropanoid metabolism (Grotewold et al., 1994; Moyano et al., 1996; Jin et al., 2000) and anthocyanin biosynthesis (Paz-Ares et al., 1987; Cone et al., 1993; Borevitz et al., 2000).
We have undertaken a systematic approach to understanding of MYB gene function in Arabidopsis. More than 70 members of the R2R3-MYB gene family were characterized and their expression examined in the major tissues and under a variety of conditions (Kranz et al., 1998). Transposon and T-DNA knockouts were obtained for many genes, and these were subsequently screened for phenotypes (Meissner et al., 1999). Here, we report the functional characterization of AtMYB61, based on the phenotype of transposon insertion mutants, and propose a role for this gene during the development of the testa epidermis. Using mutants deficient in the accumulation of seed coat polysaccharides, we also demonstrate a function for mucilage during seed germination in reduced water potential conditions. The large quantity of mucilage deposited during the development of the testa provides a significant opportunity to study polysaccharide biosynthesis and secretion in plants and the role of transcription factors in these processes.
RESULTS
Isolation of MYB61 Insertion Mutants
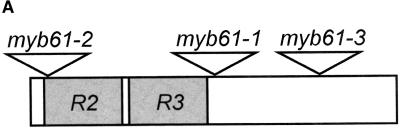
The isolation of two transposon alleles of MYB61 (At1 g09450) by use of polymerase chain reaction (PCR)–based screens from the Wisman collection has been described previously (Meissner et al., 1999). In addition, a stable allele was isolated from a hybridization screen of the Sainsbury Laboratory Arabidopsis thaliana (SLAT) collection using the Inverse Display of Insertion (IDI) filters (Tissier et al., 1999). This d/Spm insertion is located downstream of the conserved MYB helix-turn-helix repeats disrupting the codon for the isoleucine residue at position 129. Transposons in the SLAT collection are single copy and stable as the result of selection against the transposase source in trans, so this allele was chosen for further characterization and was designated myb61-1. This allele functions as a knockout at the RNA level (see Figure 4C). The two additional alleles from the Wisman collection were designated myb61-2 and myb61-3. The relative positions of the three insertions in the MYB61 gene are shown in Figure 1A.
Figure 4.
Expression of the MYB61 Gene.
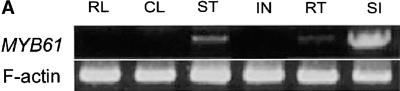
(A) RT-PCR showing expression of the MYB61 cDNA in the main tissue types. RL, rosette leaf; CL, cauline leaf; ST, inflorescence stem; IN, inflorescence; RT, root; SI, silique. F-actin transcript levels were used to normalize samples.
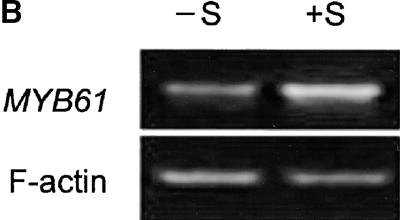
(B) The effect of sucrose on MYB61 expression in seedlings. −S, MS medium contained no sucrose; +S, MS medium contained 100 mM sucrose.
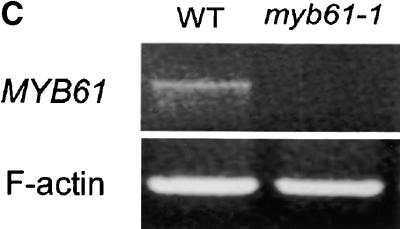
(C) RT-PCR showing undetectable expression of MYB61 in siliques of the myb61-1 mutant. WT, wild type.
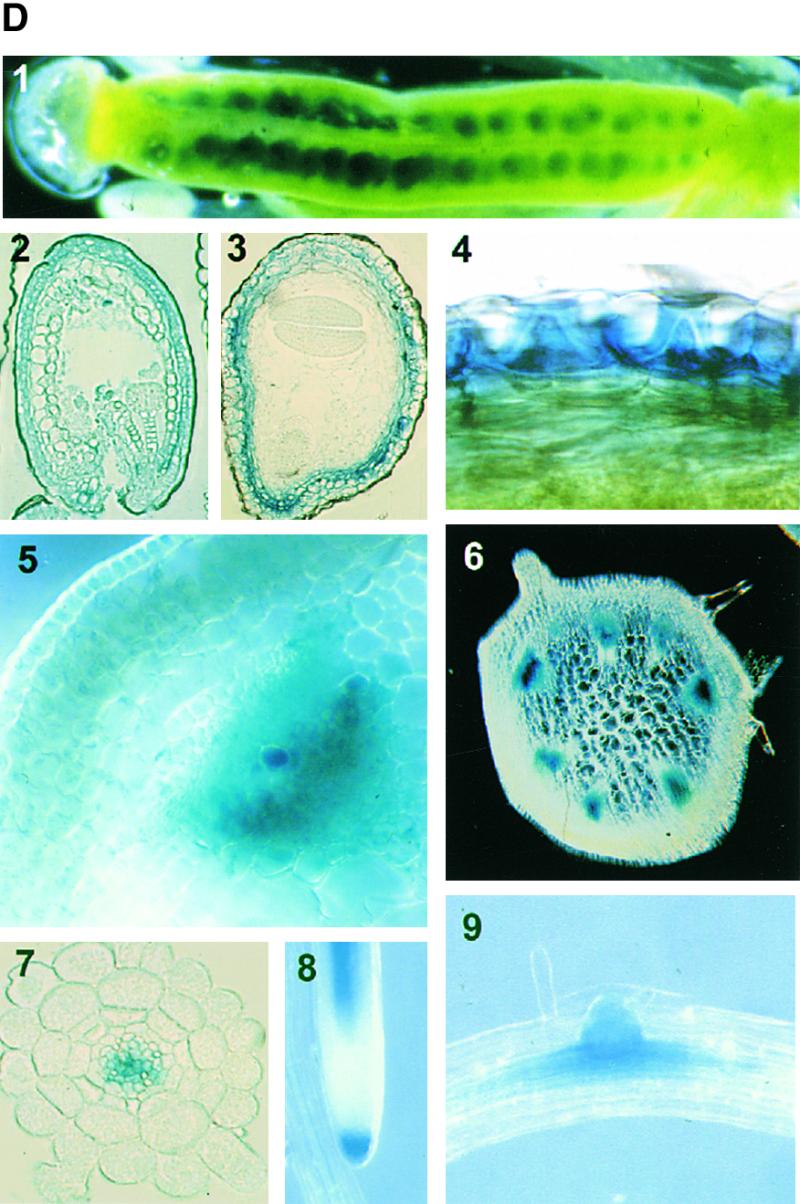
(D) Histochemical analysis of GUS reporter gene expression from the AtMYB61 promoter. (1) Expression in developing seeds within a silique; expression in the seed coat in (2) heart stage and (3) cotyledon stage seeds; (4) expression in the seed coat epidermis during late stages of seed development; and expression in vascular tissue of inflorescence stem (5 and 6), primary root (7), root cap (8), and lateral root primordia (9). Developing seed material was stained for 1.5 to 3 hr, and other tissues were stained for up to 10 hr.
Figure 1.
Three Disruptions of the MYB61 Gene Cause a Seed Mucilage Deficiency.
(A) Cartoon of the MYB61 gene showing the relative positions of the three insertions.
