Abstract
A gene encoding a cyclodextrin glucanotransferase (CGTase) from Thermococcus kodakaraensis KOD1 (CGTTk) was identified and characterized. The gene (cgtTk) encoded a protein of 713 amino acid residues harboring the four conserved regions found in all members of the α-amylase family. However, the C-terminal domain corresponding to domain E of previously known CGTases displayed a completely distinct primary structure. In order to elucidate the catalytic function of the gene product, the recombinant enzyme was purified by anion-exchange chromatography, and its enzymatic properties were investigated. The enzyme displayed significant starch-degrading activity (750 U/mg of protein) with an optimal temperature and pH of 80°C and 5.5 to 6.0, respectively. The presence of Ca2+ enhanced the enzyme activity and elevated the optimum temperature to 85 to 90°C. With the addition of Ca2+, the enzyme showed extreme thermostability, with almost no loss of enzymatic activity after 80 min at 85°C, and a half-life of 20 min at 100°C. CGTTk could hydrolyze soluble starch and glycogen but failed to hydrolyze pullulan. Most importantly, although CGTTk harbored a unique C-terminal domain, we found that the protein also exhibited significant CGTase activity, with β-cyclodextrin as the main product. In order to identify the involvement, if any, of the C-terminal region in the CGTase activity, we analyzed a truncated protein (CGTTkΔC) with 23 C-terminal amino acid residues deleted. CGTTkΔC displayed similar properties in terms of starch-binding activity, substrate specificity, and thermostability, but unexpectedly showed higher starch-degrading activity than the parental CGTTk. In contrast, the cyclization activity of CGTTkΔC was abolished. The results indicate that the presence of the structurally novel C-terminal domain is essential for CGTTk to properly catalyze the cyclization reaction.
Cyclodextrin glucanotransferases (CGTases, EC 2.4.1.19) are starch-degrading enzymes which mainly convert starch into cyclodextrins (CDs). The CDs produced by various CGTases have been found mainly to consist of six, seven, or eight α-(1→4)-linked d-glucopyranosyl units, named α-, β-, and γ-cyclodextrin, respectively. CGTases also catalyze three other reactions: (i) disproportionation, the transfer of part of a linear oligosaccharide to another oligosaccharide; (ii) coupling, the opening of a CD molecule followed by transfer to a linear oligosaccharide; and (iii) hydrolysis, the transfer of part of a linear oligosaccharide to a water molecule (24).
CGTases are members of the family 13 glycosyl hydrolases (6). Enzymes of the α-amylase family (12, 22) display a variety of reaction specificities and include isoamylase, pullulanase, amylopullulanase, neopullulanase, and branching enzyme, along with α-amylase and CGTase. We have previously proposed that these enzymes utilize the same basic mechanism for catalysis, and we have identified four regions conserved among all members (12, 15). The reactions proceed with the retention of the anomeric configuration of the substrate, supposedly through a double displacement reaction (24). At present, extensive research is in progress in order to identify the structural characteristics that lead to the distinct specificities of each enzyme.
Primary and three-dimensional structural comparisons between CGTases and α-amylases have revealed both common and distinct features among the enzymes. A comprehensive review has recently been published by Dijkhuizen and coworkers (24). Both CGTases and α-amylases share three structural domains: A, B, and C. The A domain is the catalytic domain and comprises a (β/α)8, or TIM (triosephosphate isomerase), barrel (7). The proton donor Glu257 and the bases Asp229 and Asp328 (numbering by CGTase from Bacillus circulans strain 251) are located in this domain. Domains B and C are considered to be involved in substrate binding. CGTases have two additional domains not found in α-amylases, domains D and E. The function of domain D is unknown at present, while domain E has been found to be a starch-binding domain. Two maltose-binding sites have been identified in the E domain of CGTase from B. circulans strain 251, and evidence that this domain contributes to raw-starch binding has also been obtained for other enzymes (19). Although it remains to be clarified whether the effects are direct or a consequence of indirect structural distortion, truncation of the C-terminal region of CGTase from Bacillus sp. strain 1011 led to a change in reaction specificities (9), suggesting a possible role of the C-terminal region in CGTase activity.
We have previously characterized the CGTase from Bacillus stearothermophilus (2) and the first archaeal CGTase (20) and cyclomaltodextrinase (CDase) (4) from Thermococcus sp. strain B1001, the only organism reported to possess both CGTase and CDase (4). In the present study, we have examined a CGTase from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 (CGTTk), which is quite distinct in its primary structure, particularly in the C-terminal region, from previously characterized CGTases.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacteriophages.
T. kodakaraensis KOD1 was isolated from a solfataric hot spring at a wharf in Kodakara Island, Kagoshima, Japan (14). Escherichia coli strain DH5α was used for subcloning of the gene fragments and DNA manipulations. E. coli strain BL21(DE3) (Novagen, Madison, Wis.) was used as a host, and the pET-8c vector (Novagen) was used for expression of the gene. Bacteriophage EMBL4 was used for preparation of single-stranded DNA and plaque hybridization.
DNA manipulation.
