Abstract
A strain devoid of the three hydrogenases characterized for Desulfovibrio fructosovorans was constructed using marker exchange mutagenesis. As expected, the H2-dependent methyl viologen reduction activity of the strain was null, but physiological studies showed no striking differences between the mutated and wild-type strains. The H+-D2 exchange activity measured in the mutated strain indicates the presence of a fourth hydrogenase in D. fructosovorans.
Molecular hydrogen plays an important role in the energy-generating metabolism of sulfate reducers belonging to the genus Desulfovibrio. Desulfovibrio species can alternatively utilize hydrogen as the sole source of electron and energy (2, 3) or can produce hydrogen when growing fermentatively on a suitable carbon source in the absence of sulfate as an electron acceptor (17). Furthermore, hydrogen is successively produced and consumed during the degradation of organic compounds in the presence of sulfate (7, 22, 23). It has not yet been established whether hydrogen plays only a role in the regulation of the redox state of the electron transfer chains (11) or a central role as a key intermediate in the electron transfer across the membrane (14, 15). Hydrogenases are the key enzymes of energy-generating metabolism because of their ability to catalyze the splitting or the synthesis of molecular hydrogen. The number (generally more than one), the type ([Fe], [NiFe], or [NiFeSe] on the basis of their metal contents), and the cellular location of hydrogenases vary considerably from one Desulfovibrio species to another (6, 25). This diversity makes the role of these various hydrogenases difficult to determine.
With the aim to study the role of hydrogenases in Desulfovibrio, we chose D. fructosovorans DSM 3604 (16) as a model. In this species, three hydrogenases have been already characterized: a periplasmic [NiFe] hydrogenase which represents about 1% of the total proteins (8, 20), a cytoplasmic NADP-reducing hydrogenase (13), and a periplasmic [Fe] hydrogenase (4). In order to elucidate the relative importance of these various hydrogenases in the energy-generating metabolism of D. fructosovorans, deletions were first made by marker exchange mutagenesis of the genes encoding the [NiFe] hydrogenase (19) and the NADP-reducing hydrogenase (12). All mutants (single or double) showed significant growth on organic substrates as well as on medium containing H2 as the sole energy source.
Construction and molecular characterization of a triple mutant depleted of all three hydrogenases.
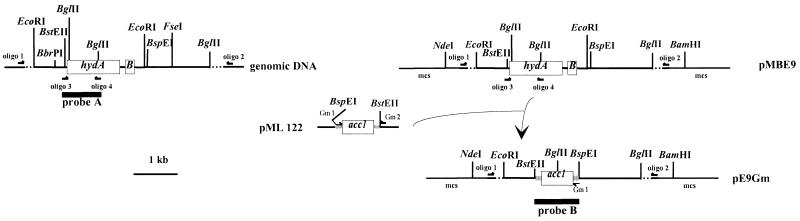
In order to perform the marker exchange experiment, a 5-kb fragment containing the two structural genes (hydAB) coding for the [Fe] hydrogenase of D. fructosovorans (obtained by PCR amplification performed on genomic DNA by using oligonucleotides 1 [5′-AAACGGCGACGCCGTGGTCGGCAAGGTCAA-3′] and 2 [5′-CGATGTCGGTGCCCGGATATTT-3′]) was cloned in pMosblue-T-vector (Amersham) to give the recombinant plasmid pMBE9 (Fig. 1). A 1.3-kb fragment containing the gentamicin resistance gene (acc1) was obtained by PCR amplification performed on pML122 (10), using two oligonucleotides, one introducing a BspEI restriction site (in boldface) (Gm 1, 5′-TTAAATCCGGATGAAGGCACGAACCCAGTT-3′) and one located downstream from the BstEII restriction site (Gm 2, 5′-GACGCTTAGCACCTCTGATAGTT-3′). The amplification product was digested with BstEII and BspEI and cloned into pMBE9 with a deletion of the BstEII-BspEI fragment containing hydAB, to give pE9Gm (Fig. 1). This recombinant suicide plasmid was introduced into the DM4 strain (ΔhynABC Kanr; ΔhndD Cmr) (12) depleted of the [NiFe] and NADP-reducing hydrogenases, using an electrotransformation procedure as previously described (19). The electrotransformed cells were first grown for 6 h without any antibiotics and then subcultured in the presence of antibiotics (50 μg of kanamycin ml−1, 34 μg of thiamphenicol ml−1, and 20 μg of gentamicin ml−1) in liquid medium. The start of growth was observed within 2 weeks. Afterwards, recombinant cultures were isolated on plates under the anaerobic atmosphere of a glove box (N2-H2, 95:5 [vol/vol]) and incubated in hyperbaric (2 × 105 Pa) (N2-CO2, 80:20 [vol/vol]) anaerobic jars. The genotype of the isolated gentamicin-resistant strain, called TM4, was then analyzed by Southern blot hybridization.
