Abstract
A cytochrome in an extremely halophilic archaeon, Haloferax volcanii, was purified to homogeneity. This protein displayed a redox difference spectrum that is characteristic of a-type cytochromes and a CN− complex spectrum that indicates the presence of heme a and heme a3. This cytochrome aa3 consisted of 44- and 35-kDa subunits. The amino acid sequence of the 44-kDa subunit was similar to that of the heme-copper oxidase subunit I, and critical amino acid residues for metal binding, such as histidines, were highly conserved. The reduced cytochrome c partially purified from the bacterial membrane fraction was oxidized by the cytochrome aa3, providing physiological evidence for electron transfer from cytochrome c to cytochrome aa3 in archaea.
Cytochrome c oxidase, a protein complex located in the inner membranes of mitochondria and many bacteria, is the terminal enzyme of most respiratory chains. The terminal oxidases of archaea have been obtained mostly from members of the orders Halobacteriales and Sulfolobales. Thermoacidophilic Sulfolobus acidocaldarius coexpresses two terminal oxidases, SoxABCD and SoxM, during heterotrophic growth. SoxABCD and SoxM are multisubunit oxidases of known primary structure (15, 16) that use caldariella-quinol as their natural substrate (1). No cytochrome c is present in the membrane, although S. acidocaldarius cytochrome oxidase was reported to oxidize bovine heart cytochrome c (24). Electron transfer in SoxABCD may also involve other substrates such as small blue copper proteins (5). In Acidianus ambivalens, a cytochrome aa3 was identified and found to oxidize caldariella-quinol but not cytochrome c (2). The primary structure of the enzyme was recently determined (20).
The respiratory systems of extremely halophilic archaea have been investigated mainly by spectroscopic methods (7, 14). Fujiwara et al. identified an aa3-type cytochrome in the membrane of Halobacterium halobium which oxidizes horse heart cytochrome c (11). However, in a later paper, they reported that a b-type cytochrome is likely to donate electrons to the cytochrome aa3 (10). This aa3-type cytochrome has a molecular mass of 40 kDa and binds carbon monoxide (9). Moreover, gene analysis showed that Halobacterium halobium cytochrome aa3 is a subunit I homologue of the heme-copper oxidase superfamily (8). Halobacterium halobium cytochrome aa3 oxidizes neither cytochrome c nor 2,3,5,6-tetramethyl-p-phenylenediamine (TMPD); therefore, its natural substrate and its physiological role in respiratory chains remain largely unknown. However, a partially purified a-type cytochrome from Halobacterium salinarum oxidizes bovine heart cytochrome c, suggesting that this a-type cytochrome from Halobacterium salinarum functions as a cytochrome c oxidase (23). On the other hand, terminal oxidase cytochrome ba3 from the archaeal halophilic species Natronobacterium pharaonis, which grows at a pH of about 9.5, was recently characterized and sequenced (18, 22). According to the redox potential of the small blue copper protein halocyanin, it is likely to serve as an electron donor for cytochrome ba3 (4, 21).
In order to determine the electron donor of halophilic archaeal oxidase, we identified the terminal oxidase of Haloferax volcanii, which, like Halobacterium species, is a member of family Halobacteriaceae, as well as cytochrome c in this bacterium. We investigated terminal oxidation in halophilic archaea.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. volcanii strain WFD (6) was purchased from the American Type Culture Collection and cultured at 40°C in a medium containing (per liter) 5 g of polypeptone, 1 g of glucose, 156 g of NaCl, 20 g of MgSO4, 13 g of MgCl2, 4 g of KCl, 1 g of CaCl2, 0.5 g of KBr, and 0.2 g of NaHCO3 at pH 7.1 with vigorous aeration in an 80-liter fermentor. Cells at the mid-logarithmic phase of growth were harvested by centrifugation and stored at −4°C.
Purification of cytochrome aa3.