Restriction enzymes and DNA polymerase were purchased from Toyobo (Osaka, Japan) as well as Takara Shuzo (Kyoto, Japan). Each enzyme was used according to the recommendations of the manufacturer. DNA ligations were performed using a DNA ligation kit (Toyobo). Genomic DNA, plasmid DNA, and phage DNA were isolated using Qiagen (Hilden, Germany) Genomic, Plasmid, and Lambda DNA isolation kits, respectively. A DNA purification kit (Toyobo) was used to recover DNA fragments from agarose gels.
Isolation and characterization of the cgtTk gene.
A 1.2-kbp HindIII DNA fragment displaying similarity to α-amylases and CGTases was used as a probe to isolate cgtTk. Probes were constructed using a DIG DNA labeling kit (Boehringer, Mannheim, Germany). DNA sequencing was performed using an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer, Foster City, Calif.). Nucleotide and deduced amino acid sequence analyses and database homology searches were performed using the Basic Local Alignment Search Tool (BLAST) program. Open reading frame searches, molecular weight calculations, and isoelectric point calculations were performed using DNASIS software (Hitachi Software, Yokohama, Japan). Multiple alignment and phylogenetic analyses were performed using the CLUSTAL W program provided by the DNA Data Bank of Japan (DDBJ).
Expression of the cgtTk gene in E. coli.
The cgtTk gene was amplified by PCR. NcoI and BamHI restriction enzyme sites were introduced at the N- and C-terminal regions, respectively. For expression of the cgtTk gene in E. coli, the PCR-amplified DNA fragment was inserted into the pET-8c expression vector (Novagen) at the NcoI and BamHI sites, and the resulting plasmid was designated pET-cgt. E. coli strain BL21(DE3) carrying the pET-cgt plasmid was grown overnight at 37°C in NZCYM medium (NZ amine, 1%; yeast extract, 0.5%; NaCl, 0.5%; Casamino Acids, 0.1%; MgSO4 · 7H2O, 0.2% [pH 7.0]) containing ampicillin (50 μg/ml). The culture was inoculated (1%) into fresh NZCYM medium containing ampicillin, and cultivation was continued until an optical density of 0.4 at 660 nm was reached. The culture was then supplemented with 1 mM (final concentration) isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated for another 4 h at 37°C for expression of the cgtTk gene. Cells were harvested by centrifugation at 6,000 × g for 10 min and washed with 50 mM Tris-HCl buffer (pH 8.0). The cell pellet was resuspended in the same buffer, and the cells were then disrupted by sonication. Soluble and insoluble fractions were separated by centrifugation (15,000 × g for 30 min). The recombinant CGTTk in the insoluble form was denatured by 6 M urea and then refolded by fractional dialysis in 3, 1.5, and 0 M urea in 50 mM Tris-HCl (pH 8.0) and 1 mM dithiothreitol. The refolded protein was purified on a HiTrap Q column (Amersham Pharmacia Biotech, Uppsala, Sweden). The purity of the protein was examined by polyacrylamide gel electrophoresis (PAGE) in the presence of 0.1% sodium dodecyl sulfate (SDS). Protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) according to the manufacturer’s instructions by using bovine serum albumin as a standard.
Determination of N-terminal amino acid sequence.
In order to determine the N-terminal amino acid sequence, the protein was electroblotted onto a polyvinylidene difluoride membrane after SDS-PAGE, and N-terminal amino acid residues were determined with a protein sequencer (model 491 cLC; Perkin-Elmer).
Plate assay for starch degradation.
A qualitative assay for starch degradation was performed using starch agar plates (0.5% starch and 2% agar). The enzyme was spotted onto the starch agar plate and incubated for 30 min at 60°C. Clear zone formation at the enzyme spots was examined after staining with an iodine solution (0.005% [wt/vol] I2 and 0.05% [wt/vol] KI).
Enzyme activity assay.
The quantitative assay for starch-degrading activity was based upon the decrease in absorbance (blue value) of the iodine-amylose complex due to starch degradation. The enzyme solution (50 μl) was incubated at 80°C for 15 min with 90 μl of 1% soluble starch solution, and the reaction was terminated by quenching on ice. The reaction mixture (10 μl) was removed and mixed with 100 μl of 0.1 N NaOH. This solution (100 μl) was mixed with 2 ml of iodine solution, and the absorbance of the mixture was measured at 660 nm.
Determination of cyclodextrin production.
Reaction mixtures (150 μl) containing 2.5% (wt/vol) soluble starch and 50 mM sodium acetate buffer (pH 5.8) were incubated with the purified enzyme (25 μg) at 80°C. Samples, after incubation, were kept on ice for 10 min. Fifty microliters of the reaction mixture was mixed with 2 μl (4 U) of glucoamylase (Toyobo), 8 μl of 2 M sodium acetate buffer (pH 5.0), and 40 μl of distilled water. The mixture was incubated at 40°C for 1 h. The reaction mixture was filtered through a membrane (pore size, 0.45 μm). The filtered sample (20 μl) was analyzed by high-performance liquid chromatography (HPLC) with a TSKgel Amide 80 column (Tosoh, Tokyo, Japan) and eluted with acetonitrile-water (60:40 [vol/vol]) at 1 ml/min. The flow cell was set at 30°C, and products were detected with a refractive index detector.
