Abstract
The NADPH-dependent l-sorbose reductase (SR) of l-sorbose-producing Gluconobacter suboxydans IFO 3291 contributes to intracellular l-sorbose assimilation. The gene disruptant showed no SR activity and did not assimilate the once-produced l-sorbose, indicating that the SR functions mainly as an l-sorbose-reducing enzyme in vivo and not as a d-sorbitol-oxidizing enzyme.
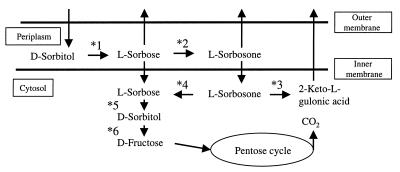
l-Sorbose is an important intermediate for industrial production of vitamin C (4) and is produced from d-sorbitol by Gluconobacter strains. During metabolic studies of l-sorbose with isotopes, it was noticed that l-sorbose was consumed to yield carbon dioxide, possibly via d-sorbitol (7). The metabolic pathways of d-sorbitol, l-sorbose, and their metabolites in Gluconobacter strains are depicted in Fig. 1 according to data from previous studies (2, 7, 12). Sugisawa et al. purified and characterized NADPH-linked l-sorbose reductase (SR) with a molecular weight of 60,000 (monomer) from Gluconobacter melanogenus N44-1 (11). The SR enzyme showed its optimum pHs for the reduction of l-sorbose and for oxidation of d-sorbitol at 7.0 and 10.0 to 10.5, respectively, and is thus termed sorbitol-sorbose oxidoreductase. Recently two sorbitol-sorbose oxidoreductases of Gluconobacter strains were also reported: NADPH-dependent SR of G. melanogenus IFO 3294 (with a molecular mass of 60 kDa and consisting of two identical subunits of 30 kDa) (1) and NADP-dependent d-sorbitol dehydrogenase (SLDH) of Gluconobacter oxydans G624) (with the calculated molecular mass of 53,634 Da and consisting of one subunit) (6). In this study, we cloned the SR gene of G. suboxydans IFO 3291, which was found to encode basically the same enzyme as the SLDH of G. oxydans G624, and constructed and characterized the gene disruptant of G. suboxydans IFO 3291 for confirming the physiological role of the SR enzyme.
FIG. 1.
The metabolic pathway of d-sorbitol, l-sorbose, and their metabolites in Gluconobacter strains. *1, membrane-bound d-sorbitol dehydrogenase (2); *2, membrane-bound l-sorbose dehydrogenase (2); *3, NAD(P)-dependent l-sorbosone dehydrogenase (2); *4, NADPH-dependent l-sorbosone reductase (2); *5, NADPH-dependent l-sorbose reductase (2,11); *6, NAD-dependent d-sorbitol dehydrogenase (1).
Plasmid pSUP202 and plasmid pSUP2021 (9) were used as a suicide vector and a vector for Tn5 mutagenesis in Gluconobacter strains. Recombinant DNA technique and conjugal mating were done as previously reported (8). No. 5 medium containing 80 g of d-sorbitol per liter (2) and SL-SCM medium containing (per liter) 3 g of yeast extract, 3 g of beef extract, 3 g of corn steep liquor, 10 g of peptone, 1 g of urea, 1 g of KH2PO4, 0.2 g of MgSO4 · 7H2O, 1 g of CaCO3 (production grade), and 20 g of d-sorbitol were used for cultivating the Gluconobacter strains.
A Tn5 mutant defective in l-sorbose reductase activity was obtained from a derivative of G. melanogenus IFO 3293 through Tn5 mutagenesis with P1::Tn5 (3) and was designated strain 26-9A. The mutant was selected as a d-sorbitol nonproducer from l-sorbose under a resting cell system (2) and was confirmed to not grow on l-sorbose (No. 5 medium). We confirmed the SR deficiency of strain 26-9A by a photometric enzyme assay (11), with strain IFO 3293 as the positive control. Strains IFO 3293 and 26-9A showed 0.20 and <0.01 U of SR activity per mg of cytosol protein, respectively.