FIG. 1.
Replacement of the hydAB genes by the acc1 gene encoding gentamicin resistance. Oligonucleotides (oligo) 1 and 2 were used for pMBE9 construction. Oligonucleotides 3, 4, Gm 1, and Gm 2 were used to obtain the probes for the Southern blot hybridizations. pE9Gm was used to perform electrotransformation and mutant construction by marker exchange. mcs, multiple cloning site.
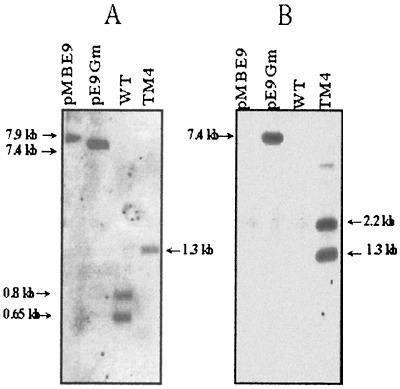
The blot of total DNA digested with EcoRI and BglII was first hybridized with a DNA fragment encompassing the majority of the hydA gene (probe A) (Fig. 2A). After removal of the probe, the blot was subsequently hybridized with the acc1 gene (probe B) (Fig. 2B). The pMBE9 and pE9Gm plasmids digested with BspEI (unique restriction site) were used as controls with the two probes. Restriction analysis of the region containing the wild-type hydAB genes revealed the existence of one EcoRI site and two BglII sites, producing three fragments (0.65, 0.8, and 1 kb) which specifically hybridized with probe A (Fig. 1). The hybridization pattern of the genomic DNA from wild-type D. fructosovorans showed the 0.8- and 0.65-kb bands (Fig. 2A). The absence of the 1-kb band can be explained by the very short sequence (52 bp) of this fragment hybridizing with probe A. In strain TM4, the replacement of hydAB by acc1 leads to the loss of one EcoRI site and one BglII site (Fig. 1). Thus, a 1.3-kb fragment should hybridize with probe A. The hybridization pattern of genomic DNA from the TM4 strain (Fig. 2A) showed that a double crossing over, rather than a single crossing over event, occurred. With probe B (Fig. 2B), two bands (1.3 and 2.2 kb) were revealed in the genomic DNA of the mutated strain TM4. Indeed, one BglII restriction site is located in the middle of the gentamicin resistance reporter gene (Fig 1). As expected, pE9Gm hybridized specifically with probe B, and neither the genomic DNA from wild-type D. fructosovorans nor the pMBE9 plasmid was detected. The results of the Southern blot experiments demonstrated that the hydAB genes were deleted and replaced by the acc1 gene. The TM4 mutant obtained is ΔhydAB Gmr in addition to the ΔhynABC Kmr and ΔhndD Tmr of the primary DM4 strain.
FIG. 2.
Southern blot hybridizations of genomic DNAs digested with EcoRI and BglII using the probe corresponding to the hydAB genes (A probe), obtained by using oligonucleotides 3 (5′-AGTATTACGTTTTGAGTGTTACGAAAT-3′) and 4 (5′-CATGCCTTCGAATTTTTTG-3′) (A), or using the probe corresponding to the acc1 gene (B probe), obtained by using oligonucleotides Gm 1 and Gm 2 (Fig. 1) (B). WT, wild type.
Methyl viologen reduction activity of the triple mutant.