Thin-layer chromatography.
The reaction products of various polysaccharides were analyzed by silica gel thin-layer chromatography. The enzyme was incubated with a 1% solution of each substrate (soluble starch, glycogen, and pullulan) in 50 mM sodium acetate buffer (pH 5.8) and 10 mM CaCl2 at 85°C. Aliquots (3 μl) of the reaction mixtures were chromatographed on a silica gel 60 plate (Merck KgaA, Darmstadt, Germany) with isopropyl alcohol-acetone-water (2:2:1 [vol/vol/vol]) as a solvent, and products were detected by spraying the plate with aniline-diphenylamine reagent (4 ml of aniline, 4 g of diphenylamine, 200 ml of acetone, and 30 ml of 85% phosphoric acid), followed by baking at 170°C.
Starch-binding assay.
The starch-binding assay was based on the cellulose-binding assay procedure, with slight modifications (3). The standard assay mixture contained 40 μl of enzyme (CGTTk and CGTTkΔC) in 50 mM Tris-HCl buffer (pH 7.5) and 40 μl of 2.5% colloidal soluble starch. After the mixture was incubated for 1 h at room temperature with occasional stirring, the supernatant containing unadsorbed protein was separated from colloidal chitin by centrifugation (15,000 × g for 10 min at 4°C). The precipitate was washed twice with 50 mM Tris-HCl buffer (pH 7.5). The supernatant and precipitate fractions were subjected to SDS-PAGE.
Circular dichroism spectra.
Circular dichroism spectra were measured on a J-820 automatic spectropolarimeter (Japan Spectroscopic Company, Hachioji, Japan) at 80°C in 10 mM Tris-HCl (pH 3.2). The protein concentration was approximately 0.2 mg/ml, and a cell with an optical path length of 1 mm was used.
Nucleotide sequence accession number.
The nucleotide sequence data of the cgtTk gene reported in this paper will appear in the DDBJ, EMBL, and GenBank DNA databases under accession number AB072372.
RESULTS
Cloning and structural analysis of cgtTk.
In the course of genome analysis of T. kodakaraensis KOD1, the nucleotide sequence of a 1.2-kbp HindIII DNA fragment was found to show similarity to starch-degrading enzymes of the α-amylase family. This DNA fragment was used as a probe to clone the full-length gene, and we found an open reading frame consisting of 2,139 nucleotides corresponding to a protein composed of 713 amino acids with a calculated molecular mass of 79,593 Da. The four conserved regions previously identified in α-amylase-family enzymes were found in CGTTk (Fig. 1). Furthermore, the seven amino acid residues that are strictly conserved in these regions (12, 15) were also completely preserved.
FIG. 1.
Alignment of CGTases from T. kodakaraensis KOD1 and B. circulans strain 251. The alanine highlighted on a solid background indicates the first residue in the numbering of the B. circulans CGTase. Asterisks indicate conserved residues between these two enzymes. Solid circles indicate the catalytic residues. Residues that are highly and uniquely conserved among CGTases and also found in CGTTk are indicated by open circles, while those not conserved in CGTTk are indicated by solid arrowheads. The four conserved motifs are shaded, and five domains are also indicated by letters A to E above the sequences.
Most microbial members of the α-amylase family are extracellular proteins and possess an N-terminal secretion signal. Little is known about the secretion mechanism of archaeal proteins, but we have previously identified an N-terminal signal sequence in an α-amylase (ApkA) from T. kodakaraensis KOD1 (21). This signal was composed of highly hydrophobic amino acid residues and was cleaved downstream of an Ala-Ser-Ala sequence during secretion of the protein. The deduced amino acid sequence of CGTTk also contained a highly hydrophobic region of 17 amino acid residues at the N terminus, followed by an identical Ala-Ser-Ala, suggesting that a similar signal sequence was present in CGTTk.
