Abstract
Two Tn5-generated mutants of Shewanella putrefaciens with insertions in menD and menB were isolated and analyzed. Both mutants were deficient in the use of several terminal electron acceptors, including Fe(III). This deficiency was overcome by the addition of menaquinone (vitamin K2). Isolated membrane fractions from both mutants were unable to reduce Fe(III) in the absence of added menaquinone when formate was used as the electron donor. These results indicate that menaquinones are essential components for the reduction of Fe(III) by both whole cells and purified membrane fractions when formate or lactate is used as the electron donor.
Fe(III) and Mn(IV) reduction are widespread anaerobic processes in both freshwater and marine environments. A large number of bacterial species, such as Geobacter metallireducens (9), Shewanella putrefaciens (14), and Pantoea agglomerans (5), are capable of coupling metal reduction to organic carbon oxidation. S. putrefaciens MR-1 is a gram-negative facultative anaerobe that can use insoluble metal oxides or oxyhydroxides as terminal electron acceptors during anaerobic respiration. It can also use a large number of soluble electron acceptors, such as fumarate, nitrate, nitrite, thiosulfate, and sulfite, for the same purpose (16). The molecular mechanisms of metal reduction are not completely known. Recent studies have suggested that the majority of the Fe(III) reductase activity is located on the outer membranes of S. putrefaciens (12) and Geobacter sulfurreducens (6). This characteristic is in contrast to that of other gram-negative bacteria that have been studied, in which components of the respiratory electron transport chains and terminal reductases are located in the cytoplasmic membranes or the periplasmic spaces.
Quinones are lipid-soluble components of electron transport chains that transfer electrons from dehydrogenases either directly or indirectly to the terminal reductases. In Escherichia coli, ubiquinone is used for aerobic and nitrate respiration, while naphthoquinones are used for fumarate, trimethylamine oxide (TMAO), and dimethyl sulfoxide (DMSO) respiration (see references 7 and 10 for reviews). S. putrefaciens strains contain both ubiquinones and naphthoquinones (menaquinone [MK] and dimethylmenaquinone) (1), and mutants deficient in MK biosynthesis have been described previously (13, 15, 17). In this paper, we describe the characterization of menD and menB S. putrefaciens mutants and show that MKs are required for metal reduction by membrane fractions when formate or NADH is used as the electron donor.
A list of bacterial strains and plasmids used in this study is given in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| S. putrefaciens | ||
| MR-1 | Wild-type strain isolated from Lake Oneida | 14 |
| MR-1R | Rifampin-resistant mutant of MR-1 | 2 |
| SR-73 | MR-1R derivative, menD::Tn5 Rifr Kmr | This work |
| SR-536 | MR-1R derivative, menB::Tn5 Rifr Kmr | This work |
| Plasmids | ||
| pSPORT1 | Cloning and sequencing vector, Ampr | BRL Life Technologies |
| pBluescript SK | Cloning and sequencing vector, Ampr | Stratagene |
| pSR731 | pSPORT1 carrying 3-kb fragment of Tn5 and adjacent menD DNA | This work |
| pSR536I | pSPORT1 carrying 3-kb fragment of Tn5 and adjacent menB DNA | This work |
| pSR73Z | 6.3-kb fragment containing menD in pBluescript SK | This work |
Identification of MK biosynthesis genes.
Mutants deficient in anaerobic respiration were isolated following Tn5 mutagenesis as described previously (2). Two mutants, SR-73 and SR-536, were analyzed, and the disrupted DNA was cloned and sequenced as described previously (2). SR-73 was found to have a Tn5 insertion in menD, while SR-536 had an insertion in menB. We cloned a 6.5-kb fragment of wild-type DNA that encompassed menD to generate the plasmid pSR73Z and sequenced 3.3 kb of this fragment (GenBank accession no. AY038363). Three open reading frames (ORFs), designated menD, menH, and cyc3, were identified.
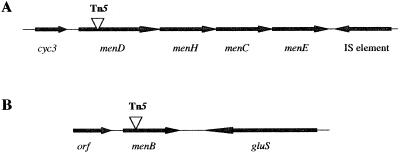
menD encodes a protein of 573 amino acids that exhibits 42 and 41% identity to MenD from Vibrio cholerae and E. coli, respectively. menH, which lies downstream of menD, encodes a putative protein of 272 amino acids that is 34% identical to MenH from E. coli (11, 20). Analysis of the genome sequence of S. putrefaciens (see the website of The Institute for Genomic Research [TIGR], http://www.tigr.org) suggests that the menDHCE genes in this organism are arranged in an operon (Fig. 1).
FIG. 1.
Maps of menD (A) and menB (B) with adjacent genes. The sites of Tn5 insertion in mutants SR-73 (A) and SR-536 (B) are indicated by triangles. IS, insertion.
We identified an ORF, designated cyc3, upstream of the men genes that encodes a putative protein of 100 amino acids and contains three heme binding sites characteristic of c cytochromes (CXXCH). Cyc3 was most similar to cytochrome c3 from Desulfovibrio africanus (27% identity and 40% similarity) (19).