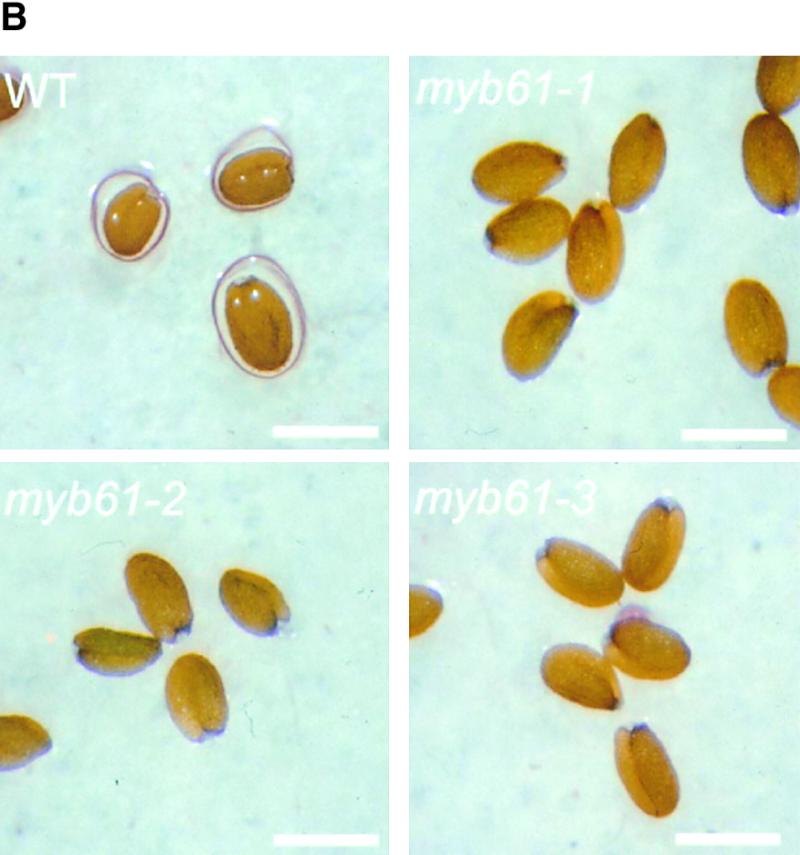
(B) Imbibed wild-type, myb61-1, myb61-2, and myb61-3 seeds stained with 0.2% ruthenium red. The extruded mucilage in wild-type seeds stains as a red halo. Bar = 0.50 mm.
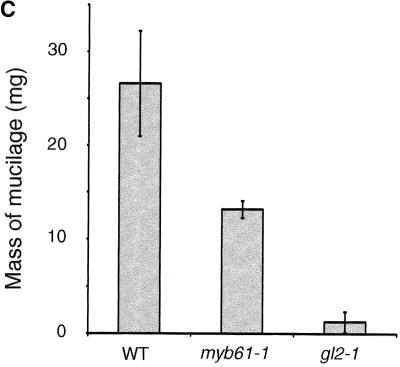
(C) Mass (mg) of freeze-dried aqueous soluble polysaccharide extracted from equal masses of ground dry seeds of the wild type and myb61-1, with gl2-1 as a negative control. Error bars indicate ±sd.
WT, wild type.
MYB61 Is Required for Seed Mucilage Extrusion during Imbibition
Lines homozygous for MYB61 insertions were subjected to systematic screens to uncover phenotypes associated with the loss of gene function. Plants carrying transposon insertions in MYB61 appeared phenotypically indistinguishable from the wild type, except for a lack of mucilage extrusion from the seeds during imbibition. Upon contact with water, Arabidopsis seeds release mucilage from the seed coat epidermis that swells to form a gelatinous coating over the seed. This was visualized by staining with ruthenium red, which stains negatively charged biopolymers such as pectin and DNA (Hanke and Northcote, 1975; Koornneef, 1981). Inspection of imbibed myb61-1, myb61-2, and myb61-3 seeds using ruthenium red indicated that they were deficient in mucilage extrusion upon hydration (Figure 1B). Aqueous extracts of ground seeds showed significantly reduced levels of soluble polysaccharides in myb61-1 seeds compared with those of the wild type (Figure 1C). To investigate the inheritance of this phenotype, we crossed myb61-1 plants with the wild type and then observed the segregation of the insertion and the phenotype. The mucilage extrusion phenotype segregated as a single recessive locus, and inheritance patterns showed that the phenotype was expressed in the sporophytic tissues of the seed. This is consistent with the inheritance of previously characterized seed coat mutants and the sporophytic origin of seed coat tissues (Léon-Kloosterziel et al., 1994). Furthermore, the mucilage phenotype faithfully co-segregated with the myb61-1 insertion (data not shown).
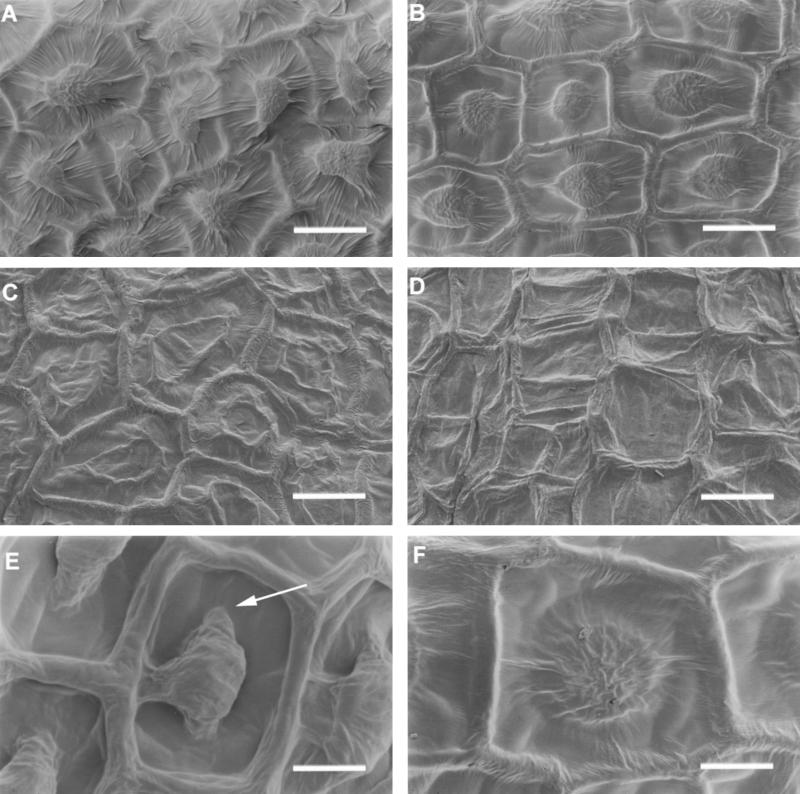
To further analyze the defect present in the myb61-1 testa epidermis, we examined seeds by scanning electron microscopy (SEM) before and after imbibition. Wild-type seeds, when viewed using SEM, exhibit a reticulate appearance as a result of the presence of the thickened radial cell walls and a raised columella in the center of each epidermal cell (Figure 2A). Examination of seeds containing MYB61 disruptions revealed that the seeds were the correct size and shape and that the radial cell walls had developed normally, but that the columellae were reduced in stature when compared with those of the wild type (Figure 2B). This contrasts with the phenotype seen in ttg1-1 and gl2-1 seeds, which lack a columella at the center of each cell (Léon-Kloosterziel et al., 1994; Figure 2C). Hence, the myb61-1 seed coat phenotype is distinct from previously characterized seed coat mutants. Observation of myb61-1 ttg1-1 double mutant seed coats revealed a more severe phenotype than did either the myb61-1 or ttg1-1 mutant alone (Figure 2D).
Figure 2.
SEM Analysis of Wild-Type and myb61-1 Seeds.
(A) Wild-type seed coat epidermis.
(B) myb61-1 seed coat epidermis.
(C) ttg1-1 seed coat epidermis.
(D) myb61-1 ttg1-1 double mutant seed coat epidermis.
(E) Wild-type seed coat epidermal cell post-imbibition. The arrow indicates the trough remaining around the central columella from which the mucilage has extruded.
(F) myb61-1 seed coat epidermal cell post-imbibition. No mucilage extrusion has occurred.
Bars in (A) to (D) = 20 μm; bars in (E) and (F) = 10 μm.
To investigate changes in seed coat appearance caused by imbibition, wild-type and myb61-1 seeds were imbibed on wet filter paper and air-dried before observation by SEM. After imbibition, deep troughs were visible around the columellae of wild-type epidermal cells (Figure 2E). These corresponded to the areas in which mucilage was deposited (Western et al., 2000) and from which it had extruded during the imbibition process. The myb61-1 seed coat was unchanged by the imbibition process (Figure 2F), indicating that no mucilage had been extruded, in agreement with observations using ruthenium red stain (Figure 1B).
Reduced Quantities of Mucilage Are Deposited during the Development of the Seed Coat Epidermis in the myb61-1 Mutants
Mucilage accumulates during the complex developmental process of the testa epidermis. This process begins with a highly vacuolated epidermal cell and is characterized by the formation of a central cytoplasmic column containing amyloplasts (Beekman et al., 2000; Western et al., 2000; Windsor et al., 2000), which begins to form at approximately the torpedo stage of embryo development, first against the outer tangential cell wall (Figure 3A). The deposition of mucilage occurs around the central column in the space between the primary cell wall and the plasma membrane (Western et al., 2000; Windsor et al., 2000; Figure 3E). After mucilage deposition is completed, the amyloplasts are degraded and a secondary cell wall is deposited, first around the plasma membrane, and then throughout the area occupied by the cytoplasm to form the columella.