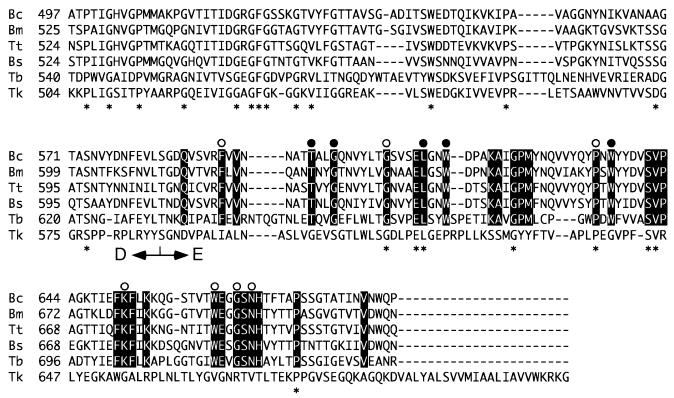
We found that the C-terminal region of CGTTk, particularly the region corresponding to domain E, displayed a primary structure completely distinct from those of other previously reported CGTases (Fig. 2). The difference does not seem to be derived from a mutation on the chromosome or a frame shift incorporated during DNA manipulation, as no reading frame showed similarity to the C-terminal domain of any CGTase. As shown in Fig. 2, the E domain, or starch-binding domain, contains many residues that are highly conserved among CGTases. Most of these residues are also conserved in the starch-binding domains of other starch-degrading enzymes, as reported by Janecek and Sevcik (8). In particular, Phe591, Thr598, Gly601, Gly608, Leu613, Trp616, Pro634, Trp636, Lys651, Trp662, Gly665, and Asn667 are completely conserved in all CGTases. Only a small number of these residues were found in the C-terminal domain of CGTTk, and hardly any of the aromatic residues were conserved (Fig. 2). It is unlikely that this is due to the fact that CGTTk is from a hyperthermophile, considering that the enzymes from Thermoanaerobacterium thermosulfurigenes EM1 (11) and Thermococcus sp. strain B1001 (20) are also highly thermostable enzymes.
FIG. 2.
Comparison of the E domains of various CGTases. The E domains of CGTases from B. circulans strain 251 (Bc) (accession no. P43379), B. marcerans (Bm) (P31835), T. thermosulfurigenes (Tt) (S17298), B. stearothermophilus (Bs) (P31797), Thermococcus sp. strain B1001 (Tb) (BAB18101), and T. kodakaraensis KOD1 (Tk) (AB072372) were aligned. Residues highlighted on a solid background were conserved in the upper five sequences. Open circles indicate residues completely conserved in previously reported CGTases, while solid circles represent residues conserved in the starch-binding domains of CGTases, α-amylases, β-amylases, maltogenic α-amylases, and glucoamylases (8).
Production and purification of CGTTk
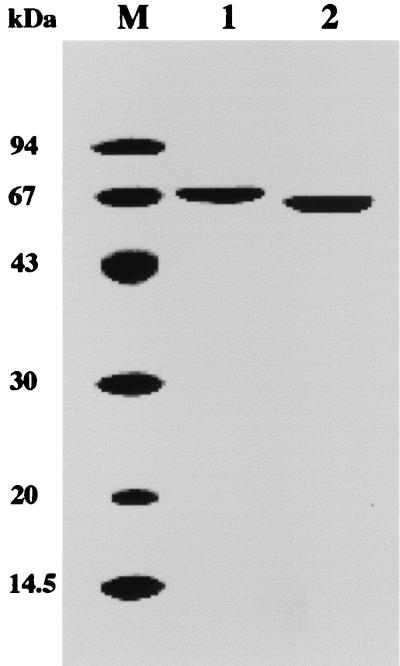
In order to characterize the protein product of the cgtTk gene, and to determine whether the enzyme was a bona fide CGTase or an α-amylase, we expressed the gene in E. coli. As the N-terminal region was considered a signal sequence for secretion, we constructed a truncated gene corresponding to a protein starting from residue 22. Two amino acid residues, Met and Gly, were added to introduce an initiation codon and an NcoI cloning site. We also constructed a truncated gene without the final 23 C-terminal residues of the native CGTTk (CGTTkΔC). When E. coli BL21(DE3) cells harboring the expression plasmids were grown at 37°C and induced with 1 mM IPTG for gene expression, the gene products were produced in an insoluble form. Gene expression was performed at a lower temperature (23°C) and lower IPTG concentrations (0.1 to 1 mM) in order to obtain soluble proteins, but these efforts were unsuccessful. Therefore, denaturation and refolding experiments were carried out under various conditions. The proteins were successfully solubilized by denaturation with 6 M urea and refolding by fractional dialysis in 3, 1.5, and 0 M urea in Tris-HCl buffer (pH 8.0) and 1 mM dithiothreitol. The solubilized proteins were purified by ion-exchange chromatography to apparent homogeneity (Fig. 3). The molecular masses of CGTTk and CGTTkΔC estimated by SDS-PAGE were slightly smaller than those deduced from their genes. We have previously encountered some cases in which archaeal proteins showed aberrant migration rates on SDS-PAGE gels, probably due to incomplete denaturation of the protein in SDS gel-loading buffer. This property has also been observed for other hyperthermophilic starch-degrading enzymes (20, 21). When CGTTk was analyzed by SDS-PAGE after denaturation with urea, the molecular mass of the protein (77 kDa) was in good agreement with the calculated value (data not shown). Furthermore, the N-terminal amino acid sequence of each purified protein was determined, confirming that we had obtained purified CGTTk and CGTTkΔC. The N-terminal 14 amino acid residues were identical to the deduced amino acid sequences for each gene.
FIG. 3.
Coomassie brilliant blue-stained SDS-PAGE gel of purified recombinant CGTTk and CGTTkΔC. Lane M, molecular mass marker; lane 1, CGTTk; lane 2, CGTTkΔC.
Effects of pH, temperature, and calcium ion concentration on enzyme activity.