Hydrogenase activity in D. fructosovorans is usually determined by measurement of hydrogen-dependent methyl viologen reduction activity in soluble cellular extract obtained from cultures grown on a 30 mM fructose–50 mM sulfate medium (4, 5, 12, 19). The presence of the hydrogenase activities in the D. fructosovorans strains was tested in native polyacrylamide gel electrophoresis (Fig. 3). None of the three hydrogenase activities which are observed in the wild-type strain were visualized in the TM4 soluble cellular extract. In addition, total methyl viologen reduction activities measured in soluble cellular extracts of different strains are given in Table 1. As expected, no hydrogenase activity was detected in the TM4 strain by using methyl viologen as a redox mediator.
FIG. 3.
Detection, by methyl viologen reduction in a native gel, of the three hydrogenase activities in the wild-type strain (WT), the triple mutant strain (TM4), and the complemented strain [TM4(pS7HF)] grown on fructose-sulfate medium. Electrophoresis was performed in a glove box under an atmosphere of N2-H2 (95:5, vol/vol). The hydrogenase activities were detected by bubbling the gel with H2 in the presence of methyl viologen and stained by adding 2,2,5-triphenyltetrazolium chloride.
TABLE 1.
Methyl viologen reduction and H+-D2 exchange activities in wild-type and mutated (DM4 and TM4) strainsa
| Strain | Methyl viologen reduction activity (U mg−1) on:
|
H+-D2 exchange activity (μmol of HD and H2 produced min−1 mg−1) on fructose-fumarate medium | |
|---|---|---|---|
| Fructose-sulfate medium | Fructose-fumarate medium | ||
| Wild type | 8 | 8.8 | 0.69 |
| DM4 | 0.15 | NDb | 0.05 |
| TM4 | 0.005 | 0.375 | 0.0634 |
Methyl viologen reduction activities were measured in soluble cellular extracts from cultures grown on fructose-sulfate or fructose-fumarate medium, and H+-D2 exchange activities were measured in whole-cell suspensions obtained from cultures grown on fructose-fumarate medium.
ND, not determined.
Complementation experiments were performed by cloning hydAB in shuttle vectors harboring chloramphenicol or streptomycin resistance (18). A synthetic linker (5′-AGCTTGGCCGGCCCTGCA-3′/5′-GGGCCGGCCA-3′), designed to introduce an FseI (boldface) site between the polylinker PstI (italics) and HindIII (underlined) sites, was cloned into plasmid pBMC6 digested with PstI and HindIII (18) to give the recombinant plasmid pC6Fse. A 2.6-kb BbrPI/FseI fragment from pMBE9 containing hydAB was cloned in pC6Fse digested with SmaI and FseI. This recombinant plasmid, pC6HF, was used to construct pS7HF. A 2.7-kb HindIII/Asp718 fragment of pC6HF containing hydAB was blunted using Klenow enzyme (Roche Molecular Biochemicals) and cloned into the SmaI-digested pBMS7 plasmid harboring the streptomycin resistance gene (18). Electrotransformation of the TM4 strain was performed with the replicative pS7HF plasmid, and transformant TM4(pS7HF) colonies were isolated in medium containing 50 μg of kanamycin ml−1, 34 μg of thiamphenicol ml−1, 20 μg of gentamicin ml−1, and 300 μg of streptomycin ml−1. On a native gel with soluble cellular extracts prepared from the TM4(pS7HF) strain, a unique hydrogenase activity corresponding to the [Fe] hydrogenase was revealed (Fig. 3).
Physiological studies.
Growth parameters of the wild-type and mutated strains were determined on fructose-sulfate medium as previously described (Table 2) (12). High-pressure liquid chromatography (Bio-Rad Aminex Fast Acid Analysis HPAH column) analysis of the metabolism products in the culture medium did not give any evidence of intermediate accumulation or that an alteration in the metabolism had occurred during the growth of the mutant strain compared to the wild-type strain (data not shown). Fructose was completely oxidized into acetate and CO2. The growth rate and the molar growth yield of strain TM4 were lower than those of the wild-type strain (Table 2), but surprisingly, no striking differences were observed compared to the results obtained in previous studies with the double mutant strain DM4 (12). Thus, the depletion of three hydrogenases did not have more of an effect on energy-generating metabolism than the depletion of two hydrogenases. It is possible to assume that the [Fe] hydrogenase is not necessary for growth on fructose-sulfate medium.