Cells obtained from 160 liters of culture were suspended in buffer A (2 M NaCl, 10 mM Tris-HCl, pH 8.0) and disrupted by sonication. Debris was removed by centrifugation at 5,000 × g for 15 min, and the supernatant was centrifuged at 100,000 × g for 40 min to collect the pellet (membrane fraction). The membrane fraction was washed once and resuspended in the same buffer at a final protein concentration of 10 mg/ml. The suspension was slowly mixed with Tween 20 to 3% (wt/vol) and stirred for 30 min on ice. The partially solubilized fraction was centrifuged at 100,000 × g for 40 min, and the resulting pellet (Tween 20-washed membranes) was resuspended in buffer A at a final protein concentration of 10 mg/ml and mixed with an equal volume of a solution containing 40% glycerol, 2% (wt/vol) deoxycholate, and 10 mM Tris-HCl (pH 8.0). After being stirred at 10°C for 24 h, the suspension was centrifuged at 150,000 × g for 1 h to remove debris. The supernatant was applied to a phenyl-Sepharose CL-4B column (4 by 15 cm; Pharmacia) equilibrated with buffer B (1 M NaCl, 20% glycerol, 1% deoxycholate, 10 mM Tris-HCl, pH 8.0). The column was washed with 50 ml of buffer B and then with 150 ml of buffer C (0.1 M NaCl, 20% glycerol, 1% deoxycholate, 10 mM Tris-HCl, pH 8.0). a-type cytochrome was eluted with an increasing gradient of Triton X-100 (0.3 to 3.5%) in buffer C at a flow rate of 1.5 ml/min. The peak fraction of a-type cytochrome was collected and applied to a DEAE-Toyopearl 650 M column (2.6 by 20 cm; Tohso) preequilibrated with buffer D (0.1 M NaCl, 20% glycerol, 0.3% Triton X-100, 10 mM Tris-HCl, pH 8.0). After a wash with buffer D, a-type cytochrome was eluted with buffer D containing 0.18 M NaCl. The peak fractions were concentrated by filtration (UP-20; Advantic Toyo) and then applied to a Superdex 200pg column (2.6 by 60 cm; Pharmacia). The a-type cytochrome eluted immediately after the void fraction. The concentration of heme a was estimated from the difference spectrum of its pyridine hemeochrome at 587 nm minus 620 nm using the extinction coefficient 25 mM−1 cm−1, and the concentration of cytochrome aa3 was estimated from the difference between the redox difference spectrum at 607 nm and that at 640 nm using the extinction coefficient 24 mM−1 cm−1 (3).
Partial purification of cytochrome c from H. volcanii.
Tween 20-washed membranes were solubilized in 1% (wt/vol) octylglucoside-1% (wt/vol) Triton X-100 for 24 h at 10°C. The solubilized membrane fraction was centrifuged at 150,000 × g for 1 h to remove debris. The suspension was diluted 10-fold with 20% glycerol-10 mM Tris-HCl (pH 8.0) and applied to a DEAE-Toyopearl 650 M column (2.6 by 20 cm) preequilibrated with buffer D. The cytochrome c fraction was eluted with an increasing gradient of NaCl ranging from 0.1 to 0.5 M in buffer D.
Enzyme assay.
Cytochrome aa3 and cytochrome c were identified spectroscopically by use of redox difference spectra and the previously determined peak wavelengths of their pyridine ferrohemeochromes (3). The redox difference spectra of cytochromes were obtained by taking the difference of spectra of the oxidized form (the resting state) and the reduced form (in the presence of 0.5 mM TMPD plus 5 mM ascorbate or dithionite). Oxidation of cytochrome c by the purified cytochrome aa3 was demonstrated by adding cytochrome aa3 (0.2 mM) to 1 ml of a TMPD-reduced cytochrome c (30 mM) suspension at room temperature and tracing the absorption spectra at 552 nm (α band of cytochrome c) for time intervals ranging from 1 to 12 min. The concentration of heme c in cytochrome c was estimated from the difference spectrum of its pyridine ferrohemeochrome at 550 and 535 nm with the extinction coefficient 24 mM−1 cm−1 (3).
Determination of partial amino acid sequences.