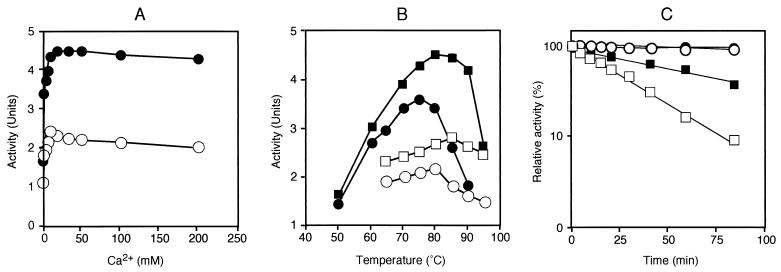
Purified CGTTk and CGTTkΔC were dialyzed, and starch-degrading activities were measured at various temperatures and pHs. CGTTk displayed maximal activity between pH 5.5 and 6.0. The pH preference of CGTTkΔC showed a slight shift toward an alkaline pH, and it displayed high activity between pH 6.0 and pH 6.5. The decrease in activity at a higher pH was more drastic in the case of CGTTk. Moreover, CGTTkΔC showed a twofold-higher starch-degrading activity than the parental CGTTk (data not shown). Because CGTTk included a Ca2+-binding region near the N terminus, we examined the effects of Ca2+ on enzyme activity. Addition of 10 mM EDTA led to 70% decreases in the starch-degrading activities of both proteins (data not shown). Enzyme activities were measured in the presence of various concentrations of Ca2+, and maximum activities were obtained between 6 and 10 mM. Enzyme activity did not decrease drastically with higher concentrations of Ca2+ up to 200 mM (Fig. 4A). We next examined the effects of temperature on the starch-degrading activities of the two proteins in the presence and absence of 10 mM Ca2+. Addition of 10 mM Ca2+ led to increases in the enzyme activities of both CGTTk and CGTTkΔC, and also resulted in elevations of the optimal temperatures (Fig. 4B). CGTTk exhibited maximal activity at 80°C when no ions were added in the reaction mixture, while it showed the highest activity at 85°C when 10 mM Ca2+ was added. CGTTkΔC showed maximum activity at 75°C without addition of Ca2+, and the optimal temperature was elevated to 80°C in the presence of 10 mM Ca2+. Again, CGTTkΔC showed higher starch-degrading activity than CGTTk at all temperatures examined. The thermostability of each protein was monitored in the presence of Ca2+, and the proteins were found to be stable at 85°C, with no decrease in activities even after 80 min of incubation at 85°C. CGTTkΔC displayed a half-life of 40 min at 100°C, while that of CGTTk was shown to be approximately 20 min at 100°C (Fig. 4C). By these measurements, we found that CGTTkΔC displayed significantly higher starch-degrading activity and slightly higher thermostability than the parental CGTTk.
FIG. 4.
Biochemical comparison of CGTTk (open symbols) and CGTTkΔC (solid symbols). (A) Effect of Ca2+ concentration on CGTTk and CGTTkΔC activities. (B) Effect of temperature on enzyme activities of CGTTk and CGTTkΔC. Activities were measured at various temperatures in the presence (squares) or absence (circles) of 10 mM CaCl2 (pH 5.8). (C) Thermostabilities of CGTTk and CGTTkΔC. The enzymes were incubated at 85°C (circles) and 100°C (squares) in the presence of CaCl2 for various lengths of time, and residual activities were measured. Four micrograms of CGTTk and CGTTkΔC was used in all cases.
Substrate specificities of CGTTk and CGTTkΔC.
The degradation of soluble starch, glycogen, and pullulan by CGTTk and CGTTkΔC was examined. Both proteins exhibited identical substrate specificities. Both proteins hydrolyzed soluble starch and glycogen to form oligosaccharides of various lengths, while they failed to utilize pullulan as a substrate (data not shown).
Cyclodextrin production.
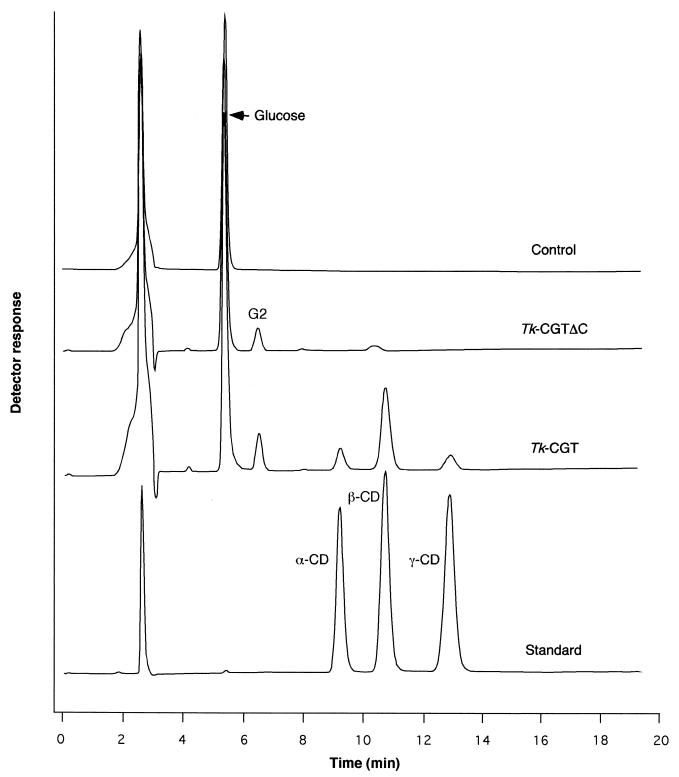
Since the primary structure of CGTTk resembled those of previously reported CGTases, we examined the production of CDs from soluble starch. Analysis by HPLC was carried out using α-, β-, and γ-cyclodextrins as standards. In the case of purified CGTTk, α-, β-, and γ-cyclodextrins were produced from soluble starch; β-cyclodextrin was the major product among the CDs (Fig. 5). In contrast, we could not detect any CD products after the reaction with purified CGTTkΔC. When EDTA was added to the reaction mixture with CGTTk, we could detect only trace amounts of CD (data not shown).