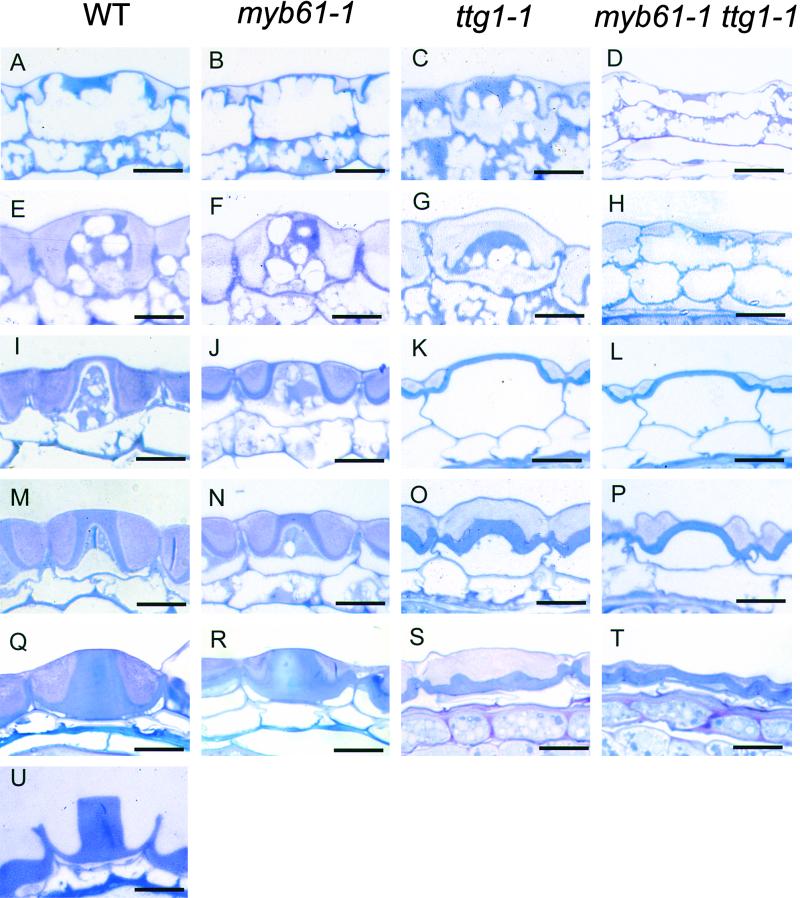
Figure 3.
The Effect of the myb61-1 and ttg1-1 Mutations on Seed Coat Development.
(A) to (D) In stage 1 seeds (torpedo stage embryo), the columella begins to form against the outer tangential cell wall accumulating amyloplasts, and mucilage begins to be secreted.
(E) to (H) At stage 2 (initiation of cotyledon formation), a cytoplasmic column extends to the base of the testa epidermal cells in wild-type and myb61-1 seeds, whereas ttg1-1 testa cells ([G] and [H]) have begun to lose the cytoplasmic column.
(I) to (L) By stage 3 (mid-cotyledon development), mucilage deposition is complete and secondary thickening of the cytoplasmic column has begun in wild-type and myb61-1 cells. In the ttg1-1 and ttg1-1 myb61-1 mutants, the vacuole expands and the mucilage is pressed to the edge of the cell, and the central cytoplasmic columns have disappeared.
(M) to (P) At stage 4 (mature embryo), secondary thickening proceeds in all mutants.
(Q) to (T) By stage 5 (maturity seed), secondary thickening is complete and wild-type cells are engorged with mucilage (Q), myb61-1 columellae have slumped (R), whereas only remnants of the columellae are visible in ttg1-1 and ttg1-1 myb61-1 ([S] and [T]).
(U) Imbibed wild-type seed from which the mucilage has extruded.
WT, wild type. Bars = 10 μm.
Although seed mucilage was not extruded during imbibition of myb61-1 seeds, mucilage deposition was observed in the course of testa development. The central cytoplasmic column appeared to form normally, but dense mucilage (revealed by light pink staining) was secreted only against the outer cell wall, whereas much of the space between the plasma membrane and the cell wall was composed of diffuse mucilage that stained faintly with toluidine blue (Figure 3F). As the cells neared maturity, a reduced volume of the cell became filled with deposited mucilage when compared with that of the wild type (cf. Figure 3Q, wild type, with Figure 3R, myb61-1). Secondary thickenings were deposited to form the columella in myb61-1, but a partial slump occurred at this stage, leading to the lower flatter structures observed under SEM (cf. Figure 2C with Figures 3N and 3R). At this stage of development, as a result of the absorption of water from the fixative (Figure 3U), it was usual for mucilage to be extruded from wild-type epidermal cells. This occurrence was rarely observed in the myb61-1 mutants, which was consistent with previous examinations using ruthenium red stain and SEM. Thus, although no mucilage was extruded from myb61-1 seeds during imbibition, reduced levels of mucilage were deposited during seed coat development.
Given the known genetic interactions between MYB-related transcription factors and the TTG1 locus, we characterized the ttg1-1 and ttg1-1 myb61-1 double mutant phenotype in the seed coat epidermis. The myb61-1 ttg1-1 double mutants were isolated using PCR to detect plants showing the ttg1-1 phenotype that were also homozygous for the myb61-1 insertion (see Methods). To minimize differences arising from the use of the two ecotypes (the myb61-1 allele is in the Columbia background, whereas ttg1-1 is in the Landsberg erecta ecotype), developing seeds from both the ttg1-1 and ttg1-1 myb61-1 plants without the erecta mutation were analyzed. Columellae began to form in ttg1-1 testa epidermal cells, and mucilage began to be deposited into the space between the plasma membrane and the outer cell wall (Figure 3C). However, the cytoplasmic column was short lived and began to lose organization before disappearing completely to leave a highly vacuolated cell (Figures 3G and 3K). The ttg1-1 mucilage was more diffuse than in either the wild-type or the myb61-1 mutants; after the loss of the columellae, only a small amount had been deposited, and this was compressed by the vacuole against the outer cell wall (Figure 3K). After this stage, secondary thickening began; because the organized cytoplasmic column had disappeared, no columella formed, but thickening was observed (Figures 3O and 3S). This gave the flattened seed surface morphology characteristic of the ttg1-1 mutant observed by SEM (Figure 2D), and no mucilage was extruded from the epidermal cells. Analyses of the ttg1-1 myb61-1 double mutant (Figures 3D to 3T) suggested an additive phenotype with less mucilage than that of the ttg1-1 single mutant and a more transient cytoplasmic column. Furthermore, secondary thickening was less well organized (Figure 3T) in the double mutant.
Analysis of Mucilage from Wild-Type myb61-1 Seeds
One explanation for the failure of mucilage extrusion in the myb61-1 mutants may be that changes in the composition or structure of the polysaccharide components might reduce the hydrophilic potential of the mucilage. To test this hypothesis, we analyzed soluble polysaccharides from the seeds of the wild type and the myb61-1 mutants. Because mucilage was not extruded from myb61-1 mutant seeds upon imbibition, soluble polysaccharides were extracted from ground seeds of mutant and wild-type lines and subjected to gas chromatography–mass spectrometry analysis. The results were normalized to the total amount of freeze-dried material extracted from each sample (Table 1). The most abundant monosaccharides in wild-type samples were rhamnose and galacturonic acid. This is consistent with previous analyses showing rhamnose as the principal monosaccharide of seed mucilage (Goto, 1985; Western et al., 2000) and suggested that seed mucilage comprised a large fraction of the polysaccharides extracted from whole seeds. In addition, there were significant amounts of other monosaccharides, including glucose, arabinose, and mannose, in the soluble extracts. Soluble polysaccharide extracts from myb61-1 mutants contained approximately half the material obtained from the wild type (Figure 1C), in agreement with microscopic observations that indicated that the myb61-1 mutant testa epidermal cells contained lower quantities of mucilage (Figure 3R). The largest reductions were seen in the rhamnose and galacturonic acid components, suggesting a reduction in the relative abundance of pectins, the major component of mucilage, in myb61-1 seeds. Levels of all sugars were reduced with the exception of arabinose, suggesting either that the arabinose content of seed mucilage is not affected by MYB61 or that the arabinose in the samples may come from elsewhere in the seed.
