Abstract
The origin and evolution of bacterial introns are still controversial issues. Here we present data on the distribution and evolution of a recently discovered divergent tRNALeu(UAA) intron. The intron shows a higher sequence affiliation with introns in tRNAIle(CAU) and tRNAArg(CCU) genes in α- and β-proteobacteria, respectively, than with other cyanobacterial tRNALeu(UAA) group I introns. The divergent tRNALeu(UAA) intron is sporadically distributed both within the Nostoc and the Microcystis radiations. The complete tRNA gene, including flanking regions and intron from Microcystis aeruginosa strain NIVA-CYA 57, was sequenced in order to elucidate the evolutionary pattern of this intron. Phylogenetic reconstruction gave statistical evidence for different phylogenies for the intron and exon sequences, supporting an evolutionary model involving horizontal intron transfer. The distribution of the tRNA gene, its flanking regions, and the introns were addressed by Southern hybridization and PCR amplification. The tRNA gene, including the flanking regions, were absent in the intronless stains but present in the intron-containing strains. This suggests that the sporadic distribution of this intron within the Microcystis genus cannot be attributed to intron mobility but rather to an instability of the entire tRNALeu(UAA) intron-containing genome region. Taken together, the complete data set for the evolution of this intron can best be explained by a model involving a nested evolution of the intron, i.e., wherein the intron has been transferred horizontally (probably through a single or a few events) to a tRNALeu(UAA) gene which is located within a unstable genome region.
The origin and evolution of introns in bacteria, and in particular the tRNALeu(UAA) self-splicing group I introns in cyanobacteria, have drawn considerable attention. The existence of related introns in the tRNALeu(UAA) gene of most green plastids, some plastids of red origin (2), and in cyanobacteria suggest that this intron could be at least 1 billion years old and possibly even a relict from an RNA world (14, 18, 30). More recently, however, an additional divergent type of tRNALeu(UAA) introns has been identified in the cyanobacterial radiation, suggesting that the evolution of tRNALeu(UAA) introns is more complex than previously anticipated (19, 21). The divergent introns were identified in both the Microcystis genus and in the Nostoc group (19). These introns have higher homology to group I introns in tRNAIle(CAU) and tRNAArg(CCU) genes than to the other tRNALeu(UAA) group I introns (19, 21).
A group I intron-encoded endonuclease specific for the anticodon region of tRNAfMet has been identified in Synechocystis sp. strain PCC 6803. The distribution pattern of this endonuclease suggests that it may be mobile (3). The presence of mobile endonucleases, or endonucleases located outside the intron, has previously been suggested based on the distribution pattern of the group I introns in cyanobacteria (19). The recent discovery of an intron-encoded endonuclease gives further support for a complex pattern of evolution of tRNA group I introns in bacteria.
The work presented here is a further step toward defining possible mechanisms for the evolution of tRNA introns in bacteria (5). We have addressed the evolution and origin of the divergent tRNALeu(UAA) intron within the Microcystis genus. The origin of the intron was investigated by comparative phylogenetic reconstructions of complete intron and the corresponding tRNALeu(UAA) exon sequences. These data support the intron being horizontally transferred between different tRNA genes. The next question we addressed was whether the sporadic intron distribution within the Microcystis genus (21) can be explained by intron mobility or, rather, by mechanisms involving instability of the whole intron-containing genomic region. Our results obtained by PCR amplification and Southern hybrization show that the whole region containing both the intron and the exon is absent in the intronless strains. Thus, the distribution of tRNALeu(UAA) group I introns within the Microcystis genus can be attributed to both intron transfer and recombination of the entire tRNA and intron-containing region.
MATERIALS AND METHODS
Cultures and sample preparation.