The 44-kDa subunit of cytochrome aa3 was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and extracted by electroelution in buffer containing 96 mM glycine, 0.05% SDS, and 12.5 mM Tris-HCl (pH 8.8). Samples were dialyzed against 200 volumes of 0.05% SDS-1 mM dithiothreitol-20 mM Tris-HCl (pH 9.0) and then incubated with lysylendopeptidase (0.2 μg) at 37°C for 3 h. The peptide fragments were separated by SDS-PAGE and electroblotted onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore) in 0.05% SDS-20% methanol-10 mM mercaptoethanol at 0.5 mA/cm2 for 8 h with a semidry electrotransfer apparatus. Peptide bands were cut from the membrane and applied to an Applied Biosystems model 476A amino acid sequencer.
Cloning of the 44-kDa subunit of cytochrome aa3.
Genomic DNA was isolated from H. volcanii strain WFD on the basis of the method of Marmur (17). Two oligonucleotide primers were synthesized: a sense primer (TCTGGTTCTTCGG[G/C]CA[C/T]CC) based on FWFFGHP, which is a highly conserved region of the cytochrome oxidase subunit I (COXI) gene, and an antisense primer (GGCGT[G/C]GT[G/C]AG[G/T/C]CGGAT[C/T]TT) based on the partial amino acid sequence of the 44-kDa subunit. PCR was carried out with EXtaq DNA polymerase (Takara) under the following conditions: 1 cycle of denaturation at 96°C for 5 min; 30 cycles of 96°C for 1 min, 56°C for 1 min, and 72°C for 1 min; and finally 1 cycle of 72°C for 10 min. PCR products were separated in 1.5% agarose and extracted by electroelution. Recovered DNA fragments were treated with Klenow fragment and then with T4 polynucleotide kinase. Blunt-ended DNA fragments were ligated into pUC18 vector cleaved with SmaI.
KpnI-digested genomic DNA was ligated into pUC18 and transformed into Escherichia coli JM109. Colony hybridization was carried out with a 32P-radiolabeled probe obtained by PCR (12). Membranes were prehybridized with 5× Denhardt’s solution-1× SSPE (0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-0.1% SDS-0.1 mg of salmon sperm DNA per ml and hybridized with the same buffer plus radiolabeled probe overnight at 40°C. Membranes were washed at 65°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 30 min. Two colonies were selected from around 3,000 colonies. The rest of the open reading frame was cloned with a SalI-digested genomic DNA library and a probe corresponding to the KpnI-SalI fragment of the cloned DNA isolated as described above. Clones were isolated by colony hybridization as described above. The DNA sequences of the plasmids were determined for both strands by the dideoxy termination method using an Alfred sequence analyzer (Amersham Pharmacia).
Reagents.
Restriction enzymes, competent cells, and plasmid vectors were purchased from Takara Shuzo Co., Ltd. (Kyoto, Japan). Other reagents were obtained from Sigma Chemical Company (St. Louis, Mo.) and Wako Chemicals (Osaka, Japan).
RESULTS
Occurrence of cytochromes in H. volcanii.
Absorption difference spectra of plasma membranes from H. volcanii reduced by ascorbate plus TMPD or dithionite showed two peaks at around 607 and 560 nm, which suggests the presence of a-type and b- or o-type cytochromes. A small shoulder at around 552 nm suggested the presence of a c-type cytochrome. Cytochrome redox difference spectra were more intense in membranes of H. volcanii harvested during exponential growth than in membranes of cells harvested during the stationary growth phase or under low aeration (data not shown).
Purification of a-type cytochrome.
a-type cytochrome was purified from aerated cultures of H. volcanii. Peripheral proteins from the crude membranes were removed with the low-solubilizing detergent Tween 20, since cytochrome oxidases are highly hydrophobic and tightly bound to membranes. Subsequent treatment of Tween 20-washed membranes with deoxycholate resulted in complete solubilization and fourfold purification of a-type cytochrome with no loss of enzyme activity. No further purification was achieved by salting out with ammonium sulfate, most likely because of the already high salt concentration of the solubilized protein. Triton X-100 gradient elution and stepwise detergent substitution elution (19) were equally efficient for purification of cytochromes by phenyl-Sepharose CL-4B hydrophobic chromatography. In the second chromatographic step, cytochrome was eluted from DEAE-Toyopearl 650 M with 0.18 M NaCl. Subsequent gel filtration chromatography of the cytochrome fraction on Superdex 200pg resulted in purification to apparent homogeneity as determined by SDS-PAGE and coelution of 44- and 35-kDa polypeptides (data not shown). Although these two polypeptides tended to aggregate, the enzymatic activity was retained at 4°C for at least 1 week.