TABLE 2.
Growth parameters of D. fructosovorans wild-type and mutated (DM4 and TM4) strainsa
| Strain | Growth rate (h−1) | Yield relative to fructose (g mol−1) |
|---|---|---|
| Wild type | 0.05 ± 0.001 | 36 ± 2.8 |
| DM4b | 0.041 ± 0.005 | 25.6 ± 2.8 |
| TM4 | 0.037 ± 0.006 | 29.1 ± 1.5 |
Values were measured with 10, 20, and 30 mM fructose and 50 mM sulfate and are reported as means ± standard deviations.
Data are from reference 12.
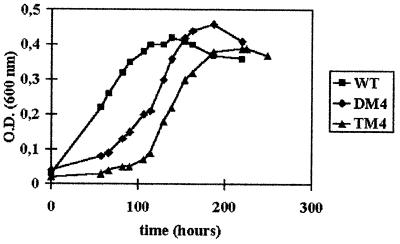
The wild-type and TM4 strains were grown mixotrophically on medium containing H2 as the sole energy source, 50 mM acetate and CO2 as carbon sources, and 20 mM sulfate as a terminal electron acceptor. Under these conditions (cultures were gassed periodically with H2-CO2 [80:20, vol/vol] at a pressure of 2 × 105 Pa), the growth was linear rather than exponential (Fig. 4), which prevented us from determining the growth rates. Surprisingly, except for a slower growth at the beginning, the kinetics of the mutated strain was quite similar to those of the wild type and DM4. In all cases, the growth stopped when sulfate was exhausted, and the molar growth yields relative to sulfate were found to be 6.17 ± 0.74 g mol−1, 6.05 ± 0.3 g mol−1, and 5.48 ± 0.73 g mol−1 for the wild-type, DM4, and TM4 strains, respectively. This growth, with H2 as the sole electron donor, can be explained only by the presence of an enzyme able to split the H2 molecule, i.e., a fourth hydrogenase.
FIG. 4.
Kinetics of mixotrophic growth on medium containing H2 as the sole energy source (H2-acetate-CO2-sulfate medium) of the wild-type (WT), double mutant (DM4), and triple mutant (TM4) strains. O.D., optical density.
Search for a remaining hydrogenase activity in the triple mutant.
The proton-deuterium (H+-D2) exchange reaction in whole-cell suspensions was measured by a mass spectrometric method (9). Cells were grown in 20 mM fructose–20 mM fumarate medium to an optical density at 600 nm of 1.3. Fumarate, rather than sulfate, was used to prevent formation of sulfur precipitates. Argon was bubbled in the reaction vessel filled with 1 ml of 50 mM Tris-HCl (pH 8) until saturation to eliminate the majority of O2, and then 0.5 ml of the culture was added to the reaction vessel and D2 was bubbled until saturation. Changes in concentrations of gases (D2, HD, H2, and O2) were automatically scanned and recorded immediately after the vessel was closed, as described previously (24). Production of H2 and HD was used to calculate the exchange activity. The results presented in Table 1 show that an exchange reaction occurred in the triple mutant strain (TM4), which was about 10-fold lower than that in the wild-type strain but equivalent to the activity measured in the double mutant strain (DM4).
As methyl viologen reduction activity was null for soluble cellular extracts of the TM4 strain grown on fructose-sulfate medium and H+-D2 exchange activity could be measured in whole cells of the same strain grown on fructose-fumarate medium (Table 1), we measured methyl viologen reduction in cultures grown on fructose-fumarate medium. Results obtained with whole-cell suspensions and soluble cellular extracts were quite similar (data not shown). Interestingly, a significant methyl viologen reduction activity was measured in the triple mutant TM4, representing 4% of the wild-type activity (Table 1). Thus, H+-D2 exchange activity and methyl viologen reduction activity in cells grown on fructose-fumarate indicated the presence of a fourth hydrogenase in D. fructosovorans. This activity was not seen in strain TM4 grown on fructose-sulfate medium. Thus, it can be assumed that the induction or the derepression of a gene encoding a fourth hydrogenase occurred when TM4 was cultivated in fructose-fumarate medium. In this medium, the metabolism of the bacteria is mostly fermentative, as the amount of exogenous fumarate (electron acceptor) is limiting. Thus, the energy-generating metabolism may be quite different than that in fructose-sulfate medium. It is possible to assume that the fermentative growth has induced the expression of one enzyme presenting an hydrogenase activity in order to dispose of an excess of reducing power. On the other hand, this hydrogenase is likely to be responsible for the growth on H2 as a sole energy source.