The organisms used in this work were classified according to the criteria given in Bergey’s Manual of Systematic Bacteriology (4). Most of the strains investigated are maintained at the Norwegian Institute for Water Research (NIVA). The strains used were as follows: Microcystis aeruginosa NIVA-CYA (N-C) 57, 228/1, and 31; Microcystis flos-aquae N-C 144; Microcystis viridis N-C 122/2 and 169/7; Microcystis botrys N-C 264, Nostoc sp. strain N-C 308; Aphanizomenon gracile N-C 103; and Anabaena lemmermannii N-C 83/1. The cultures are maintained at constant temperature at 17 ± 2°C in growth medium Z8 (17). The strains were originally isolated from their natural habitat as single cells or filaments. The cultures at NIVA are routinely examined for contamination by microscopy. Absence or low levels of contaminants were confirmed through direct sequencing of 16S ribosomal DNA (rDNA) without cloning through the application of primers located in conserved regions of the gene (24). Based on these criteria, the cultures are confirmed as unialgal (19). DNA extraction for PCR was done as described previously (24). For restriction enzyme digestion and Southern blotting, DNA was isolated from ∼50-mg (wet weight) cell pellets by a standard phenol-chloroform method (22).
PCR amplification, cloning, and sequencing.
The PCR primers used in this work are described in Table 1. Between 30 and 40 cycles were used in the amplification reactions, with a denaturation at 95°C for 30 s, annealing for 30 s at the temperatures described in Table 1, and extension at 72°C for 30 s to 1 min. All reactions were initiated by a 4-min denaturation at 94°C and terminated with a 7-min extension at 72°C. The amplified products were cloned by using the TA-cloning pGEM-T Vector system (Promega, Madison, Wis.). The sequencing was done with the Model 373 DNA Sequencer (Applied Biosystems, Foster City, Calif.). The TA-cloning and subsequent sequencing were done according to standard protocols (Promega and Applied Biosystems, respectively).
TABLE 1.
Primer sequences with corresponding Tm values
| Name | Sequence | Tma (°C) |
|---|---|---|
| EG | 5′-ggg gac ttg aac ccc cam ggg-3′ | 67.9 |
| EB | 5′-gaa ccc cca agg caa agc ccg-3′ | 69.2 |
| EE | 5′-ttc gcc acg tcc gca tag cca-3′ | 61.9 |
| OSF1 | 5′-cat cca tca agc gaa atc aga-3′ | 57.4 |
| OSF3 | 5′-gtt cct tga gcg ggt tct tg-3′ | 58.4 |
| EF | 5′-acg tgg cga aat cgg tag acg c-3′ | 66.1 |
| OSF2 | 5′-caa gaa ccc gct caa gga ac-3′ | 58.4 |
| OSF4 | 5′-act acc ttg tcc cct ttt tcg-3′ | 56.1 |
| CB | 5′-tgg ggg tgg ggg gac ttg a-3′ | 66.4 |
| DH | 5′-gtg gtc tgg act atc cct tc-3′ | 52.1 |
| ED | 5′-tcg ggc wac cga gtg gcg g-3′ | 72.0 |
| EC | 5′-cgc cag cgg ggc acg gtg a-3′ | 73.5 |
| DG | 5′-gtg gtc tgg act atc cct tc-3′ | 52.1 |
| CA | 5′-gcg gaa tgg tag acg cta cgg a-3′ | 64.1 |
| OSF5 | 5′-gtt cgc ttg aat atg caa agg a-3′ | 58.0 |
| OSF6 | 5′-atc tga ttt cgc ttg atg gat g-3′ | 57.8 |
| CAB | 5′-cga aat cgg tag acg caa cgg-3′ | 63.4 |
| B-CBB | 5′-cgg acg ggg gga ctt gaa c-3′ | 63.4 |
The annealing temperature is 5°C below the Tm of the primer with the lowest melting temperature in the PCR reaction when the primer pairs were used in various combinations.
Southern hybridization.
DNA samples of ca. 1 μg were separately digested overnight at 37°C with 1 U of HindIII and AluI (Promega), respectively. The restricted DNA were then separated on a 1.5% agarose gel at 45 V overnight. Transfer of DNA and subsequent cross-linking to GeneScreen hybridization membranes were performed as recommended by the manufacturer (NEN, Boston, Mass.). The membranes were hybridized as described by Galau et al. (10). The hybridizations were done overnight at 50°C for the tRNA exon probes and at 55°C for the intron probes; the 16S rDNA probes were hybridized at 65°C.