Table 1.
Cell Wall Polysaccharides from Extracted myb61-1 versus Wild-Type Seed Soluble Polysaccharide a
| Sugar | Wild Type b | myb61-1b |
|---|---|---|
| Rha | 2.96 ± 0.11 | 1.10 ± 0.01 |
| Ara | 0.99 ± 0.04 | 0.98 ± 0.06 |
| Xyl | 0.27 ± 0.02 | 0.14 ± 0.04 |
| Man | 0.94 ± 0.03 | 0.49 ± 0.01 |
| Gal | 0.68 ± 0.06 | 0.45 ± 0.02 |
| Glu | 1.37 ± 0.07 | 0.57 ± 0.03 |
| Fuc | tr | tr |
| GalA | 1.72 ± 0.03 | 0.68 ± 0.03 |
a Data are normalized to the total mass of seed extracted. The mole percentages of sugars were calculated from total ion counts between m/z 50 and m/z 350 on the basis of response factors from authentic sugar standards.
b Values are the mean ±sd of three (wild type) or four (myb61-1) samples; mg/100 mg seed.
Rha, rhamnose; Ara, arabinose; Xyl, xylose; Man, mannose; Gal, galactose; Glu, glucose; Fuc, fucose; GalA, galacturonic acid; tr, trace.
The major rhamnose-containing pectin in cotyledonous plants, RG I, consists of a backbone of alternating (1→2)-linked rhamnose and (1→4)-linked galacturonic acid residues, with side branches consisting of arabinan and arabinogalactan moieties attached to varying numbers of the rhamnose residues (Carpita and Gibeaut, 1993). To investigate the structure of seed mucilage RG I in the wild type and the myb61-1 mutant, linkage analysis was performed on soluble polysaccharides extracted from wild-type and myb61-1 seeds (Table 2; see Methods). Wild-type mucilage contained (1→2)-rhamnose and (1→4)-galacturonic acid, consistent with the presence of the rhamnose and galacturonic acid in the pectin RG I. This was not significantly esterified. Only small amounts of rhamnose branch point residues were detected, suggesting that the frequency of arabinan and arabinogalactan side branching in seed mucilage was low. No detectable changes in the linkage profile of seed polysaccharides in myb61-1 were found, but various sugar components of myb61-1 polysaccharides were reduced, consistent with the analyses in Table 1. These findings suggest that reduced mucilage secretion in myb61-1 seeds is not associated with changes in polysaccharide structure.
Table 2.
Comparison of Cell Wall Polysaccharides Extracted from myb61-1 and Wild-Type Seed Coatsa
| Sugar and Linkage | Wild Type | myb61-1 |
|---|---|---|
| Fucose | ||
| t-Fuc | tr | tr |
| Rhamnose | ||
| 2-Rha | 32.0 | 24.4 |
| 2,3-Rha | 0.5 | tr |
| 2,4-Rha | 0.9 | 0.5 |
| Arabinose | ||
| t-Ara | 5.0 | 6.9 |
| 2-Ara | 0.6 | 0.9 |
| 3-Ara | 1.2 | 4.9 |
| 5-Ara | 0.9 | 2.9 |
| 2,5-Ara | 3.3 | 6.0 |
| 3,5-Ara | 0.2 | 0.6 |
| Xylose | ||
| t-Xyl | 0.2 | tr |
| 2-Xyl | tr | tr |
| 4-Xyl | 2.3 | 2.6 |
| 2,4-Xyl | 0.6 | 0.5 |
| Mannose | ||
| t-Man | 2.1 | 0.5 |
| 4-Man | 7.3 | 9.7 |
| 4,6-Man | 1.2 | 0.9 |
| Galactose | ||
| t-Gal | 0.8 | 1.1 |
| 3-Gal | 0.9 | 0.2 |
| 4-Gal | tr | tr |
| 6-Gal | 1.4 | 1.9 |
| 3,4-Gal | ND | ND |
| 3,6-Gal | 4.6 | 7.1 |
| Glucose | ||
| t-Glc | 2.7 | 0.5 |
| 4-Glc | 11.0 | 10.7 |
| 4,6-Glc | 1.8 | 1.7 |
| Galacturonic acid | ||
| 4-GalA | 19.4 | 15.5 |
| 4-GalA (methyl ester) | tr | tr |
The mole percent values are the average of two independent trials; variance was <5% for all linkage groups.
tr, trace; ND, not determined.
MYB61 Gene Is Expressed in the Developing Seed Coat and Is Sucrose Induced
Reverse transcription (RT)–PCR was used to assay MYB61 transcript abundance in several tissues. Relatively high MYB61 expression was detected in developing siliques, with reduced expression in both stem and root tissue (Figure 4A). This is in agreement with previous observations using reverse RNA gel blot analysis that found the most abundant MYB61 expression in siliques, with lower levels in stem and young leaf tissue (Kranz et al., 1998). It is likely that the expression in roots detected by RT-PCR was beyond the threshold detectable by the reverse RNA gel blot approach. Because MYB61 was implicated in the accumulation of polysaccharides, the potential for regulation of the MYB61 gene by carbohydrates was investigated in seedlings. RT-PCR analysis revealed that MYB61 expression was increased in seedlings grown on medium containing 100 mM sucrose (Figure 4B). In addition, transcripts from the myb61-1 allele were not detectable in silique RNA, demonstrating that this allele leads to a likely loss of function (Figure 4C).
To localize the expression of MYB61 during silique development, the β-glucuronidase (GUS) reporter gene (Jefferson et al., 1987) was fused to the MYB61 promoter. Using PCR, a 2.2-kb fragment corresponding to the MYB61 promoter was amplified, fused to the GUS reporter gene, and transformed into Arabidopsis using the pGREEN II vector (Hellens et al., 2000). Four independent transformants expressed the GUS transgene in a similar pattern, although at slightly different levels. The main sites of GUS activity were in developing seeds and the vascular system (Figure 4D). GUS expression directed by the MYB61 promoter in siliques was primarily restricted to the seed coat (Figure 4D, 1 to 4), although low levels of expression were detected in the funiculi and the silique vascular tissue (data not shown). Expression was observed initially after fertilization of the ovule and was present in all cell layers of the developing seed coat, from the globular stage of development until the cotyledon stage. During the cotyledon stage of embryo development, GUS expression disappeared from all but the testa epidermal cell layer, where it persisted until the final stages of embryo maturation (Figure 4D, 4). In addition to being observed in the seed coat, MYB61:GUS expression was observed in the developing vascular tissue in both the root and inflorescence stem (Figure 4D, 5 to 7), where expression was relatively high in developing tracheary elements (Figure 4D, 5). GUS expression was also detected in the root cap and lateral root primordia (Figure 4D, 8 and 9). The patterns of expression of the MYB61:GUS transgene were consistent with those observed for MYB61 using RT-PCR as shown in Figure 4A. No phenotypes associated with myb61 disruptions were observed in root or vascular development.
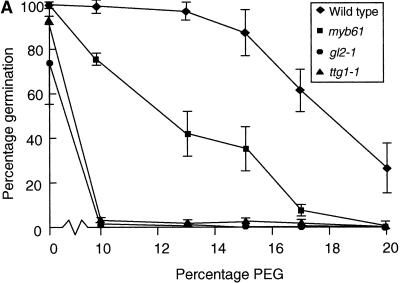
Figure 5.
Seedling Establishment of Arabidopsis Seed Mucilage Mutants on Media Containing Polyethylene Glycol.
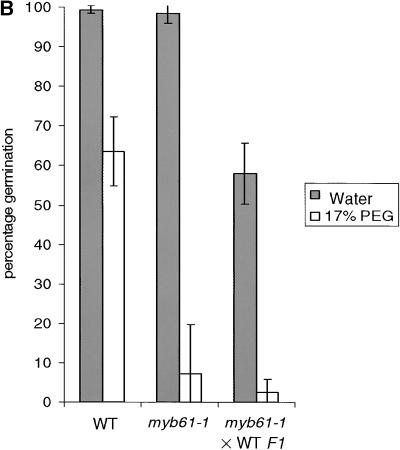
(A) Comparison of the germination of the wild type and seed mucilage mutants on increasing concentrations of PEG.