The transposon insertion in SR-536, the second mutant analyzed, was in menB, which encodes a protein of 300 amino acids. S. putrefaciens MenB exhibited 68 and 66% identity to MenB from Halobacterium sp. strain NRC-1 (18) and Mycobacterium tuberculosis (4), respectively. The complete sequence of menB and adjacent DNA was obtained from the TIGR website. menB does not appear to be closely associated with other MK biosynthesis genes. It is flanked by an ORF that is similar to gluS from V. cholerae (8) and an ORF of unknown function (Fig. 1).
The genome sequence of S. putrefaciens also contains menG, menA, and menF homologs. With the exception of menDHCE, it appears that the MK biosynthesis genes of S. putrefaciens are not closely linked. This is in contrast to the genes of E. coli and Bacillus megaterium, for example, which are found in two clusters (10, 21). It is interesting to note that menB was highly similar to genes from archaebacteria and gram-positive bacteria. This was not expected, since S. putrefaciens, which is a member of the γ group of the Proteobacteria, is more closely related to E. coli and Vibrio species.
Phenotypic analysis of SR-73 and SR-536.
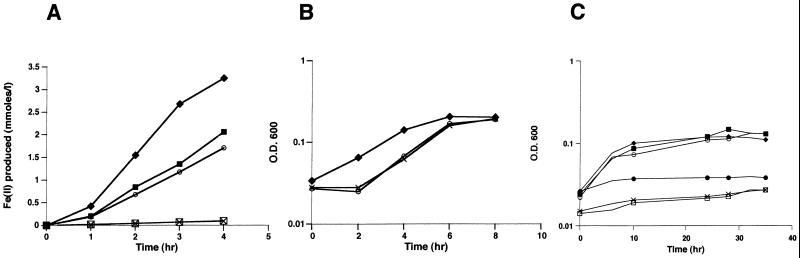
SR-73 and SR-536 were grown anaerobically in LM medium plus 50 mM lactate or LM medium plus 50 mM formate supplemented with the different electron acceptors used by the wild type, as described previously (2, 3). As shown in Fig. 2A, both mutants were deficient in Fe(III) reduction. The addition of 0.05 mM vitamin K2 (MK-4) to the growth medium resulted in a marked increase in the ability of SR-73 and SR-536 to reduce ferric iron. Similar results were obtained when the mutants were tested for the reduction of Mn(IV), nitrite, thiosulfate, sulfite, and to a lesser extent nitrate (data not shown). To determine the involvement of MKs in TMAO and DMSO reduction, anaerobic growth of both wild-type and mutant strains with a 10 mM concentration of each electron acceptor was monitored at A600 (Fig. 2B and C). Both mutants were able to grow with TMAO but not with DMSO. The addition of vitamin K2 restored the abilities of SR-73 and SR-536 to grow with DMSO as the terminal electron acceptor.
FIG. 2.
(A) Fe(III) reduction levels by wild-type S. putrefaciens (MR-1) and MK biosynthesis mutants (SR-73 and SR-536). The addition of vitamin K2 restores the abilities of both mutants to reduce Fe(III). (B) Anaerobic growth of wild-type and MK biosynthesis mutants of S. putrefaciens with TMAO as the terminal electron acceptor. (C) Anaerobic growth of wild-type and mutant strains with DMSO as the terminal electron acceptor. ⧫, MR-1; □, SR-73; ○, SR-73 supplemented with vitamin K2; ×, SR-536; ▪, SR-536 supplemented with vitamin K2; •, MR-1 grown without added electron acceptor. OD600, optical density at 600 nm.
Fe(III) reduction by membrane fractions of S. putrefaciens.
Total membrane fractions were obtained from wild-type and mutant strains as follows. Cultures were incubated in a Coy anaerobic chamber overnight, and the cells were harvested by centrifugation. Membrane fractions were obtained following the lysis of the cells with a French pressure cell. Unlysed cells were removed by centrifugation at 12,000 × g, and the membranes were recovered by centrifugation of the resulting supernatant at 100,000 × g for 1 h. Fe(III) reductase activity was measured as described previously with formate as the electron donor (3, 12). As shown in Table 2, both mutants, SR-73 and SR-536, exhibited very low levels of Fe(III) reductase activity. The addition of vitamin K2 restored this activity in mutant membrane fractions almost to wild-type levels. These results confirm that MKs are essential components of the electron transport chain that leads to Fe(III) reduction. The residual activity detected in the membrane fractions of the mutants may represent assimilatory Fe(III) reduction that does not require the activity of the electron transport chain components.
TABLE 2.