Single-stranded 32P-labeled probes were generated from PCR-amplified DNA by using a random primer DNA labeling kit (Boehringer Mannheim, GmbH, Mannheim, Germany) as described in Espelund et al. (6). The regions used for probes are displayed in Fig. 1. The exon probe for tRNALeu(UAA) was made with a synthetic tRNALeu(UAA) type B gene (19) without intron as a template.
FIG. 1.
Cloned flanking region, included probe, and primer regions for the primers used to generate the probes for M. aeruginosa N-C 57 (EMBL accession no. AJ307004). Two partial ORFs (RF1 and RF2) were identified in the flanking region. The primer sites and orientation are indicated with arrows, while the probe regions are indicated with bars. The type B tRNALeu(UAA) exon probe is denoted with a bar with a stippled line to indicate that stippled region is not present in the probe.
Phylogenetic reconstruction.
The sequences were aligned by using CLUSTAL X software (28) and then manually edited by the program GeneDoc (K. Nicholas and H. Nicholas [GeneDoc Multiple Sequence and Alignment Editor and Shading Utility, v.2.5.002]). The ambiguously aligned sites were denoted as “N” to obtain maximal phylogenetic information from the alignment. A total of 275 aligned positions were considered for the intron, while 100 positions were used in the phylogenetic reconstruction for the exon.
Separate phylogenetic trees were constructed by using the maximum-parsimony method (9) and the distance method (25) provided in the software package PAUP*4.0 developed by D. L. Swoford (Florida State University, Tallahassee) and distributed by Sinauer Associates, Inc. (Sunderland, Mass.). In addition, maximum-likelihood methods were provided in the Puzzle 4.0.2 software (27). Search for the maximum-parsimony tree was done heuristically. Both maximum-likelihood distances with empirically determined base frequencies (7) and LogDet distances were used for the minimum evolution distance trees. We used the model developed by Hasegawa et al. (11) for the maximum-likelihood analysis. For the parsimony, and the distance methods consensus trees were constructed from 1,000 bootstrap replicates (8), while for the maximum-likelihood analysis 1,000 quarter puzzling steps were used (27). The tree length skewness, determined by the third momentum statistics (g1) for 10,000 randomly generated trees, was used to investigate the phylogenetic structure of the data. The results obtained were compared to critical values given by Hillis and Huelsenbeck (12).
RESULTS
The rationale for this study was to employ phylogenetic analyses of the tRNALeu(UAA) exons and introns, separately. Together with the distribution of these elements, as determined by PCR and Southern analyses, the evolution of the tRNALeu(UAA) exon and/or intron region was reconstructed.
Evolutionary origin of M. aeruginosa tRNALeu(UAA) exon sequences.
The intron-containing Microcystis tRNALeu(UAA) gene has the typical cloverleaf secondary structure of tRNA genes (Fig. 2). To determine the evolutionary origin of the intron-containing Microcystis tRNALeu(UAA) gene, a representative set of known tRNA genes were selected from the tRNA database (26). The tRNA sequences were aligned both manually and by using the information obtained from the alignment provided in the tRNA database.
FIG. 2.
Secondary structure of the intron-containing tRNALeu(UAA) gene. The intron insertion site is indicated by an arrow. The structure was obtained by a combination of MFOLD computer prediction (GCG Package; Genetic Computer Group, Madison, Wis.) and manual editing.
The g1 (10,000 randomly generated trees) for the tRNA data set was 1.15, which is significant (P = 0.01) according to the criteria given by Hillis and Huelsenbeck (12). The tRNA alignment supported a monophyletic origin of tRNALeu(UAA) genes and showed, in addition, that the tRNAIle(CAU) and tRNAArg(CCU) genes are relatively distantly related to all known tRNALeu(UAA) genes. Furthermore, the intron-containing tRNALeu(UAA) gene in Microcystis formed a group with the other cyanobacterial and chloroplast tRNALeu(UAA) genes (Fig. 3A). We have previously demonstrated that all Microcystis strains with an intron-containing tRNALeu(UAA) gene also contained a tRNALeu(UAA) gene without an intron. These two gene clusters, however, are quite evolutionarily distinct, indicating that they are not the same gene with and without an intron (19). The intron-containing tRNALeu(UAA) gene has previously been denoted as type B, while the tRNA gene without an intron was denoted as type A (19). We used the same nomenclature in the work presented here.