After confirming that the Tn5 insertion in 26-9A caused SR deficiency (below 0.01 U/mg of cytosol protein) by reconstructing the Tn5 mutant with the DNA fragment containing Tn5, we determined the nucleotide sequence of the Tn5-inserted region. The region encoded a polypeptide belonging to the mannitol dehydrogenase superfamily including mannitol-2-dehydrogenase of Rhodobacter sphaeroides (accession number P33216 [5]), mannonate oxidoreductase of Escherichia coli (P39160), and mannitol-1-phosphate 5-dehydrogenases of, for example, Enterococcus faecalis (P27543). The mannitol dehydrogenase (MDH) (EC 1.1.1.67) is a mannitol-fructose oxidoreductase. The SR enzyme of the Gluconobacter strain is a sorbitol-sorbose/mannitol-fructose oxidoreductase (11). The amino acid sequences deduced from the SR nucleotide sequences around the Tn5 insertion point were aligned with those belonging to the MDH superfamily (data not shown). In the SR sequence, the MDH signature of PS00974 in the protein motif database PROSITE was found. The corresponding sequence from strain 26-9A, FPNGMVDRITP, and the other region showing a high homology, MTITEGGY, were selected for designing the set of primers for PCR.
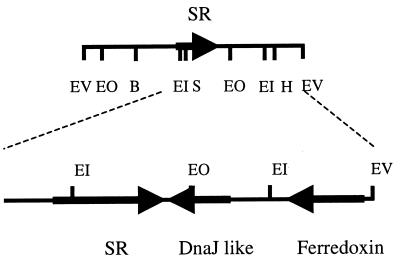
We cloned the partial SR gene of G. suboxydans IFO 3291 (ca. 300 bp) through PCR amplification with the primers 5′-ATGAC(C/G)AT(C/T)AC(C/G)GA(A/G)GG(A/C/T)GG(A/C/T)TA and 5′-CG(A/G)TC(A/C/G)ACCAT(A/G/T)CC(A/G)TT(A/G/C)GGGAA and then obtained the complete gene with the PCR product as the probe in an 8.0-kb EcoRV fragment. The complete nucleotide sequence of the open reading frame (ORF) was 1,455 bp (accession number AB063188), encoding 485 amino acids; the calculated Mr of 53,541 agrees with that of the purified enzyme (60,000 [11]). Computer analysis of the sequence including the upstream and downstream regions showed that there are two ORFs, DnaJ-like protein and ferredoxin, downstream in the direction opposite to that of the SR gene (as shown in Fig. 2), suggesting that there are no ORFs included in an operon with the SR gene.
FIG. 2.
Computer analysis of the sequences of upstream and downstream regions of the SR gene. B, BamHI; EI, EcoRI; EO, EcoO1091; EV, EcoRV; H, HindIII; S, SmaI.
Homology search with the nucleotide sequence of the SR gene was done with the Blastx program; recently published sequences of genes encoding NADP-SLDH of G. oxydans strain G624 (AB028937[6]) and NAD-MDH sequences, including that from Pseudomonas aeruginosa (AE004660 [10]), were found in addition to that from R. sphaeroides. The amino acid sequence of SR showed identities of 84.5, 42.6, and 39.6%, respective to the order of the nucleotide sequences described above. SR from G. suboxydans IFO 3291 should be an ortholog of NADP-SLDH from G. oxydans G624.