N-terminal amino acid sequence analysis of the 44-kDa subunit was unsuccessful, which suggests that the N-terminal amino acid residue was blocked. After lysylendopeptidase digestion, the N terminus of one fragment was determined to be IRLTTPMF, which is related to the intermembrane region between helix VIII and helix IX of Halobacterium halobium COXI (8).
Spectral properties of H. volcanii a-type cytochrome.
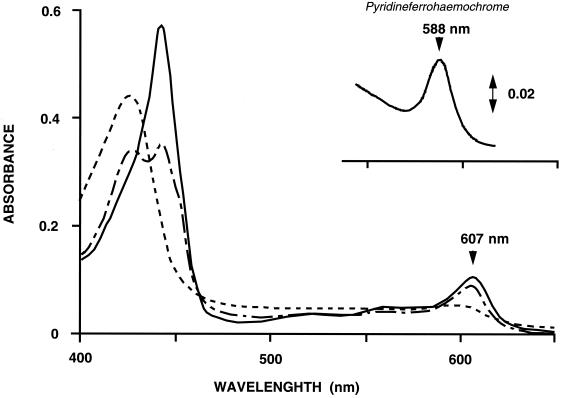
Pyridine ferrohemeochrome of the purified cytochrome showed absorption peaks at 432 and 588 nm, confirming that the cytochrome carries heme a as the prosthetic group (Fig. 1, inset). H. volcanii a-type cytochrome showed absorption peaks at 427 and 598 nm in the oxidized form and at 442 and 607 nm in the reduced form (Fig. 1). The redox difference spectrum of the enzyme appeared to be unchanged after solubilization. Addition of 5 mM KCN to the oxidized enzyme caused an increase and shift from 427 to 429 nm in the absorbance peak (data not shown). The difference spectrum of oxidized a-type cytochrome with or without 5 mM KCN showed peaks at 442 and 614 nm and a trough at 408 nm (data not shown). Upon subsequent addition of Na2S2O4 to the cyanide-enzyme complex, absorption peaks appeared at 427, 442, and 607 nm (Fig. 1). The reduced enzyme in the presence of 5 mM KCN showed absorption peaks at 442 and 607 nm and an additional peak at 587 nm under anaerobic conditions. These spectral features are similar to those of Halobacterium salinarum (9) and indicate that the cytochrome purified from H. volcanii is also an aa3-type cytochrome. However, unlike for Halobacterium cytochrome aa3, a broad peak was observed at 830 nm in the resting condition (data not shown), suggesting that the cytochrome contains copper atoms.
FIG. 1.
Absorption spectra of H. volcanii oxidized (---) and dithionite-reduced (—) cytochrome aa3. The oxidized cytochrome fraction was supplemented with KCN and then with dithionite (---). The inset shows the pyridine hemeochrome spectrum of the purified cytochrome. The cytochrome fraction was dialyzed in distilled water; treated with 200 mM NaOH, 20% pyridine, and 0.3 mM K3Fe(CN)6; and then supplemented with solid sodium dithionite.
Partial purification of cytochrome c from H. volcanii.