FIG. 3.
Phylogenetic reconstruction of tRNA exon (A) and intron (B) sequences. The trees were built by using both distance and maximum-parsimony methods. The trees shown in panels A and B (based on 100 and 275 aligned positions, respectively) were built by the neighbor-joining method using maximum-likelihood distances. The distances are expressed as substitutions per nucleotide in the neighbor-joining tree. Numbers at the nodes (expressed as maximum likelihood/LogDet distances/maximum-likelihood distances/heuristic search for maximum parsimony trees) indicate the percentage of Puzzle (for maximum likelihood) and bootstrap trees for the others in which the cluster descending from the node was found. Numbers are only shown for nodes supported by ≥50% for all of the methods tested. Each tRNA gene is annotated in panel A, while the three clusters of introns identified (panel B) are indicated by I, II, and III.
Evolutionary origin of M. aeruginosa tRNALeu(UAA) intron sequences.
The conserved motifs in the primary sequence were used to guide the intron alignments. Secondary structure alignments were avoided for the introns due to the uncertainties and theoretical nature of these structures. The positions that could not be unambigiously aligned were coded as N in order to obtain maximum phylogentic information from the alignment (19).
The intron data set (10,000 randomly generated trees) also produced a significant (P = 0.01) g1 of 0.77 according to the criteria given by Hillis and Huelsenbeck (12). The tRNA introns identified within the Microcystis genus are closely related, with a homology of >99% (results not shown). The intron alignment supported a clustering of the Microcystis introns with the introns in tRNAIle(CAU) and tRNAArg(CCU) genes. The other cyanobacterial and chloroplast tRNALeu(UAA) introns, on the other hand, formed a separate cluster (Fig. 3B).
Flanking regions for tRNALeu(UAA) intron-containing gene.
The generated sequences were deposited in the database with EMBL accession no. AJ307004. A partial open reading frame (ORF) of 218 amino acids was identified upstream of the intron-containing tRNA gene (Fig. 1). No homology to other amino acid sequences (ORFs) were identified by a TBLASTN search through all six reading frames in the NCBI database (www.ncib.nlh.gov; 1 May 2001). The most closely related sequence was a region on chromosome 1 in Deinococcus radiodurans R, sequence section 209, nucleotides 6238 to 6414 (accession no. AE002072). There was a 33% identity in this region. A 48-amino-acid partial ORF was identified on the complementary strand downstream of the tRNALeu(UAA) gene. No significant matches were found in the database with a TBLASTN search with this partial ORF.
Distribution of tRNALeu(UAA) introns, flanks, and exons.
The distribution of the intron, flank, and exon sequences were accessed by both PCR amplification and Southern hybridization analyses. We constructed specific PCR primers (CAB and B-CBB) for selective amplification of the intron-containing tRNALeu(UAA) gene. This primer pair was then used in a PCR screening for isolates containing tRNALeu(UAA) genes of type B. All type B tRNALeu(UAA) genes identified contained introns, including the Nostoc strain N-C 308. The strains without identified introns (19, 21) did not yield any amplification products with this primer pair (including several strains belonging to the Nostoc group), suggesting the absence of type B tRNA genes (Fig. 4). The products were sequence verified, and the sequences obtained were confirmed through comparison to the published intron sequences sequences: accession no. y13474 for M. aeruginosa N-C 57, accession no. AJ228695 for M. aeruginosa N-C 228/1, and accession no. AJ228712 for Nostoc sp. strain N-C 308.
FIG. 4.
PCR amplification analyses with the primer pair CAB-B-CBB (A) and through control amplification of 16S rDNA with the primer pair CC-CD (B). All samples were electrophoresed in 1.5% agarose gels for 30 min at 100 V. Twenty percent of the amplification products was loaded in each lane. MW, molecular weight marker.