(B) Germination of heterozygous (phenotypically wild type [WT]) embryos in myb61-1 seed coats on 17% PEG.
Data points represent the mean and standard deviation of three independent experiments in both panels.
Seed Mucilage Is Required for Efficient Germination and Seedling Establishment in Low–Water Potential Conditions
The hygroscopic properties of mucilage suggest that it may function to enhance water uptake during germination. Therefore the efficiency of germination and seedling establishment of the wild type and seed mucilage mutants were tested in conditions of reduced water potential. Seeds were germinated on filter paper moistened with water or aqueous solutions of polyethylene glycol (PEG) 8000 at varying concentrations. Germination and seedling establishment were scored by the appearance of green seedlings after 5 days (Figure 5A). Germination and establishment of wild-type seedlings were only slightly affected by PEG concentrations up to 15%, beyond which the frequency of green seedlings steadily decreased. In contrast, germination and seedling establishment of the ttg1-1 and gl2-1 mutants were almost completely inhibited by 10% PEG. The myb61-1 mutants displayed levels of germination and seedling establishment intermediate between those of the wild type and ttg1-1 and gl2-1 seeds. This is consistent with observations showing that the myb61 phenotype is intermediate between those of the wild type and ttg1-1 in terms of mucilage deposition, and it suggests that seed mucilage can enhance seedling establishment when water is a limiting factor. Because mucilage was not detectably extruded upon imbibition in myb61 and ttg1-1 seeds, these data also suggest that the reduced level of unextruded mucilage in myb61 seeds still contributed to increasing seedling establishment in conditions of lowered water potential. myb61-1 plants were fertilized with wild-type pollen to create phenotypically wild-type embryos inside myb61-1 seed coats. These germinated with an efficiency comparable to that of myb61-1 seeds on 17% PEG, demonstrating that seed coat characteristics are responsible for the germination phenotypes observed (Figure 5B). No significant difference in germination on PEG was noted between ttg1-1 and myb61-1 ttg1-1 double mutant seeds (data not shown).
DISCUSSION
A screen for transposon-mediated disruptions in Arabidopsis led to the isolation of three insertions in the coding regions of the MYB61 gene (At1 g09450) that are likely to be loss-of-function alleles (Meissner et al., 1999). Systematic phenotype screens revealed a deficiency in mucilage extrusion from the testa epidermis during imbibition in each of the three myb61 alleles, and this phenotype segregated with the myb61-1 insertion. No other visible phenotypes were observed. Expression analysis using RT-PCR and GUS reporter gene fusions with the MYB61 promoter showed that the MYB61 gene is most abundantly expressed in the seed coat. Together these observations demonstrate that the seed mucilage deficiency is a consequence of loss of MYB61 function. In view of the known genetic interactions between MYB transcription factors and the TTG1 locus (Oppenheimer et al., 1991; Larkin et al., 1999; Lee and Schiefelbein, 1999), we also undertook a detailed characterization of the ttg1-1 mutant phenotype in the seed coat. The myb61 gene disruptions were found to have a phenotype distinct from that of the ttg1-1 mutant, whereas the effect of combining the ttg1-1 and myb61-1 mutations was additive. These results show that MYB61 performs a novel role in the development of the testa epidermis by regulating processes involved in seed mucilage deposition and extrusion.
Role of MYB61 in Seed Coat Development
The absence of mucilage extrusion from the seed coat was the only observed phenotype in myb61 knockout lines in routine screens. This phenotype has been observed in plants with lesions at three known loci (TTG1, GL2, and AP2; Koornneef, 1981; Koornneef et al., 1982; Jofuku et al., 1994) and was thought to be the result of the absence of mucilage deposition in the testa epidermis. Sections of developing myb61-1 seed coats revealed the deposition of mucilage, but in reduced amounts when compared with that of the wild type. This observation was supported by biochemical analyses of soluble polysaccharides from ground seeds that showed a 50% reduction in soluble polysaccharides in myb61-1 seeds. Deposition of mucilage in myb61-1 seed coats was characterized by areas that stained only faintly with toluidine blue compared with the wild type, and these may represent areas in which the mucilage is diffuse and possibly swollen by absorbed water during the fixation of sections. Such differential mucilage depositions have been observed in other plant species, including Hibiscus and Cinnamon (Bakker and Baas, 1993). Mucilage deposition was also observed in the ttg1-1 (and gl2-1; S. Penfield and M.W. Bevan, unpublished data) seed coats, although only in small quantities.
Mucilage extrusion occurs upon hydration of the mucilage as a result of the rupture of the primary cell wall under hydrostatic pressure (Western et al., 2000). The reduced levels of mucilage probably contribute to reduced pressure on the primary cell wall in the myb61 and ttg1-1 mutants and the lack of extrusion at imbibition. The unchanged structure of mucilage components in the myb61-1 mutant (Table 2) suggests that it is unlikely that the structure of mucilage components is responsible for reduced extrusion by the mucilage. The observation that mucilage does not extrude even from immature wild-type cells suggests that the mucilage or the epidermal cells may be modified during the final stages of testa maturation. One possibility is a partial hydrolysis of the primary wall to ease the extrusion process. This could require the secretion of hydrolytic enzymes and may be defective in both the myb61 and ttg1-1 mutants. Hence, they may promote the deposition of both mucilage polysaccharides and cell wall–modifying proteins between the wall and plasma membrane. GUS activity expressed from a MYB61:GUS transgene was detected in the developing seed coat, consistent with the data obtained by RT-PCR. This expression pattern conforms to the proposed role of MYB61 in seed coat development and mucilage deposition.
Another feature of the myb61-1 phenotype was the slump of the columella during secondary thickening, which may be because mucilage is required to stabilize the columella structure as thickening proceeds. Brassica campestris mucilage is secreted against the center of the outer tangential cell wall, and no columellae are formed (Van Caeseele et al., 1981), suggesting a correlation between the area in which mucilage is secreted and the final morphology of the seed coat epidermis. There is considerable variation in seed coat morphology throughout the Brassicacae (Vaughan and Whitehouse, 1971), including species such as Capsella, in which the seed coat resembles the myb61-1 mutant phenotype. It is possible that changes in the expression or activity of MYB transcription factors may play an important role in the evolution of adaptive seed coat traits.
MYB61 Promotes the Accumulation of Linear Rhamnogalacturonans
Both wild-type and myb61-1 mucilage were rich in the pectin RG I, as demonstrated by the presence of (1→2)-linked rhamnose and (1→4)-linked galacturonic acid residues as the most abundant monosaccharides (Carpita and Gibeaut, 1993). Linkage analysis demonstrated that the frequency of side branching is low in Arabidopsis seed mucilage RG I. This agrees with and extends previous analyses that have shown that rhamnose is the main neutral sugar component of Arabidopsis mucilage (Goto, 1985; Western et al., 2000). The monosaccharide composition of mucilage in myb61-1 lines showed a reduction of both rhamnose and galacturonic acid in whole-seed soluble polysaccharides, suggesting reduced abundance of RG I in myb61-1 seeds. Linkage analysis demonstrated that there were no detectable differences in the branching structure of RG I in wild-type and myb61-1 seeds. Mucilage RG I was not subject to substantial esterification in either wild-type or myb61-1 seeds, and it is similar to the non-methylesterified pectin in mucilage secreted from clover root cap cells (Lynch and Staehelin, 1992). RG I molecules are also released from in vitro–dif-ferentiating Zinnia elegans tracheids (Stacey et al., 1995). MYB61 was also expressed at lower levels in developing vascular tissue and in the root cap (Figure 4D), suggesting a potential link between MYB61 expression and pectin synthesis, secretion, or deposition. This suggests a more specific role for MYB61 in regulating RG I pectin levels in the developing testa. However, MYB61 is also expressed in other cell layers of the seed coat during early seed development. Little is known about the synthesis of polysaccharides in these cells, but it is possible that MYB61 has either a function in pectin accumulation in these cells or more general functions, including pectin accumulation.
Similarities in the Development of Vascular and Testa Tissues
Although there is little vascular development in the Arabidopsis seed coat, the seed coats of many dicotyledonous species exhibit varying degrees of vascularization (Fahn, 1990), suggesting that vascular tissue and the seed coat may share elements of a common developmental pathway. There are several similarities between vascular development and seed coat development: in Arabidopsis, both cell types exhibit pectin secretion, secondary cell wall deposition, primary cell wall modification, and eventual cell death (Fukuda, 1997). It is possible that MYB61 plays a role in the morphogenesis of cell types that share this set of developmental characteristics. As described above, this role may include promotion of secretory processes.