FIG. 5.
Detection of cyclodextrins by HPLC analysis. The samples applied to HPLC analysis are indicated on the right. Reaction mixtures containing 2.5% (wt/vol) soluble starch and 50 mM sodium acetate buffer (pH 5.8) were incubated with CGTTk and CGTTkΔC (25 μg) at 80°C. Production of cyclodextrins was determined by HPLC as described in Materials and Methods. The cyclodextrin standard is a mixture of α-, β-, and γ-cyclodextrins. The control contains all the reagents but without enzyme.
Starch-binding activities of CGTTk and CGTTkΔC.
Because the C-terminal regions of various CGTases were shown to be involved in binding to starch, we compared the starch-binding abilities of CGTTk and CGTTkΔC. Both proteins displayed high affinities toward starch, and the C-terminal truncation of CGTTk seemed to have no effect on its starch-binding ability (data not shown).
Circular dichroism spectra of CGTTk and CGTTkΔC.
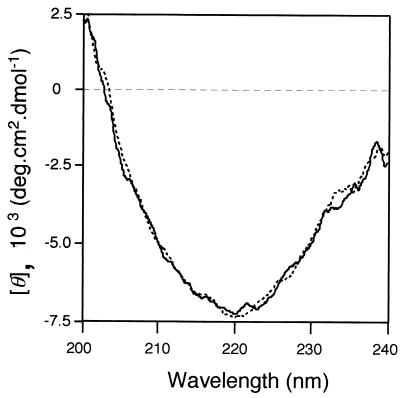
We measured the circular dichroism spectra of CGTTk and CGTTkΔC at 80°C in the presence of 10 mM Ca2+. We could not detect any significant differences in the two spectra, suggesting that the two proteins had similar secondary structures (Fig. 6).
FIG. 6.
Circular dichroism spectra of CGTTk and CGTTkΔC. Spectra were measured at 80°C. Solid line, circular dichroism spectrum of CGTTk; dotted line, circular dichroism spectrum of CGTTkΔC.
DISCUSSION
We have characterized a CGTase (CGTTk from a hyperthermophilic archaeon, T. kodakaraensis KOD1. The primary structure of the protein displayed low similarity with those of previously characterized starch-degrading enzymes. The most similar protein was CGTase from the hyperthermophilic archaeon Thermococcus sp. strain B1001 (40%) (20). CGTTk displayed 35% identity with Novamyl, an α-amylase from B. stearothermophilus (accession number S28784), and 30.4 and 29% identities to the CGTases from T. thermosulfurigenes (accession number S17298) and B. circulans strain 251 (CGTBc, accession number P43379), respectively. Motifs I to IV, conserved in all members of the α-amylase family (12, 15), were found in CGTTk. The three-dimensional structures of CGTBc complexed with acarbose (17), maltoheptaose (13, 23), maltononaose (18), and α- or β-cyclodextrin (10, 16) have revealed interactions between these saccharides and specific enzyme residues. In the A1 domain, the calcium-binding ligands Asp27, Asn32, Asn33, and Asp53 (residue numbers of mature CGTBc) and Arg47, which binds to cyclic oligosaccharides in CGTBc, were conserved. However, Cys43 and Cys50, and moreover the unique CGTase residue Leu46, were not found in CGTTk. In the B domain, the region that binds glucose at subsite −7 (His140 to Glu153) in the case of CGTBc was significantly shorter in CGTTk, resembling that of the CGTase from B. firmus/lentus strain 290-3 (accession number CAA01436). Although most of the unique CGTase residues were conserved in CGTTk, Phe136, Gly165, and Phe175 were replaced by Tyr, Thr, and Tyr, respectively, the same replacements found in the CGTase of Klebsiella pneumoniae (accession number P08704), an enzyme with exceptionally low similarity to all other CGTases. Tyr195, whose phenyl ring is located at the center of the active site, was conserved in CGTTk. This tyrosine residue is replaced by a much smaller residue, Gly, Leu, Ser, Thr, or Val, in the case of α-amylases (24). In the A2 domain, catalytic residues Glu257, Asp229, and Asp328 were conserved. However, the C-terminal region of CGTTk, particularly the region corresponding to domain E, displayed a primary structure distinct from those of previously reported CGTases. The C-terminal region did not display significant similarity to those of any proteins in the databases.