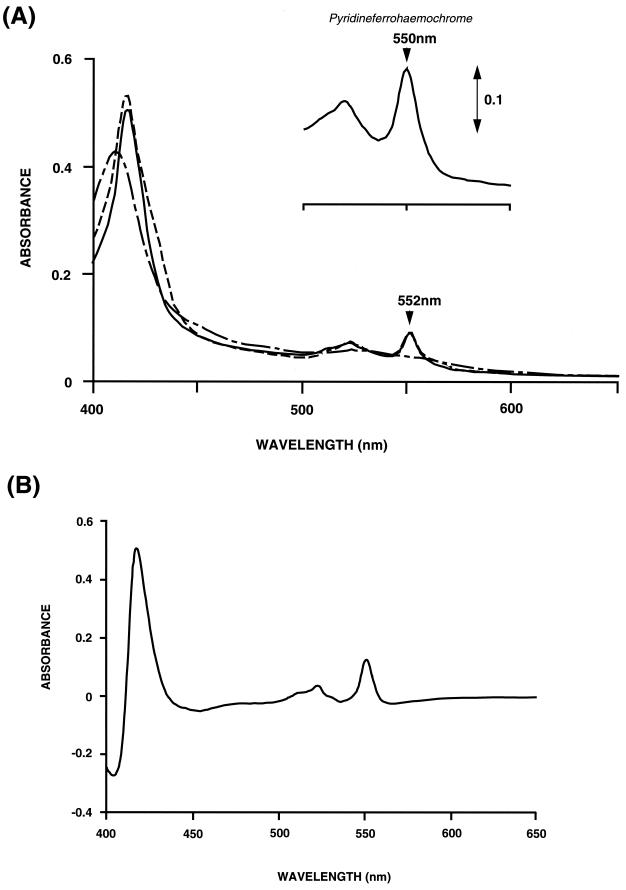
The redox difference spectrum of the crude membrane showed a small shoulder at 552 nm. This shoulder was still present in the Tween 20-washed membranes. Putative cytochrome c of H. volcanii was partially purified by chromatography with DEAE-Toyopearl 650 M. The pyridine ferrohemeochrome of the resulting fraction had a peak at 550 nm, which is typical for cytochrome c (Fig. 2A, inset). The reduced form of the cytochrome c fraction showed absorbance at 416, 522, and 552 nm (Fig. 2A). Differential spectra of the cytochrome with or without TMPD plus ascorbate showed peaks at 418, 522, and 552 nm and a trough at 404 nm (Fig. 2B). The spectrum of the fraction reduced with dithionite showed small shoulders at 430 and 560 nm, which were possibly due to contaminating b-type cytochrome. These shoulders disappeared when the cytochrome fraction was reduced with TMPD plus ascorbate. These results indicate that cytochrome c was contained predominantly in the membrane fraction. The partially purified cytochrome c was stable at 4°C for more than 1 week.
FIG. 2.
Absorption spectra of H. volcanii cytochrome c. (A) The cytochrome fraction was diluted in buffer C and reduced with TMPD plus ascorbate (—) or sodium dithionite (---) or oxidized with air (---). The inset shows the pyridine hemeochrome spectrum of cytochrome c. The cytochrome fraction was dialyzed in distilled water; treated with 200 mM NaOH, 20% pyridine, and 0.3 mM K3Fe(CN)6; and then supplemented with solid sodium dithionite. (B) TMPD-ascorbate reduced-minus-oxidized difference spectrum of cytochrome c.
Oxidase activity of cytochrome aa3.
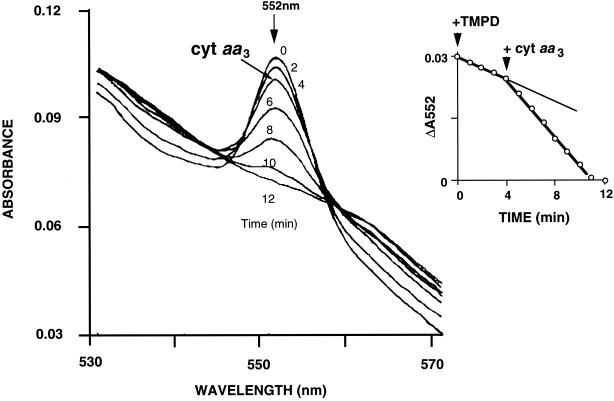
Changes in absorbance of partially purified cytochrome c were monitored spectrophotometrically between 530 and 570 nm at fixed time intervals after addition of equimolar TMPD (Fig. 3). The peak absorbance at 552 nm of the reduced cytochrome c spontaneously and continuously decreased, possibly due to auto-oxidation. Upon addition of purified cytochrome aa3, the change in absorbance at 552 nm increased and remained linear until the measured absorbance was no longer detectable (Fig. 3, inset). Cytochrome aa3-catalyzed oxidation of reduced partially purified cytochrome c was reproducibly observed.