Role of TTG1 in Seed Coat Epidermal Development
The TTG1 gene is required for the patterning and initiation of trichomes, root atrichoblasts, and flavonoid biosynthesis as well as mucilage secretion (Koornneef, 1981; Lloyd et al., 1992). Formation of cytoplasmic columns and secretion of mucilage began normally in the ttg1-1 mutant, suggesting that TTG1 is not required for the initiation of testa epidermal cell development. However, ttg1-1 cells cease mucilage secretion during early stages of testa development, and the cytoplasmic columns degrade before secondary thickening of the collumella occurs, whereas the vacuole expands to occupy most of the space within the cell. This state is similar to the morphology of the subepidermal palisade cell layer at this stage and suggests a progressive loss of epidermal identity of the mucilage-secreting cells. The mucilage secreted in ttg1-1 appears to swell and absorb water, filling a large volume of the epidermal cells, but it is not extruded through the primary cell wall. This contrasts with both wild-type and myb61-1 cells. However, it is clear that when the vacuole expands and the mucilage is compressed, little mucilage is deposited, even compared with the myb61-1 mutants. The extra swelling may be the result of the presence of more space in the cells as a result of the degradation of the cytoplasmic columns that constrain the mucilage in wild-type and myb61-1 testa cells. The observation that secondary thickening occurs in the remaining cytoplasm of both ttg1-1 and myb61-1 mutants suggests that this process is genetically separable from earlier events in the maturation of the testa epidermis.
These observations are consistent with a model in which TTG1 activity is not required for the early stages of specification of the seed coat epidermis but is required to maintain the differentiated state of the epidermal cells after the assumption of the mucilage-secreting cell fate. Further support for the hypothesis that TTG1 activity is not required for the earliest events of epidermal cell differentiation comes from the analysis of an atrichoblast-specific Green Fluorescent Protein marker J2301 in roots (Berger et al., 1998). Its expression is confined to atrichoblasts even in the ttg1-1 mutant background, demonstrating the occurrence of atrichoblast-specific events in the absence of TTG1 function (S. Costa and L. Dolan, personal communication). Hence, further factors must be necessary for the initial specification of mucilage cell fate. These may include the AP2 gene product, strong alleles of which cause a more severe epidermal cell phenotype than those of ttg1-1 or gl2-1 (Jofuku et al., 1994). Alternately, it is possible that one or more bHLH transcription factors, known to interact with the TTG1 protein (Payne et al., 2000), may perform this function.
Analysis of myb61-1 ttg1-1 double mutants showed an additive effect of these genes on columella development and mucilage accumulation at later stages of testa development. The myb61-1 ttg1-1 double mutants secreted less mucilage than either myb61-1 or ttg1-1 alone, and this was accompanied by a more severe columella defect. Thus, MYB61 may function in a genetic pathway independent of TTG1, yet it is also possible that both MYB61 and TTG1 act together in a genetic pathway that still retains some function in the ttg1-1 mutant background. It is also possible that MYB61 function may overlap with that of closely related MYB transcription factors, and consequently the phenotype of the myb61 mutants may not reflect that of a complete loss-of-function mutant. The possible residual function of the ttg1-1 allele and the time and place of MYB61 and TTG1 function during testa development will need to be established to distinguish between these possibilities.
Seed Mucilage Facilitates Germination and Seedling Establishment in Dry Conditions
Seed mucilage is a natural example of a hydrogel (Zwieniecki et al., 2001) and is an efficient absorber of water. We therefore tested the hypothesis that mucilage takes up water to increase and stabilize water potential surrounding the seed, thereby promoting efficient germination and seedling establishment. The seed mucilage mutants tested germinated and grew normally under standard laboratory conditions, but as the water potential of the medium was lowered, establishment of ttg1-1and gl2-1 seedlings was strongly reduced compared with that of the wild type, whereas myb61-1 seeds displayed intermediate levels of seedling establishment. This effect was unrelated to effects of the ttg1-1 and gl2-1 mutants on seed dormancy, because they have been reported to exhibit decreased and increased seed dormancy, respectively (Debeaujon et al., 2000a). The stronger phenotype of ttg1-1 and gl2-1 compared with myb61-1 may reflect the smaller quantity of mucilage deposited or, alternately, the ease of mucilage extrusion from the testa epidermal cells. This experiment also demonstrates that the remaining mucilage in myb61-1 mutants performs a partial function. The definition of plant seed mucilage function during germination suggests that the production of seed mucilage is an adaptation to environments of low or variable water availability, such as those encountered during the evanescent growth of Arabidopsis. Further definition of the roles of MYB61 in this process may suggest ways in which seed mucilage production could be modified to alter seedling establishment patterns.
Testa as a Model System
The development of the Arabidopsis seed coat epidermis is an excellent model system for establishing links between the regulation of cell differentiation and control of the biosynthesis and exocytosis of polysaccharides. Copious amounts of mucilage are secreted that are easily extractable for biochemical analysis, and facile mutant screens should identify a number of genes required for these processes. In addition, similarities between seed coat and xylem suggest that understanding morphogenesis of the testa epidermis may be relevant to defining mechanisms controlling the development of vascular cells. Future work aims to establish the sets of genes regulated by MYB61. Among these may be genes encoding components of polysaccharide synthesis and secretion. The isolation and characterization of additional testa mutants should improve our understanding of the complex processes underlying polysaccharide secretion and its contribution to plant cell differentiation and architecture.
METHODS
Plant Materials
Arabidopsis thaliana plants carrying the myb61-1 allele in the Columbia background were obtained from the Sainsbury Laboratory Arabidopsis thaliana (SLAT) population using Inverse Display of Insertion filter screens with a digoxygenin-labeled (Roche, Lewes, UK) MYB61-specific probe (Meissner et al., 1999; Tissier et al., 1999). The isolation of the myb61-2 and myb61-3 alleles has been described by Meissner et al. (1999). The ttg1-1 allele was obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, UK).
Ruthenium Red Staining of Seed Mucilage
Whole seeds were allowed to imbibe on moist filter paper for between 5 min and 1 hr, before the application of 0.2% w/v aqueous ruthenium red (Sigma) solution. Seeds were photographed with a WILD MZ8 dissecting microscope (Leica UK Ltd., Milton Keynes, UK).
Microscopy and Histology
For scanning electron microscopy (SEM), dry seeds were gold coated using a High Resolution Sputter Coater (Agar, Redding, CA) and imaged in a Philips (FEI UK Ltd., Cambridge, UK) XL30 scanning electron microscope with an accelerating voltage of 3 kV. Imbibed seeds were air dried overnight on filter paper and then treated as dry seeds. For thin sections of seed coats, whole siliques were fixed in 2.5% glutaraldehyde (Sigma Aldrich) in 0.05 M sodium cacodylate buffer, which was vacuum infiltrated before dehydration in a graded ethanol series, and infiltrated with LR White resin (London Resin Company). Polymerization was performed overnight at 60°C. Sections of between 200 and 400 nm in thickness were made with a Reichert Ultramicrotome (Leica UK Ltd., Milton Keynes, UK) and stained with toluidine blue.
AtMYB61 Promoter::GUS Fusion
Standard molecular biology techniques were employed as described (Sambrook et al., 1989). The MYB61 promoter was amplified as a 2.2-kb XhoI-NcoI fragment from genomic DNA by use of primers F61P (5′-CCTCGAGCTACACTTTCTGACCAAGAC-3′) and R61P (5′-CCATGGTTAGTTATTCACAGCTGCAATG-3′) and cloned into the pGEM-T vector (Promega, Southampton, UK) according to the manufacturer's instructions. The β-glucuronidase (GUS) gene fused to the nopaline synthase terminator was subcloned from the pRAJ275 vector (Jefferson et al., 1987) as a HindIII-EcoRI fragment into the pGREEN II vector (Hellens et al., 2000) to make plasmid pGREENGUS. The XhoI-NcoI MYB61 promoter was then subcloned upstream of the GUS gene in pGREENGUS. This was transformed into Arabidopsis Columbia ecotype by Agrobacterium-mediated floral dip (Clough and Bent, 1998). Histochemical detection of GUS expression was performed as described (Jefferson et al., 1987). Seeds and roots were fixed in 2.5% glutaraldehyde (Sigma) in 0.05% sodium cacodylate buffer before dehydration in a graded ethanol series and embedding in Technovit Historesin (Kulzer Heraeus, Wehreim, Germany) according to the manufacturer's protocol. Hand-cut sections were made from GUS-stained stem tissue and photographed on a Microphot (Nikon, Kingston, UK) 600 microscope. Whole-mount tissues were photographed using a WILD MZ8 dissecting microscope (Leica).