Southern hybridization analyses were, in addition to the PCR screening, conducted in order to verify the distribution of the intron-containing tRNALeu(UAA) gene region within the Microcystis genus. Hybridization with an exon probe for non-intron-containing tRNALeu(UAA) genes (19) to HindIII-digested DNA gave hybridizing bands for all of the strains tested (Fig. 5A). Hybridization bands of >20 kb were identified for strains N-C 57 and 228/1 with tRNA type B probe (Fig. 5B), the intron probe (Fig. 5C), and the flank 1 probe (see Fig. 1 for the probe locations), while no hybridization was observed with these probes for the other strains tested (Fig. 5B to D). Hybridization with a 16S rDNA probe was used to standardize the amount of template, as well as to confirm that no significant commensals were present in the preparations. Multiple hybridizing bands would have been expected if commensals were present in the samples. Multiple bands could, however, also have been present if there are differences among the ribosomal operons in a single strain. A single 16S rDNA band was identified for the intron-containing strains N-C 57 and 228/1, in addition to strains N-C 31, 144, and 169/7 (Fig. 5E). The only strains with two hybridizing bands were strains N-C 43 and 324/1 (probably due to operon differences [see results below]). The absence of commensals has also been determined through direct sequencing of a 16S rDNA fragment amplified with the universal primer pair CC and CD (results not shown).
FIG. 5.
Southern hybridization analysis of selected Microcystis strains. Genomic DNA digested with HindIII was separated, blotted, and hybridized as described in Materials and Methods. The same membrane was used in each of the hybridizing experiments, providing an exact assignment of the hybridizing bands from different experiments. The hybridizing probes were as follows: flank 1 (A), tRNA type A (B), tRNA type B (C), intron (D), and 16S rDNA (E).
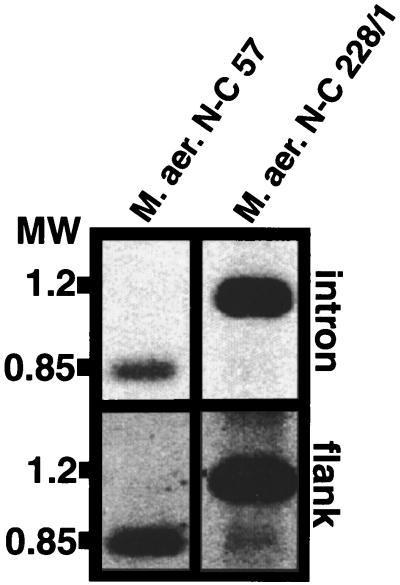
Restriction cutting with the enzyme AluI was conducted in order to obtain a better resolution in the intron-containing tRNA region. Hybridization studies with the intron and the flank 2 probe strongly suggest that these regions are located on the same restriction fragment with sizes of approximately 850 and 1,200 bp, respectively, for the two intron-containing strains N-C 57 and 228/1 (Fig. 6).
FIG. 6.
Southern hybridization analysis with AluI-digested DNA for the M. aeruginosa strains N-C 57 and 228/1. Digested genomic DNA was separated, blotted, and hybridized as described in Materials and Methods. The following probes were used: flank 2 (A) and intron (B).
DISCUSSION
As more details about the cyanobacterial tRNALeu(UAA) group I introns are accumulated, a more complex intron evolutionary pattern emerges. On the basis of the data presented here, we describe an evolutionary model involving nested evolution of the tRNALeu(UAA) group I intron involving both horizontal intron transfer and recombination of the entire tRNA locus.
Phylogenetic reconstruction support intron transfer.
Previously, data have been presented that support horizontal intron transfer between tRNALeu(UAA), tRNAIle(CAU) and/or tRNAArg(CCA) genes (21). These results, however, were based on partial tRNA sequences (only 28 aligned positions) which do not yield sufficient information for rigid conclusions. Here we have cloned the complete tRNALeu(UAA) exon sequence from the intron-containing tRNA gene in the M. aeruginosa strain N-C 57 (20). The comparison of complete exon sequences gave further evidence for separate clustering of tRNALeu(UAA) apart from the tRNAIle(CAU) and tRNAArg(CCA) genes (see Fig. 3A).
The sequences for the tRNALeu(UAA) intron in Microcystis, on the other hand, have a clear affiliation with the introns in tRNAIle(CAU) and tRNAArg(CCA) (see Fig. 3B). Theoretically, it cannot be excluded that there has been concerted selection on certain primary sequence elements, resulting in substitutions that do not reflect the evolutionary history. Such an argument, however, questions the whole basis of modern phylogeny (for microorganisms, see the review by Woese [29]). The underlying assumption is that the majority of the genetic changes are due to neutral and not to selective substitutions (13). If we assume that the majority of genetic changes in our data set are neutral, then the most likely explanation for the different phylogenies for tRNALeu(UAA) exons and introns is horizontal intron transfer.