An SR gene disruptant was constructed with pSUP202-SR::Km, which has a Kmr gene cassette from pUC4K (Amersham Pharmacia Biotech, Uppsala, Sweden) as an EcoRI fragment in an EcoRI site of the cloned SR gene. The gene disruption was confirmed by Southern blot hybridization and was designated SR3. In a spectrophotometric assay, strains SR3 and IFO 3291 showed SR activities of 0.02 (negligible) and 0.92 U/mg of cytosol protein, respectively. l-Sorbose assimilation and growth profiles of strains SR3 and IFO 3291 were evaluated with the SL-SCM medium (Table 1). Both converted d-sorbitol nearly stoichiometrically in 9 to 12 h; IFO 3291 consumed l-sorbose accompanying further cell growth, while SR3 neither consumed it nor grew further. The reason why SR3 consumed d-sorbitol faster, resulting in faster growth and faster production of l-sorbose than IFO 3291, is unknown. Sugar and sugar alcohol assimilation abilities of both strains were further confirmed by using a minimal agar medium containing 2.5% l-sorbose, d-sorbitol, or d-fructose together with 1% Casamino Acids. Strain IFO 3291 grew on all the carbon sources, while SR3 grew on d-sorbitol and d-fructose but not on l-sorbose; this result agrees with the pathway shown in Fig. 1.
TABLE 1.
l-Sorbose assimilation and growth of G. suboxydans IFO 3291 and SR3a
| Time (h) | Assimilation (g/liter) of:
|
Growth (OD600) of:
|
||||
|---|---|---|---|---|---|---|
|
l-Sorbose
|
d-Sorbitol
|
IFO 3291 | SR3 | |||
| IFO 3291 | SR3 | IFO 3291 | SR3 | |||
| 0 | 0.0 | 0.0 | 20.0 | 20.0 | 0.1 | 0.1 |
| 3 | 1.9 | 4.5 | 20.7 | 14.5 | 0.3 | 0.5 |
| 6 | 3.8 | 9.2 | 17.7 | 11.0 | 0.6 | 1.0 |
| 9 | 9.5 | 17.7 | 10.2 | 1.9 | 1.2 | 2.3 |
| 12 | 18.3 | 17.6 | 1.2 | 0.0 | 2.6 | 2.8 |
| 15 | 16.4 | 19.0 | 0.0 | 0.0 | 3.9 | 2.6 |
| 18 | 14.0 | 18.1 | 0.0 | 0.0 | 4.7 | 2.5 |
| 23 | 9.2 | 18.1 | 0.0 | 0.0 | 5.9 | 2.5 |
The strains were cultivated in 500-ml flasks containing 50 ml of SL-SCM medium at 30°C on a rotary flask shaker at 180 rpm. OD600, optical density at 600 nm.
A database search of MedLine with the keyword sorbose gave us one sorbose assimilation pathway via l-sorbose-1-phosphate in E. coli, Klebsiella pneumoniae, and Lactobacillus casei; there were no references describing l-sorbose assimilation via d-sorbitol, with the exception of studies by us and coworkers (2, 7, 11). Adachi et al. (1) supposed from its enzymatic properties that the physiological role of NADPH-SR of G. melanogenus IFO 3294 would be assimilation of l-sorbose. In this report, we first genetically confirmed that SR triggers the l-sorbose assimilation via d-sorbitol, not via l-sorbose-1-phosphate. In addition, we revealed that the disruptant SR3 showed strong d-sorbitol dehydrogenase activity to produce l-sorbose comparable to that of strain IFO 3291 (Table 1), suggesting that SR does not function as a main d-sorbitol-oxidizing enzyme in vivo. This result is reasonable, because the pH of a cytosol is usually around 7, optimal for SR to function as a reductase rather than a dehydrogenase. Considering the existence of NADPH-dependent SR (a dimer with an Mr of 60,000) in G. melanogenus IFO 3294 (1), this SR could also exist in strains IFO 3291 and IFO 3293 and in their derivatives and could function weakly as the second pathway in assimilating l-sorbose for maintenance of life. On the other hand, NADP-SLDH of G. oxydans G624 (with a calculated Mr of 53,642) should be a synonym of SR of G. suboxydans IFO 3291 (with a calculated Mr of 53,541). In summary, in vivo SR should mainly be responsible for l-sorbose assimilation, not for l-sorbose production. Since l-sorbose is hard for most microorganisms to assimilate, whereas d-sorbitol is easily assimilated, this deposit (d-sorbitol to l-sorbose) and withdrawal (l-sorbose to d-sorbitol) system of sugars and sugar alcohols is a clever strategy adopted by Gluconobacter strains to survive in mixed populations of the microbial world.