FIG. 3.
Oxidation of cytochrome c by cytochrome (cyt) aa3 from H. volcanii. Reduced cytochrome c (30 μM) was prepared by addition of equimolar TMPD and scanned spectrophotometrically at 2-min intervals. Cytochrome aa3 was added to the reaction mixture at 4 min. The inset shows the time course of oxidation of cytochrome c monitored by measurement of peak absorbance at 552 nm.
Cloning of 44-kDa subunit gene in H. volcanii.
Using the degenerate PCR product, we cloned the entire open reading frame of the 44-kDa subunit gene. The genomic sequence and PCR fragment sequence (nucleotides 860 to 1225) are identical, and the predicted polypeptide sequence corresponds to the partial amino acid sequence determined from a lysylendopeptidase-digested peptide fragment (GenBank accession number AF334819). The cytochrome aa3 44-kDa subunit gene encoded a protein of 586 amino acid residues with a calculated molecular mass of 64,308 Da, which is larger than the 44-kDa subunit based on SDS-PAGE. Like COXI of Halobacterium salinarum, the H. volcanii 44-kDa subunit is homologous with human (41% identity), maize (45% identity), and E. coli (36% identity) COXI. Moreover, the H. volcanii 44-kDa subunit is 69% identical with Halobacterium salinarum COXI. The six highly conserved histidine residues that serve binding to heme a, heme a3, and CuB (13) are also found in the H. volcanii 44-kDa subunit. The hydropathy profile suggests that the subunit has 12 transmembrane segments, which resemble those in members of the heme-copper oxidase superfamily. A short hydrophobic segment at the N terminus of the H. volcanii 44-kDa subunit is likely to serve as a signal sequence (data not shown).
DISCUSSION
Cytochrome c as an electron carrier.
c-type cytochromes of extreme halophiles have been characterized spectroscopically in whole cell membranes or partially purified membrane fractions of Halobacterium salinarum (7, 14, 23). These crude preparations also contained b-type cytochrome; thus, it is not clear whether c-type cytochrome is a terminal electron donor for a terminal oxidase or a component of the cytochrome bc1 complex. In the present study, the partially purified Haloferax cytochrome c fraction contained a very low level of b-type cytochrome, demonstrating that it is not a component of a respiratory complex such as cytochrome bc1 but is instead an independent electron carrier. Reduced cytochrome c of H. volcanii had absorbance peaks at 416, 522, and 552 nm, which are similar to those of cytochrome c from Bacteria and Eucarya. Remarkably, cytochrome c did not seem to be a peripheral component like mitochondrial or bacterial cytochrome c, since the protein still resided in the membrane fraction even after removal of peripheral proteins with Tween 20. The extremely saline halobacterial environment may require integral association of cytochrome c with the membrane of H. volcanii to prevent detachment. Moreover, reduced cytochrome c was oxidized upon addition of purified cytochrome aa3, indicating that H. volcanii cytochrome c transferred an electron to purified H. volcanii cytochrome aa3. Therefore, we conclude that the electron transport system in extremely halophilic archaea uses a cytochrome c as an independent electron donor for cytochrome aa3.
Cytochrome aa3 in H. volcanii.
A 40-kDa carbon monoxide binding cytochrome aa3 was previously purified from Halobacterium halobium membranes. Halobacterium halobium cytochrome aa3 consisted of a single polypeptide that is homologous with COXI but contains no copper atoms, based on near-infrared spectroscopy (9). In Haloferax, another genus of the Halobacteriales family, an isolated membrane-bound cytochrome showed absorption spectra that are characteristic of aa3-type cytochromes. Unlike Halobacterium cytochrome aa3, H. volcanii cytochrome aa3 consists of a 44-kDa subunit and a 35-kDa subunit as evidenced by coelution of these two proteins from the Superdex 200pg gel filtration column. The multisubunit structure that we detected is more likely to be the structure of halophilic cytochrome oxidase, and like most cytochrome oxidases, these isozymes are also likely to contain copper. Thus, in earlier studies describing cytochrome oxidases consisting of a single polypeptide, a subunit was probably lost during the purification process, resulting in loss of endogenous copper and catalytic activity.