Reverse Transcription–Polymerase Chain Reaction
RNA was isolated using TRIZOL reagent (Gibco BRL) from aerial tissues of greenhouse-grown plants and from roots grown for 3 weeks in liquid culture (Kranz et al., 1998), using the manufacturer's protocol. Seedlings were grown for 7 days in tissue culture on Murashige and Skoog (Duchefa, Haarlem, The Netherlands) media with or without 100 mM sucrose (Sigma). First-strand cDNA was synthesized from 5 μg of RNA using M-MLV Reverse Transcriptase (Gibco BRL) according to the manufacturer's instructions. Reverse transcription–polymerase chain reaction (RT-PCR) for the MYB61 gene used primers F61Bam (5′-GGATCCATGGGGAGACATTCTTTGCTGTTAC-3′) and R61Eco (5′-GAATTCTAAAGGGACTGACCAAAAGAGAC-3′), using 1 μL of the first-strand reaction on a Touchdown thermocycler (Hybaid, UK) under 90°C for 2 min, and then 35 cycles of the following: 90°C for 40 sec, 65°C for 1 min, 72°C for 2 min, and then 72°C for 5 min. The actin control was amplified using primers ACT1 (5′-GCC-AAAGCAGTGATCTCTTTGCTC-3′) and ACT2 (5′-GTGTTGGAC-TCTGGAGATGGTGTG-3′), using the above reaction conditions with either 25 or 35 amplification cycles.
Isolation of myb61-1 ttg1-1 Double Mutants
ttg1-1 plants were crossed to myb61-1, and double mutants were isolated from the resulting F2 population by PCR. Plants showing the ttg1-1 phenotype were tested using primers F61Bam and R61Eco and the above PCR conditions to detect the wild-type allele, whereas R61Eco and the En8130 primer (Wisman et al., 1998) were used to amplify a product from the myb61-1 insertion.
Seed Mucilage Extraction
Equal masses of ground dry wild-type and myb61-1 seeds were initially extracted with 2 M imidazole, pH 7.5; insoluble material was removed by centrifugation, and the supernatant was precipitated with 5 volumes of ethanol. The precipitated material was redissolved in water; insoluble material was removed by centrifugation and precipitated with 5 volumes of ethanol. This was repeated three times before freeze-drying the soluble polysaccharide before measuring yield.
Determination of Sugar and Polymer Composition of the Pectic and Alkali-Soluble Polysaccharides
Uronic acid units in polysaccharides in ammonium oxalate extracts of freeze-dried soluble polysaccharides were activated by the water-soluble diimide, 1-cyclo-3-(2-morpholinoethyl)carbodiimide metho-p-toluenesulfonate (CMC), and reduced with NaBD4 to their respective 6,6-dideuterio neutral sugars, as described by Kim and Carpita (1992). One to two mg of each fraction was hydrolyzed with 2 M tri-fluoroacetic acid (TFA) containing 1 μmol of myo-inositol (internal standard) for 90 min at 120°C. The TFA was evaporated under a stream of nitrogen, and the sugars were converted to alditol acetates (Gibeaut and Carpita, 1991). The alditol acetates were separated by gas-liquid chromatography (GLC) on a 0.25-mm × 30-m vitreous silica capillary column of SP-2330 (Supelco, Bellefonte, PA). Temperature was programmed from 170°C to 240°C at 5°C per min with a 6-min hold at the upper temperature. The 6,6-dideuteriogalactosyl units, diagnostic of GalA, were determined by the shift of 2 amu of the secondary fragments from electron-impact mass spectrometry (EIMS) from those of Gal. Diagnostic pairs used were m/z 189/191, m/z 217/219, m/z 259/261, and m/z 289/291, and amounts were calculated by the equation described by Kim and Carpita (1992).
Linkage Analyses
Polysaccharides, with uronic acids that had been reduced with NaBD4 to their respective 6,6-dideuterio sugars, were per-O-methylated with Li+ methylsulfinylmethanide and methyl iodide according to Gibeaut and Carpita (1991). The per-O-methylated polymers were recovered after addition of water to the mixture and partitioning into chloroform. The chloroform extracts were washed five times with a threefold excess of water, and the chloroform was evaporated. The permethylation step was repeated, and the methylated polymers were purified by chloroform partitioning and were hydrolyzed in 2 M TFA for 90 min at 120°C. The TFA was evaporated in a stream of nitrogen gas, and the sugars were then reduced with NaBD4 and acetylated. The partly methylated alditol acetates were separated on the column as the alditol acetates but in a temperature program of 160°C to 210°C at 2°C per min, then to 240°C at 5°C per min with a hold of 5 min at the upper temperature. GLC-EIMS analysis was used to verify all derivative structures (Carpita and Shea, 1989).
Determination of Degree of Esterification
The esterified uronic acid residues, regardless of the leaving group, were chemically reduced to their respective neutral sugars by addition of sodium borohydride (Maness et al., 1990). We devised a variation of this method whereby the pectic material was dissolved or suspended in 4 mL of 25 mM sodium acetate, pH 4.6 (Carpita and McCann, 1996). The suspension was split into two equal samples, chilled to ice temperature, and while stirring continuously, 1 mL of 2 M imidazole (HCl), pH 7.0, and 300 mg of sodium borohydride or sodium borodeuteride were added, respectively, to the two samples. The mixtures were slowly warmed and incubated at ambient temperature with continuous stirring. The reactions were stopped by addition of glacial acetic acid to destroy the excess borohydride and bring the pH to below 5. The samples were dialyzed overnight against running deionized water. The samples then were returned to 25 mM sodium acetate, pH 4.6, with continuous stirring, and the remaining unesterified uronic acids were activated with CMC. The mixtures were chilled to ice temperature, and 1 mL of 2 M imidazole (HCl), pH 7.0, and 300 mg of sodium borodeuteride or sodium borohydride were added, respectively, to the samples. The samples were incubated, and the reactions stopped as described previously; after extensive dialysis against running deionized water, the materials were freeze-dried. As described above, alditol acetate derivatives were prepared and separated by GLC, and the mole fractions of 6,6-dideuteriogalactose were determined by EIMS. The mole fraction of 6,6-dideuteriogalactose deduced from the primary borodeuteride reduction–secondary borohydride reduction represented the esterified uronic acid, whereas the amount deduced from primary borohydride reduction–secondary borodeuteride reduction represented the amount of free acid; the remainder was nascent galactose.
Germination on Polyethylene Glycol
Two-week-old seeds were placed on 9-cm filter paper discs sealed in an upturned Petri dish and moistened with 1 mL of water or polyethylene glycol (PEG) 8000 solution from 10 to 20% concentration (Sigma). Seeds were stratified for 3 days at 4°C. Seedling establishment was scored as the appearance of green seedlings after 5 days. Each treatment was performed in triplicate with ∼50 seeds per genotype per treatment.
Acknowledgments
The technical support of Caroline Smith is gratefully acknowledged. We thank Bernd Weisshaar for generously sharing the myb61-3 line. S.P. was supported by a Biological and Biotechnological Sciences Research Council Committee Studentship, and R.C.M. was supported by European Commission Contract CT95-0129.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010265.
References
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Bakker, M.E., and Baas, P. (1993). Cell walls in oil and mucilage cells. Acta Bot. Neerl. 42, 133–139. [Google Scholar]
- Beekman, T., De Rycke, R., Viane, R., and Inzé, D. (2000). Histological study of seed coat development in Arabidopsis thaliana. J. Plant Res. 113, 139–148. [Google Scholar]
- Bender, J., and Fink, G. (1998). A MYB homolog, ATR1, activates tryptophan gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 5655–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, F., Hung, C.Y., Dolan, L., and Schiefelbein, J. (1998). Control of cell division in the root epidermis of Arabidopsis thaliana. Dev. Biol. 194, 235–245. [DOI] [PubMed] [Google Scholar]
- Borevitz, J.O., Xia, Y.J., Blount, J., Dixon, R.A., and Lamb, C. (2000). Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12, 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M.E., Barley, R., Curtis, M., Arroyo, J.M., Dunham, M., Hudson, A., and Martienssen, R.A. (2000). Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408, 967–971. [DOI] [PubMed] [Google Scholar]
- Carpita, N.C., and Gibeaut, D.M. (1993). Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30. [DOI] [PubMed]
- Carpita, N.C., and McCann, M.C. (1996). Some new methods to study plant polyuronic acids and their esters. In Progress in Glycobiology, R. Townsend and A. Hotchkiss, eds (New York: Marcel Dekker), pp. 595–611.