No homology to other known sequences for the identified reading frames.
The inferred amino acid sequence of the partial ORFs upstream and downstream of the tRNALeu(UAA) gene did not show any significant similarities with previously characterized amino acid sequences. The potential functions can therefore not be identified from previously characterized genetic elements in the database. Research addressing whether or not the genetic elements in the flanking regions of the intron-containing tRNALeu(UAA) gene are important for the intron distribution is thus called for. The presence of potentially mobile endonucleases without homology to other known sequences has recently been identified for a cyanobacterial group I intron (3).
The distribution of tRNALeu(UAA) introns, exons, and flanking regions in the Microcystis genus suggests instability of the whole region.
Both the flanks and the intron-containing tRNA gene are present in the strains N-C 57 and 228/1 but absent in the other investigated strains (see Fig. 5). These results strongly suggest that the observed intron distribution in the Microcystis genus is not due to the mobility of the intron itself but rather to instability of the whole intron-containing region. We do not know whether this distribution pattern is due to insertions or deletions or if the intron-containing tRNA gene is located on an episomal element. However, we conclude that relatively recent event(s) have caused the distribution pattern since the different Microcystis strains are closely related (23, 24).
Model for the evolution of the Microcystis tRNALeu(UAA) group I introns.
We have presented the first statistically supported phylogenetic evidence for the horizontal transfer of group I introns been between different tRNA genes in bacteria. Furthermore, we have linked the location of the intron-containing tRNA gene to a region in the genome prone to recombinations. Our data support that the tRNA introns can evolve both by being localized in an unstable genetic element or region and via an intrinsic property of (infrequent) intron mobility (1, 15) (Fig. 7).
FIG. 7.
Model for the evolution of the Microcystis tRNALeu(UAA) group I intron. The model shows that the process of intron homing is relatively infrequent (A) and that the process of recombination of the entire tRNALeu(UAA) genome region is relatively frequent (B). Taken together, these two processes can explain the origin of the Microcystis introns and the current intron distribution within this genus.
The distribution and evolution of introns in bacteria have recently been thoroughly reviewed by Edgell et al. (5). They conclude that the evolution of introns in bacterial tRNA genes is unique. No introns have until now been isolated from tRNA genes in mobile elements. The intron-containing tRNA gene we identified, however, has a distribution pattern that resembles a pattern expected for a mobile element. Future research will reveal whether tRNA group I introns also will be identified among the diversity of introns identified in mobile entities (16).
Acknowledgments
We thank Olav M. Skulberg and Randi Skulberg for providing the cyanobacterial strains used in this study.
The work was supported by grant 107622/420 from the Norwegian Research Council to K.S.J and in part by a research levy on certain agricultural products.
REFERENCES
- 1.Belfort, M., M. E. Reaban, T. Coetzee, and J. Z. Dalgaard. 1995. Prokaryotic introns and inteins: a panoply of form and function. J. Bacteriol. 177:3897–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besendahl, A., Y. L. Qiu, J. Lee, J. D. Palmer, and D. Bhattacharya. 2000. The cyanobacterial origin and vertical transmission of the plastid tRNA(Leu) group-I intron. Curr. Genet. 37:12–23. [DOI] [PubMed] [Google Scholar]
- 3.Bonocora, R. P., and D. A. Shub. 2001. A novel group I intron-encoded endonuclease specific for the anticodon region of tRNA fMet genes. Mol. Microbiol. 39:1299–1306. [DOI] [PubMed] [Google Scholar]
- 4.Castenholz, R. W., and J. B. Waterbury. 1989. Group I. Cyanobacteria, p.1710–1728. In N. Pfenning and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology. The Williams & Wilkins Co., Baltimore, Md.