The presence of multiple hydrogenases in bacteria is relatively widespread. Escherichia coli possesses four hydrogenases (1). In D. vulgaris, three hydrogenases of different types have been characterized ([Fe], [NiFe], and [NiFeSe]) (6), and a gene encoding a potential fourth one has been isolated (21). Thus, it is not surprising for D. fructosovorans to possess a fourth hydrogenase activity. The enzyme responsible for the fourth hydrogenase activity may be a cryptic hydrogenase induced to compensate for the mutations. Alternatively, we cannot rule out the possibility that this enzyme might not be a typical hydrogenase but might be an enzyme exhibiting this activity under particular conditions.
Acknowledgments
We gratefully acknowledge Odile Valette for technical help, Bernard Dimon and Patrick Carrier for the mass spectrometric measurements, the Laboratoire des Champignons Filamenteux (INRA) for access to the high-pressure liquid chromatograph, and Jean-Pierre Belaïch for help and support.
REFERENCES
- 1.Andrews, S. C., B. C. Berks, J. McClay, A. Ambler, M. A. Quail, P. Golby, and J. R. Guest. 1997. A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology 143:3633–3647. [DOI] [PubMed] [Google Scholar]
- 2.Badziong, W., R. K. Thauer, and J. G. Zeikus. 1978. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch. Microbiol. 116:41–49. [DOI] [PubMed] [Google Scholar]
- 3.Brandis, A., and R. K. Thauer. 1981. Growth of Desulfovibrio species on hydrogen and sulfate as sole energy source. J. Gen. Microbiol. 126:249–252. [Google Scholar]
- 4.Casalot, L., C. E. Hatchikian, N. Forget, P. de Philip, Z. Dermoun, J. P. Bélaïch, and M. Rousset. 1998. Molecular study and partial characterization of iron-only hydrogenase in Desulfovibrio fructosovorans. Anaerobe 4:45–55. [DOI] [PubMed] [Google Scholar]
- 5.De Luca, G., P. de Philip, M. Rousset, J. P. Belaïch, and Z. Dermoun. 1998. The NADP-reducing hydrogenase of Desulfovibrio fructosovorans: evidence for a native complex with hydrogen-dependent methyl viologen-reducing activity. Biochem. Biophys. Res. Commun. 248:591–596. [DOI] [PubMed] [Google Scholar]
- 6.Fauque, G., H. D. Peck, Jr., J. J. Moura, B. H. Huynh, Y. Berlier, D. V. Der Vartanian, M. Teixeira, A. E. Przybyla, P. A. Lespinat, and I. Moura. 1988. The three classes of hydrogenases from sulfate-reducing bacteria of the genus Desulfovibrio. FEMS Microbiol. Rev. 4:299–344. [DOI] [PubMed] [Google Scholar]
- 7.Hatchikian, E. C., M. Chaigneau, and J. Le Gall. 1976. Analysis of gas production by growing culture of the three species of sulfate-reducing bacteria, p.109–118. In H. G. Schlegel, G. Gottschalk, and N. Pfenning (ed.), Microbial production and utilization of gases. Goltza, K. G., Göttingen, Germany.