Fe(III) reductase activity of the wild type and MK-deficient mutants of S. putrefaciens
| S. putrefaciens strain | Fe(III) reductase activity (nmol/min/mg) (mean ± SD) |
|---|---|
| MR-1 (wild type) | 455.6 ± 148.9 |
| SR-73 | 21.3 ± 13.3 |
| SR-73 + vitamin K2 | 396.8 ± 196.8 |
| SR-536 | 47.3 ± 14.5 |
| SR-536 + vitamin K2 | 299.9 ± 40.1 |
Unlike other organisms studied to date, metal-reducing bacteria use insoluble terminal electron acceptors during anaerobic respiration. Previous cell fractionation studies have suggested that Fe(III) reductase activity is primarily associated with the outer membrane of S. putrefaciens. This was based on the detection of low levels of Fe(III) reductase activity [14 to 21 nmol of Fe(II) produced/min/mg] in outer membrane fractions supplemented with formate or NADH as the electron donor (12). We obtained similarly low levels of activity in membrane fractions of mutants that lack MKs (Table 2), compared to levels of ∼400 nmol of Fe(II) produced/min/mg in wild-type or complemented mutant membrane fractions. The MK requirement for Fe(III) reductase activity in membrane fractions calls into question the conclusions drawn from earlier experiments (12). Given the role that quinones play in proton translocation across the cytoplasmic membrane, it is unlikely that they are located in the outer membrane. The use of formate or NADH as the electron donor requires the presence of the respective dehydrogenase for the oxidation of formate or NADH and the concomitant reduction of quinones. It is unlikely that both dehydrogenases (formate and NADH) are also located in the outer membrane. The Fe(III) reductase activity that was detected in outer membrane fractions of S. putrefaciens when formate was used as the electron donor could have been due to low levels of cytoplasmic membrane contamination of these fractions. The results presented in this paper suggest that the assays which are used to determine the levels of Fe(III) reduction without the addition of MK are not appropriate for the measurement of enzyme activity in purified outer membrane fractions of S. putrefaciens. Our results, however, do not rule out the possibility that some components required for metal reduction are found in the outer membrane. We have recently described two genes that encode outer membrane-associated proteins involved in metal reduction (2, 3). Further investigation will be needed to determine the actual location of the terminal metal reductases.
Acknowledgments
This work was supported by National Science Foundation grant MCB 9604298 and Department of Energy grant DE-FG02-00ER15068. Preliminary sequence data was obtained from the TIGR website.
We thank M. McBride for helpful comments and critical reading of the manuscript.
REFERENCES
- 1.Akagawa-Matsushita, M., T. Itoh, Y. Katayama, H. Kuraishi, and K. Yamasato. 1992. Isoprenoid quinone composition of some marine Alteromonas, Marinomonas, Deleya, Pseudomonas, and Shewanella species. J. Gen. Microbiol. 138:2275–2281. [Google Scholar]
- 2.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beliaev, A., D. Saffarini, J. McLaughlin, and D. Hunnicut. 2001. MtrC, an outer membrane decaheme c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722–730. [DOI] [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. [DOI] [PubMed] [Google Scholar]
- 5.Francis, C. A., A. Y. Obraztsova, and B. M. Tebo. 2000. Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl. Environ. Microbiol. 66:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaspard, S., F. Vazquez, and C. Holliger. 1998. Localization and solubilization of the iron(III) reductase of Geobacter sulfurreducens. Appl. Environ. Microbiol. 64:3188–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gennis, R. B., and V. Stewart. 1996. Respiration, p.217–261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington D.C.
- 8.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, et al. 2000. DNA sequence of both chromsomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovely, D., S. Giovannoni, D. White, J. Champine, and E. Phillips. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336–344. [DOI] [PubMed] [Google Scholar]
- 10.Meganathan, R. 1996. Biosynthesis of isoprenoid quinones menaquinone (vitamin K2) and ubiquinone (coenzyme Q), p.642–656. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington D. C.
- 11.Meganathan, R. 2001. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q): a perspective on enzymatic mechanisms. Vitam. Horm. 61:173–218. [DOI] [PubMed] [Google Scholar]
- 12.Myers, C., and J. Myers. 1993. Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 108:15–22. [Google Scholar]
- 13.Myers, C., and J. Myers. 1993. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 114:215–222. [Google Scholar]
- 14.Myers, C., and K. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. [DOI] [PubMed] [Google Scholar]
- 15.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nealson, K., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311–343. [DOI] [PubMed] [Google Scholar]
- 17.Newman, D., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94–97. [DOI] [PubMed] [Google Scholar]
- 18.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, et al. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norager, S., P. Legrand, L. Pieulle, C. Hatchikian, and M. Roth. 1999. Crystal structure of the oxidized and reduced acidic cytochrome c3 from Desulfovibrio africanus. J. Mol. Biol. 290:881–902. [DOI] [PubMed] [Google Scholar]
- 20.Sharma, V., K. Suvarna, R. Meganathan, and M. E. S. Hudspeth. 1992. Menaquinone (vitamin K2) biosynthesis: nucleotide sequence and expression of the menB gene from Escherichia coli. J. Bacteriol. 174:5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taber, H. W. 1993. Respiratory chains, p.199–212. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.