Acknowledgments
We thank Takahide Kon and Noribumi Tomiyama for their technical contribution and helpful discussion.
REFERENCES
- 1.Adachi, O., Y. Ano, D. Moonmangmee, E. Shinagawa, H. Toyama, G. Theeragool, N. Lotong, and K. Matsushita. 1999. Crystallization and properties of NADPH-dependent l-sorbose reductase from Gluconobacter melanogenus IFO 3294. Biosci. Biotechnol. Biochem. 63:2137–2143. [DOI] [PubMed] [Google Scholar]
- 2.Hoshino, T., T. Sugisawa, M. Tazoe, M. Shinjoh, and A. Fujiwara. 1990. Metabolic pathway for 2-keto-l-gulonic acid formation in Gluconobacter melanogenus IFO 3293. Agric. Biol. Chem. 54:1211–1218. [Google Scholar]
- 3.Manning, R. F., and M. Kahn. January 1992. Biosynthesis of 2-keto-l-gulonic acid. U.S. patent 5,082,785.
- 4.Reichstein, T., A. Grüssner, and R. Oppenauer. 1933. Synthese der d- und l-ascorbinsäure (C-Vatamin). Helv. Chem. Acta 16:1019–1033. [Google Scholar]
- 5.Schneider, K. H., F. Giffhorn, and S. Kaplan. 1993. Cloning, nucleotide sequence and characterization of the mannitol dehydrogenase gene from Rhodobacter sphaeroides. J. Gen. Microbiol. 139:2475–2484. [DOI] [PubMed] [Google Scholar]
- 6.Shibata, T., C. Ichikawa, M. Matsuura, Y. Takata, Y. Noguchi, Y. Saito, and M. Yamashita. 2000. Cloning of a gene for d-sorbitol dehydrogenase from Gluconobacter oxydans G624 and expression of the gene in Pseudomonas putida IFO3738. J. Biosci. Bioeng. 89:463–468. [DOI] [PubMed] [Google Scholar]
- 7.Shinjoh, M., Y. Setoguchi, T. Hoshino, and A. Fujiwara. 1990. l-Sorbose dissimilation in 2-keto-l-gulonic acid-producing mutant UV10 derived from Gluconobacter melanogenus IFO 3293. Agric. Biol. Chem. 55:2257–2263. [Google Scholar]
- 8.Shinjoh, M., N. Tomiyama, A. Asakura, and T. Hoshino. 1995. Cloning and nucleotide sequencing of the membrane-bound l-sorbosone dehydrogenase gene of Acetobacter liquefaciens IFO 12258 and its expression in Gluconobacter oxydans. Appl. Environ. Microbiol. 61:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784–791. [Google Scholar]
- 10.Stover, C. K., X. Q. T. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrook-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. M. Lim, K. A. Smith, D. H. Spencer, G. K. S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959–964. [DOI] [PubMed] [Google Scholar]
- 11.Sugisawa, T., T. Hoshino, and A. Fujiwara. 1991. Purification and properties of NADPH-linked l-sorbose reductase from Gluconobacter melanogenus N44-1. Agric. Biol. Chem. 55:2043–2049. [Google Scholar]
- 12.Sugisawa, T., T. Hoshino, S. Masuda, S. Nomura, Y. Setoguchi, M. Tazoe, M. Shinjoh, S. Someha, and A. Fujiwara. 1990. Microbial production of 2-keto-l-gulonic acid from l-sorbose and d-sorbitol by Gluconobacter melanogenus. Agric. Biol. Chem. 54:1201–1209. [Google Scholar]