- 8.Hatchikian, E. C., S. A. Traore, M. Fernandez, and R. Cammack. 1990. Characterization of the nickel-iron periplasmic hydrogenase from Desulfovibrio fructosovorans. Eur. J. Biochem. 187:635–643. [DOI] [PubMed] [Google Scholar]
- 9.Jouanneau, Y., B. C. Kelley, Y. Berlier, P. A. Lespinat, and P. Vignais. 1980. Continuous monitoring, by mass spectrometry, of H2 production and recycling in Rhodopseudomonas capsulata. J. Bacteriol. 143:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labes, M., A. Pühler, and R. Simon. 1990. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for Gram-negative bacteria. Gene 89:37–46. [DOI] [PubMed] [Google Scholar]
- 11.Lupton, F. S., R. Conrad, and J. G. Zeikus. 1984. Physiological function of hydrogen metabolism during growth of sulfidogenic bacteria on organic substrates. J. Bacteriol. 159:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malki, S., G. De Luca, M. L. Fardeau, M. Rousset, J. P. Bélaïch, and Z. Dermoun. 1997. Physiological characteristics and growth behavior of single and double hydrogenase mutants of Desulfovibrio fructosovorans. Arch. Microbiol. 167:38–45. [DOI] [PubMed] [Google Scholar]
- 13.Malki, S., I. Saimmaime, G. De Luca, M. Rousset, Z. Dermoun, and J. P. Bélaïch. 1995. Characterization of an operon encoding an NADP-reducing hydrogenase in Desulfovibrio fructosovorans. J. Bacteriol. 177:2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguera, D. R., G. A. Brusseau, B. E. Rittmann, and D. A. Stahl. 1998. A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol. Bioeng. 59:733–746. [DOI] [PubMed] [Google Scholar]
- 15.Odom, J. R., and H. D. Peck, Jr. 1981. Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria Desulfovibrio sp. FEMS Microbiol. Lett. 12:47–50. [Google Scholar]
- 16.Ollivier, B., R. Cord-Ruwisch, E. C. Hatchikian, and J. L. Garcia. 1988. Characterization of Desulfovibrio fructosovorans sp. nov. Arch. Microbiol. 150:26–31. [Google Scholar]
- 17.Postgate, J. R. 1952. Growth of sulfate-reducing bacteria in sulfate-free media. Research (London) 5:189–190. [Google Scholar]
- 18.Rousset, M., L. Casalot, B. J. Rapp-Giles, Z. Dermoun, P. de Philip, J. P. Bélaïch, and J. D. Wall. 1998. New shuttle vectors for the introduction of cloned DNA in Desulfovibrio. Plasmid 39:114–122. [DOI] [PubMed] [Google Scholar]
- 19.Rousset, M., Z. Dermoun, M. Chippaux, and J. P. Bélaïch. 1991. Marker exchange mutagenesis of the hydN genes in Desulfovibrio fructosovorans. Mol. Microbiol. 5:1735–1740. [DOI] [PubMed] [Google Scholar]
- 20.Rousset, M., Z. Dermoun, C. E. Hatchikian, and J. P. Bélaïch. 1990. Cloning and sequencing of the locus encoding the large and small subunit genes of the periplasmic [NiFe] hydrogenase from Desulfovibrio fructosovorans. Gene 94:95–101. [DOI] [PubMed] [Google Scholar]
- 21.Stokkerman, J., W. van Dongen, A. Kaaa, W. van den Berg, and C. Veeger. 1989. hydγ, a gene from Desulfovibrio vulgaris (Hildenborough), encodes a polypeptide homologous to the periplasmic hydrogenase. FEMS Microbiol. Lett. 49:217–222. [DOI] [PubMed] [Google Scholar]
- 22.Traore, A. S., C. E. Hatchikian, J. P. Bélaïch, and J. Le Gall. 1981. Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. J. Bacteriol. 154:101–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji, K., and T. Yagi. 1980. Significance of hydrogen burst from growing cultures of Desulfovibrio vulgaris Miyazaki and the role of hydrogenase and cytochrome c3 in energy production system. Arch. Microbiol. 125:35–42. [Google Scholar]
- 24.Vignais, P. M., B. Dimon, N. A. Zorin, A. Colbeau, and S. Elsen. 1997. HupUV proteins of Rhodobacter capsulatus can bind H2: evidence from the H-D exchange reaction. J. Bacteriol. 179:290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voordouw, G., V. Nivière, F. G. Ferris, P. M. Fedorak, and D. W. S. Westlake. 1990. Distribution of hydrogenase genes in Desulfovibrio spp. and their use in the identification of species from the oil field environment. Appl. Environ. Microbiol. 56:3748–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]