Purification and enzymatic characterization of CGTTk has revealed that the enzyme is a bona fide CGTase, producing β-cyclodextrin as the major product from starch. We have previously characterized the first archaeal CGTase from Thermococcus sp. strain B1001 (CGTTb) (20). The product specificities of the two archaeal enzymes were distinct, as CGTTb catalyzed the cyclization reaction leading to α-cyclodextrin as the major product (20). Domain E of CGTTb displayed a structure similar to those of previously characterized CGTases, indicating that the C-terminal domain of CGTTk is an exception, and not a common feature of archaeal or hyperthermophilic CGTases.
CGTTkΔC, with a truncation of 23 amino acids at the extreme C terminus of CGTTk, displayed an increase in starch-degrading activity compared to the parental enzyme, but cyclization activity was abolished. This result itself indicates a direct function of the C-terminal region in cyclization activity. However, the roles of the C-terminal region have been a focus of research on CGTases (24), and many studies have reported various effects of C-terminal deletions (1, 5, 9). At present, with an accumulation of biochemical and structural data, it is supposed that the E domain acts predominantly as a starch-binding domain and is not directly involved in catalysis (24). C-terminal truncations of CGTase may lead to conformational changes in the enzymes, or may affect their structural integrity, resulting in an indirect abolishment of cyclization activity. Nevertheless, since the C-terminal domain of CGTTk did not show similarity to those of previously identified CGTases, we cannot conclude that this is also the case for CGTTk. The thermostability of CGTTkΔC was as high as that of CGTTk, suggesting that the C-terminal truncation did not drastically alter the conformation of the enzyme, at least not to an unstable conformation. This is further strongly supported by the comparison of the circular dichroism spectra of the two proteins at 80°C, which did not reveal any significant differences in secondary structure. We also found that the C-terminal truncation did not affect the starch-binding ability of CGTTk.
Our results have indicated the presence of an archaeal CGTase that harbors a novel C-terminal domain, distinct in primary structure from the well-conserved E domains of previously reported CGTases. A 23-residue truncation resulted in abolishment of the cyclization activity but showed little effect on other biochemical properties of the enzyme such as secondary structure, thermostability, and starch-binding activity. The truncation did lead to an increase in starch-degrading activity. Our results, along with those of previous studies, indicate that even subtle changes in structure lead to alterations in the reaction specificities of enzymes in the α-amylase family. Further biochemical and structural studies will be necessary to elucidate the direct and/or indirect roles of this region in the cyclization activity of CGTTk.
Acknowledgments
This work was partly supported by the Japan Science and Technology Corporation (JST) for Core Research for Evolutional Science and Technology (CREST) (to T. Imanaka).
REFERENCES
- 1.Bender, H. 1990. Studies of the mechanism of the cyclization reaction catalyzed by the wild-type and a truncated α-cyclodextrin glycosyltransferase from Klebsiella pneumoniae strain M5, and the β-cyclodextrin glycosyltransferase from Bacillus circulans strain 8. Carbohydr. Res. 206:257–267. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara, S., H. Kakihara, K. Sakaguchi, and T. Imanaka. 1992. Analysis of mutations in cyclodextrin glucanotransferase from Bacillus stearothermophilus which affect cyclization characteristics and thermostability. J. Bacteriol. 174:7478–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilkes, N. R., R. A. J. Warren, R. C. J. Miller, and D. G. Kilburn. 1988. Precise excision of the cellulose binding domains from two Cellulomonas fimi cellulases by a homologous protease and the effect on catalysis. J. Biol. Chem. 263:10401–10407. [PubMed] [Google Scholar]
- 4.Hashimoto, Y., T. Yamamoto, S. Fujiwara, M. Takagi, and T. Imanaka. 2001. Extracellular synthesis, specific recognition, and intracellular degradation of cyclomaltodextrins by the hyperthermophilic archaeon Thermococcus sp. strain B1001. J. Bacteriol. 183:5050–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellman, J., M. Wahlberg, M. Karp, T. Korpela, and P. Mantsala. 1990. Effects of modification at the C terminus of cyclomaltodextrin glucanotransferase from Bacillus circulans var. alkalophilus on catalytic activity. Biotechnol. Appl. Biochem. 12:387–396. [Google Scholar]