- 5.Edgell, D. R., M. Belfort, and D. A. Shub. 2000. Barriers to intron promiscuity in bacteria. J. Bacteriol. 182:5281–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espelund, M., R. A. P. Stacy, and K. S. Jakobsen. 1990. A simple method for generating single-stranded DNA probes labeled to high activities. Nucleic Acids Res. 18:6157–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17:368–376. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. [DOI] [PubMed] [Google Scholar]
- 9.Fitch, W. M. 1977. On the problem of discovering the most parsimonious tree. Am. Naturalist 111:223–257. [Google Scholar]
- 10.Galau, G. A., D. W. Huges, and L. Dure III. 1986. Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) mRNAs. Plant Mol. Biol. 7:155–170. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa, M., Y. Iida, T. Yano, F. Takaiwa, and M. Iwabuchi. 1985. Phylogenetic relationships among eukaryotic kingdoms inferred from ribosomal RNA sequences. J. Mol. Evol. 22:32–38. [DOI] [PubMed] [Google Scholar]
- 12.Hillis, D. M., and J. P. Huelsenbeck. 1992. Signal, noise, and reliability in molecular phylogenetic analyses. J. Hered. 83:189–195. [DOI] [PubMed] [Google Scholar]
- 13.Kimura, M. 1983. The neutral theory of molecular evolution, p.208–233. In M. Nei and R. K. Koehn. (ed.), Evolution of genes and proteins. Sinauer Associates, Sunderland, Mass.
- 14.Kuhsel, M. G., R. Strickland, and J. D. Palmer. 1990. An ancient group I intron shared by eubacteria and chloroplasts. Science 250:1570–1573. [DOI] [PubMed] [Google Scholar]
- 15.Lambowitz, A. M., and M. Belfort. 1993. Introns as mobile genetic elements. Annu. Rev. Biochem. 62:587–622. [DOI] [PubMed] [Google Scholar]
- 16.Landthaler, M., and D. A. Shub. 1999. Unexpected abundance of self-splicing introns in the genome of bacteriophage Twort: introns in multiple genes, a single gene with three introns, and exon skipping by group I ribozymes. Proc. Natl. Acad. Sci. USA 96:7005–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norwegian Institute for Water Research. 1990. Culture collection of algae: catalogue of strains. Norwegian Institute for Water Research, Oslo, Norway.
- 18.Paquin, B., S. D. Kathe, S. A. Nierzwicki-Bauer, and D. A. Shub. 1997. Origin and evolution of group I introns in cyanobacterial tRNA genes. J. Bacteriol. 179:6798–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudi, K., and K. S. Jakobsen. 1999. Complex evolutionary patterns of tRNALeu(UAA) group I introns in the cyanobacterial radiation. J. Bacteriol. 181:3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudi, K., T. Fossheim, and K. S. Jakobsen. 1999. Restriction cutting-independent method for cloning genomic DNA segments outside the boundaries of known sequences. BioTechniques 27:1170–1177. [DOI] [PubMed] [Google Scholar]
- 21.Rudi, K., and K. S. Jakobsen. 1997. Cyanobacterial tRNA(Leu)(UAA) group I introns have polyphyletic origin. FEMS Microbiol. Lett. 156:293–298. [DOI] [PubMed] [Google Scholar]
- 22.Rudi, K., M. Kroken, O. J. Dahlberg, A. Deggerdal, K. S. Jakobsen, and F. Larsen. 1997. Rapid, universal method to isolate PCR-ready DNA using magnetic beads. BioTechniques 22:506–511. [DOI] [PubMed] [Google Scholar]
- 23.Rudi, K., O. M. Skulberg, and K. S. Jakobsen. 1998. Evolution of cyanobacteria by exchange of genetic material among phyletically related strains. J. Bacteriol. 180:3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudi, K., O. M. Skulberg, F. Larsen, and K. S. Jakobsen. 1997. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl. Environ. Microbiol. 63:2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425. [DOI] [PubMed] [Google Scholar]
- 26.Sprinzl, M., C. Steegborn, F. Hubel, and S. Steinberg. 1996. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 24:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strimmer, K., and A. von Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964–969. [Google Scholar]
- 28.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, M. Q., S. D. Kathe, H. Goodrich-Blair, S. A. Nierzwicki-Bauer, and D. A. Shub. 1990. Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science 250:1566–1570. [DOI] [PubMed] [Google Scholar]