We showed that addition of purified cytochrome aa3 accelerated oxidation of cytochrome c, suggesting that cytochrome aa3 is a cytochrome c oxidase. However, it is also possible that some other molecule may mediate this redox reaction, since we were not using pure, homogeneous cytochrome c preparations. The redox reaction between cytochrome aa3 and cytochrome c is not likely to involve b-type cytochrome, which was a contaminant in the cytochrome c preparation, since the b-type cytochrome was not reduced by TMPD (Fig. 2A). A small blue copper protein, which is a possible electron donor for cytochrome ba3 in N. pharaonis, was not detected in our cytochrome c preparation as evidenced by the absence of an absorbance peak at around 600 nm in the redox difference spectrum (4). Thus, these results supports direct electron transfer from cytochrome aa3 to cytochrome c in halophilic archaea.
The salt concentration appears to greatly influence solubilized halophilic cytochrome c oxidase activity. For example, Halobacterium salinarum cytochrome oxidase activity assayed with bovine cytochrome c greatly increases with decreasing NaCl concentration (23). Moreover, H. volcanii cytochrome aa3 stored in buffer containing 0.18 M NaCl retains high cytochrome oxidase specific activity for at least 1 week at 4°C. The increased activity and stability of halophilic cytochrome oxidase seen with low NaCl concentrations seems counterintuitive based on the extremely saline conditions (3 M NaCl) under which halophilic organisms thrive. However, halophilic cytochrome oxidases are membrane bound in the native state, and therefore the behavior of these proteins under saline conditions was probably altered by detergent solubilization.
Amino acid sequence comparisons indicate that the 44-kDa subunit of H. volcanii cytochrome aa3 is homologous with subunit I of the COXI superfamily of heme-copper oxidases. The COXI of H. volcanii cytochrome aa3 is very similar to that of Halobacterium salinarum cytochrome aa3, indicating that these proteins belong to the same phylogenetic branch. Since cytochrome c is oxidized by both H. volcanii and Halobacterium salinarum cytochromes aa3, these proteins are likely to function as terminal oxidases that catalyze transfer of electrons from cytochrome c.
REFERENCES
- 1.Anemuller, S., and G. Schafer. 1990. Cytochrome aa3 from Sulfolobus acidocaldarius. A single-subunit, quinol-oxidizing archaebacterial terminal oxidase. Eur. J. Biochem. 191:297–305. [DOI] [PubMed] [Google Scholar]
- 2.Anemuller, S. C. L., I. Pacheco, G. Schafer, and M. Teixeira. 1994. A cytochrome aa3-type quinol oxidase from Desulfurolobus ambivalens, the most acidophilic archaeon. FEMS Microbiol. Lett. 117:275–280. [Google Scholar]
- 3.Berry, E. A., and B. L. Trumpower. 1987. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161:1–15. [DOI] [PubMed] [Google Scholar]
- 4.Brischwein, M., B. Scharf, M. Engelhard, and W. Mantele. 1993. Analysis of the redox reaction of an archaebacterial copper protein, halocyanin, by electrochemistry and FTIR difference spectroscopy. Biochemistry 32:13710–13717. [DOI] [PubMed] [Google Scholar]
- 5.Castresana, J., M. Lubben, and M. Saraste. 1995. New archaebacterial genes coding for redox proteins: implications for the evolution of aerobic metabolism. J. Mol. Biol. 250:202–210. [DOI] [PubMed] [Google Scholar]
- 6.Charlebois, R. L., J. D. Hofman, L. C. Schalkwyk, W. L. Lam, and W. F. Doolittle. 1989. Genome mapping in halobacteria. Can. J. Microbiol. 35:21–29. [DOI] [PubMed] [Google Scholar]
- 7.Cheah, K. S. 1970. The membrane-bound carbon monoxide-reactive hemoproteins in the extreme halophiles. Biochim. Biophys. Acta 197:84–86. [DOI] [PubMed] [Google Scholar]