- Carpita, N.C., and Shea, E.M. (1989). Linkage structure of carbohydrates by gas chromatography–mass spectrometry (GC-MS) of partially methylated alditol acetates. In Analysis of Carbohydrates by GLC and MS, C.J. Biermann and G.D. McGinnis, eds (Boca Raton, FL: CRC Press), pp. 157–216.
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thal-iana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cone, K.C., Cocciolone, S.M., Moehlenkamp, C.A., Weber, T., Drummond, B.J., Tagliani, L.A., Bowen, B.A., and Perrot, G.H. (1993). Role of the regulatory gene pl in the photocontrol of maize anthocyanin pigmentation. Plant Cell 5, 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon, I., Léon-Kloosterziel, K.M., and Koornneef, M. (2000. a). Influence of the testa on seed dormancy, germination and longivity in Arabidopsis. Plant Physiol. 122, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn, A. (1990). Plant Anatomy. 4th ed. (Oxford: Pergamon Press).
- Fukuda, H. (1997). Xylogenesis: Initiation, progression and cell death. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 299–325. [DOI] [PubMed] [Google Scholar]
- Gibeaut, D.M., and Carpita, N.C. (1991). Tracing the biosynthesis of the cell wall in intact cells and plants. Selective turnover and alteration of cytoplasmic and cell wall polysaccharides of proso millet cells in liquid culture and Zea mays seedlings. Plant Physiol. 97, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, N. (1985). A mucilage polysaccharide secreted from testa of Arabidopsis thaliana. Arabidopsis Inf. Serv. 22, 1–4. [Google Scholar]
- Grotewold, E., Drummond, B.J., Bowen, B., and Peterson, T. (1994). The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76, 543–553. [DOI] [PubMed] [Google Scholar]
- Gutterman, Y., and Shemtov, S. (1996). Structure and function of the mucilaginous seed coats of Plantago coronopus inhabiting the Negev Desert of Israel. Israel J. Plant Sci. 44, 125–133. [Google Scholar]
- Hanke, D.E., and Northcote, D.H. (1975). Molecular visualization of pectin and DNA by ruthenium red. Biopolymers 14, 1–17. [DOI] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGREEN: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42, 819–832. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., Cominelli, E., Pailey, P., Parr, A., Mehrtens, F., Tonelli, C., Weisshaar, B., and Martin, C. (2000). Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku, D., den Boer, B.G.W., Van Montagu, M., and Okamuro, J.K. (1994). Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.B., and Carpita, N.C. (1992). Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol. 98, 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M. (1981). The complex syndrome of the ttg mutants. Arabidopsis Inf. Serv. 18, 45–51. [Google Scholar]
- Koornneef, M., Dellaert, L.W.M., and Vanderveen, J.H. (1982). EMS-induced and radiation-induced mutation frequencies at individual loci in Arabidopsis-thaliana (L) heynh. Mutat. Res. 93, 109–123. [DOI] [PubMed] [Google Scholar]
- Kranz, H.D., et al. (1998). Towards a functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J. 16, 263–276. [DOI] [PubMed] [Google Scholar]
- Larkin, J.C., Walker, J.D., Bolognesi-Winfield, A.C., Gray, J.C., and Walker, A.R. (1999). Allele-specific interactions between ttg and gl1 during trichome development in Arabidopsis thaliana. Genetics 151, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.M., and Schiefelbein, J. (1999). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99, 473–483. [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel, K.M., Keijer, C.J., and Koornneef, M. (1994). A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, A.M., Walbot, V., and Davis, R.W. (1992). Anthocyanin production in dicots activated by the maize anthocyanin-specific regulators, R and C1. Science 258, 1773–1775. [DOI] [PubMed] [Google Scholar]
- Lynch, M.A., and Staehelin, L.A. (1992). Domain-specific and cell type-specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J. Cell Biol. 118, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness, N.O., Ryan, J.D., and Mort, A.J. (1990). Determination of the degree of methyl esterification of pectins in small samples by selective reduction of esterified galacturonic acid to galactose. Anal. Biochem. 185, 346–352. [DOI] [PubMed] [Google Scholar]
- Martin, C., and Paz-Ares, J. (1997). MYB transcription factors in plants. Trends Genet. 13, 67–73. [DOI] [PubMed] [Google Scholar]
- Meissner, R.C., et al. (1999). Function search in a large transcription factor gene family in Arabidopsis: Assessing the potential of reverse genetics to identify insertion mutants in R2R3 MYB genes. Plant Cell 11, 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano, E., Martinez-Garcia, J.F., and Martin, C. (1996). Apparent redundancy in MYB gene function provides gearing for the control of flavonoid biosynthesis in Antirrhinum flowers. Plant Cell 8, 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer, D.G., Herman, P.L., Sivakumaran, S., Esch, J., and Marks, M.D. (1991). A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67, 483–493. [DOI] [PubMed] [Google Scholar]
- Payne, C.T., Zhang, F., and Lloyd, A.M. (2000). GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares, J., Ghosal, D., Wienand, U., Peterson, P.A., and Saedler, H. (1987). The regulatory c1 locus of Zea mays encodes a protein with homology to MYB proto-oncogene products and with similarities to transcriptional activators. EMBO J. 7, 3553–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerie, W.G., Feldmann, K.A., and Marks, M.D. (1994). The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Genes Dev. 8, 1388–1399. [DOI] [PubMed] [Google Scholar]
- Riechmann, J.L., and Ratcliffe, O.J. (2000). A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 3, 423–434. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Frisch, E.F., and Maniatis, T. (1989). Molecular Cloning. A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Stacey, N.J., Roberts, K., Carpita, N.C., Wells, B., and McCann, M.C. (1995). Dynamic changes in cell surface molecules are very early events in the differentiation of mesophyll cells from Zinnia elegans into tracheary elements. Plant J. 8, 891–906. [Google Scholar]
- Tissier, A.F., Marillonnet, S., Klimyuk, V., Patel, K., Torres, M.A., Murphy, G., and Jones, J.D.G. (1999). Multiple independent defective suppressor–mutator transposon insertions in Arabidopsis: A tool for functional genomics. Plant Cell 11, 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Caeseele, L., Mills, J.T., Sumner, M., and Gillespie, R. (1981). Cytology of mucilage production in the seed coat of Candle canola (Brassica campestris). Can. J. Bot. 59, 291–300. [Google Scholar]
- Vaughan, J.G., and Whitehouse, J.M. (1971). Seed structure and the taxonomy of the Cruciferae. Bot. J. Linn. Soc. 64, 383–409. [Google Scholar]
- Walker, A.R., Davison, P.A., Bolognesi-Winfield, A.C., James, C.M., Srinivasan, N., Blundell, T.L., Esch, J.J., Marks, D.M., and Gray J.C. (1999). The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11, 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western, T.L., Skinner, D.J., and Haughn, G.W. (2000). Differentiation of mucilage secretary cells of the Arabidopsis seed coat. Plant Physiol. 122, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, J.B., Vaughan-Symonds, V., Mendenhall, J., and Lloyd, A.M. (2000). Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 22, 483–493. [DOI] [PubMed] [Google Scholar]
- Wisman, E., Hartmann, U., Sagasser, M., Baumann, E., Palme, K., Hahlbrock, K., Saedler, H., and Weisshaar, B. (1998). Knockout mutants from an En-1 mutagenised Arabidopsis thaliana population generate phenylpropanoid biosynthesis phenotypes. Proc. Natl. Acad. Sci. USA 95, 12432–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki, M.A., Melcher, P., and Holbrook, N.M. (2001). Hydrogel control of xylem hydraulic resistance in plants. Science 291, 1059–1062. [DOI] [PubMed] [Google Scholar]