- 6.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janecek, S. 1994. Parallel β/α-barrels of α-amylase, cyclodextrin glycosyltransferase and oligo-1,6-glucosidase versus the barrel of β-amylase: evolutionary distance is a reflection of unrelated sequences. FEBS Lett. 353:119–123. [DOI] [PubMed] [Google Scholar]
- 8.Janecek, S., and J. Sevcik. 1999. The evolution of starch binding domain. FEBS Lett. 456:119–125. [DOI] [PubMed] [Google Scholar]
- 9.Kimura, K., S. Kataoka, A. Nakamura, T. Takano, S. Kobayashi, and K. Yamane. 1989. Functions of the COOH-terminal region of cyclodextrin glucanotransferase of alkalophilic Bacillus sp. #1011: relation to catalyzing activity and pH stability. Biochem. Biophys. Res. Commun. 161:1273–1279. [DOI] [PubMed] [Google Scholar]
- 10.Knegtel, R. M. A., B. Strokopytov, D. Penninga, O. G. Faber, H. J. Rozeboom, K. H. Kalk, L. Dijkhuizen, and B. W. Dijkstra. 1995. Crystallographic studies of the interaction of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 with natural substrates and products. J. Biol. Chem. 270:29256–29264. [DOI] [PubMed] [Google Scholar]
- 11.Knegtel, R. M. A., R. D. Wind, H. J. Rozeboom, K. H. Kalk, R. M. Buitelaar, L. Dijkhuizen, and B. W. Dijkstra. 1996. Crystal structure at 2.3 Å resolution and revised nucleotide sequence of the thermostable cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. J. Mol. Biol. 256:611–622. [DOI] [PubMed] [Google Scholar]
- 12.Kuriki, T., and T. Imanaka. 1999. The concept of the α-amylase family: structural similarity and common catalytic mechanism. J. Biosci. Bioeng. 87:557–565. [DOI] [PubMed] [Google Scholar]
- 13.Lawson, C. L., R. van Montfort, B. Strokopytov, H. J. Rozeboom, K. H. Kalk, G. E. de Vries, D. Penninga, L. Dijkhuizen, and B. W. Dijkstra. 1994. Nucleotide sequence and X-ray structure of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 in maltose-dependent crystal form. J. Mol. Biol. 236:590–600. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa, M., Y. Izawa, N. Rashid, T. Hoaki, and T. Imanaka. 1994. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl. Environ. Microbiol. 60:4559–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima, R., T. Imanaka, and S. Aiba. 1986. Comparison of amino acid sequences of eleven different α-amylases. Appl. Microbiol. Biotechnol. 23:355–360. [Google Scholar]
- 16.Schmidt, A. K., S. Cottaz, H. Driguez, and G. E. Schulz. 1998. Structure of cyclodextrin glycosyltransferase complexed with a derivative of its main product β-cyclodextrin. Biochemistry 37:5909–5915. [DOI] [PubMed] [Google Scholar]
- 17.Strokopytov, B., D. Penninga, H. J. Rozeboom, K. H. Kalak, L. Dijkhuizen, and B. W. Dijkstra. 1995. X-ray structure of cyclodextrin glycosyltransferase complexed with acarbose. Implications for the catalytic mechanism of glycosidases. Biochemistry 34:2234–2240. [DOI] [PubMed] [Google Scholar]
- 18.Strokopytov, B., R. M. A. Knegtel, D. Penninga, H. J. Rozeboom, K. H. Kalk, L. Dijkhuizen, and B. W. Dijkstra. 1996. Structure of cyclodextrin glycosyltransferase complexed with maltononaose inhibitor at 2.6 Å resolution. Implications for product specificity. Biochemistry 35:4241–4249. [DOI] [PubMed] [Google Scholar]
- 19.Svensson, B., H. Jespersen, M. R. Sierks, and E. A. MacGregor. 1989. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem. J. 264:309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tachibana, Y., A. Kuramura, N. Shirasaka, Y. Suzuki, T. Yamamoto, S. Fujiwara, M. Takagi, and T. Imanaka. 1999. Purification and characterization of an extremely thermostable cyclomaltodextrin glucanotransferase from a newly isolated hyperthermophilic archaeon, a Thermococcus sp. Appl. Environ. Microbiol. 65:1991–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana, Y., M. M. Leclere, S. Fujiwara, M. Takagi, and T. Imanaka. 1996. Cloning and expression of the α-amylase gene from the hyperthermophilic archaeon Pyrococcus sp. KOD1, and characterization of the enzyme. J. Ferment. Bioeng. 82:224–232. [Google Scholar]
- 22.Takata, H., T. Kuriki, S. Okada, Y. Takesada, M. Iizuka, N. Minamiura, and T. Imanaka. 1992. Action of neopullulanase: neopullulanase catalyzes both hydrolysis and transglycosylation at α-(1–4)- and α-(1–6)-glucosidic linkages. J. Biol. Chem. 267:18447–18452. [PubMed] [Google Scholar]
- 23.Utidehaag, J. C. M., G. W. M. van Alebeek, B. A. van der Veen, L. Dijkhuizen, and B. W. Dijkstra. 2000. Structures of maltohexaose and maltoheptaose bound to the donor sites of cyclodextrin glycosyltransferase give insight into the mechanisms of transglycosylation activity and cyclodextrin size specificity. Biochemistry 39:7772–7780. [DOI] [PubMed] [Google Scholar]
- 24.van der Veen, B. A., J. C. Uitdehaag, B. W. Dijkstra, and L. Dijkhuizen. 2000. Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim. Biophys. Acta 1543:336–360. [DOI] [PubMed] [Google Scholar]