- 8.Denda, K., T. Fujiwara, M. Seki, M. Yoshida, Y. Fukumori, and T. Yamanaka. 1991. Molecular cloning of the cytochrome aa3 gene from the archaeon (Archaebacterium) Halobacterium halobium. Biochem. Biophys. Res. Commun. 181:316–322. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara, T., Y. Fukumori, and T. Yamanaka. 1989. Purification and properties of Halobacterium halobium “cytochrome aa3 ” which lacks CuA and CuB. J. Biochem (Tokyo) 105:287–292. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara, T., Y. Fukumori, and T. Yamanaka. 1993. Halobacterium halobium cytochrome b-558 and cytochrome b-562: purification and some properties. J. Biochem. (Tokyo) 113:48–54. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, T., Y. Fukumori, and T. Yamanaka. 1987. aa3-type cytochrome c oxidase occurs in Halobacterium halobium and its activity is inhibited by higher concentrations of salts. Plant Cell Physiol. 28:29–36. [Google Scholar]
- 12.Grunstein, M., and D. S. Hogness. 1975. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc. Natl. Acad. Sci. USA 72:3961–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm, L., M. Saraste, and M. Wikstrom. 1987. Structural models of the redox centres in cytochrome oxidase. EMBO J. 6:2819–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanyi, J. K. 1968. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch. Biochem. Biophys. 128:716–724. [DOI] [PubMed] [Google Scholar]
- 15.Lubben, M., S. Arnaud, J. Castresana, A. Warne, S. P. Albracht, and M. Saraste. 1994. A second terminal oxidase in Sulfolobus acidocaldarius. Eur. J. Biochem. 224:151–159. [DOI] [PubMed] [Google Scholar]
- 16.Lubben, M., B. Kolmerer, and M. Saraste. 1992. An archaebacterial terminal oxidase combines core structures of two mitochondrial respiratory complexes. EMBO J. 11:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from bacteria. J. Mol. Biol. 3:208–218. [Google Scholar]
- 18.Mattar, S., and M. Engelhard. 1997. Cytochrome ba3 from Natronobacterium pharaonis—an archaeal four-subunit cytochrome-c-type oxidase. Eur. J. Biochem. 250:332–341. [DOI] [PubMed] [Google Scholar]
- 19.Nagasawa, T., H. Nagasawa-Fujimori, and P. C. Heinrich. 1979. Hydrophobic interactions of cytochrome c oxidase. Application to the purification of the enzyme from rat liver mitochondria. Eur. J. Biochem. 94:31–39. [DOI] [PubMed] [Google Scholar]
- 20.Purschke, W. G., C. L. Schmidt, A. Petersen, and G. Schafer. 1997. The terminal quinol oxidase of the hyperthermophilic archaeon Acidianus ambivalens exhibits a novel subunit structure and gene organization. J. Bacteriol. 179:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharf, B., and M. Engelhard. 1993. Halocyanin, an archaebacterial blue copper protein (type I) from Natronobacterium pharaonis. Biochemistry 32:12894–12900. [DOI] [PubMed] [Google Scholar]
- 22.Scharf, B., R. Wittenberg, and M. Engelhard. 1997. Electron transfer proteins from the haloalkaliphilic archaeon Natronobacterium pharaonis: possible components of the respiratory chain include cytochrome bc and a terminal oxidase cytochrome ba3. Biochemistry 36:4471–4479. [DOI] [PubMed] [Google Scholar]
- 23.Sreeramulu, K., C. L. Schmidt, G. Schafer, and S. Anemuller. 1998. Studies of the electron transport chain of the euryarcheon Halobacterium salinarum: indications for a type II NADH dehydrogenase and a complex III analog. J. Bioenerg. Biomembr. 30:443–453. [DOI] [PubMed] [Google Scholar]
- 24.Wakagi, T., T. Yamauchi, T. Oshima, M. Muller, A. Azzi, and N. Sone. 1989. A novel a-type terminal oxidase from Sulfolobus acidocaldarius with cytochrome c oxidase activity. Biochem. Biophys. Res. Commun. 165:1110–1114. [DOI] [PubMed] [Google Scholar]