Abstract
Streptococcus salivarius is a lactose- and galactose-positive bacterium that is phylogenetically closely related to Streptococcus thermophilus, a bacterium that metabolizes lactose but not galactose. In this paper, we report a comparative characterization of the S. salivarius and S. thermophilus gal-lac gene clusters. The clusters have the same organization with the order galR (codes for a transcriptional regulator and is transcribed in the opposite direction), galK (galactokinase), galT (galactose-1-P uridylyltransferase), galE (UDP-glucose 4-epimerase), galM (galactose mutarotase), lacS (lactose transporter), and lacZ (β-galactosidase). An analysis of the nucleotide sequence as well as Northern blotting and primer extension experiments revealed the presence of four promoters located upstream from galR, the gal operon, galM, and the lac operon of S. salivarius. Putative promoters with virtually identical nucleotide sequences were found at the same positions in the S. thermophilus gal-lac gene cluster. An additional putative internal promoter at the 3′ end of galT was found in S. thermophilus but not in S. salivarius. The results clearly indicated that the gal-lac gene cluster was efficiently transcribed in both species. The Shine-Dalgarno sequences of galT and galE were identical in both species, whereas the ribosome binding site of S. thermophilus galK differed from that of S. salivarius by two nucleotides, suggesting that the S. thermophilus galK gene might be poorly translated. This was confirmed by measurements of enzyme activities.
Streptococcus salivarius is an oral bacterium that is phylogenetically closely related to Streptococcus thermophilus, which is used in food fermentation (16, 17, 25, 29). Both species were initially placed in the S. salivarius taxon as S. salivarius subsp. salivarius and S. salivarius subsp. thermophilus (7) but were regarded as distinct species by Schleifer et al. (28) on the basis of both genetic and phenetic criteria. These two species, together with Streptococcus vestibularis, form a distinct cluster within the streptococcal phylogenetic tree (13, 16, 25). Lactose, the principal energy source used by S. thermophilus for growth in milk, is transported into the cell by a permease (LacS) belonging to the glycoside-pentoside-hexuronide–cation symporter family (23). Lactose is hydrolyzed within the cell into glucose and galactose by β-galactosidase. Glucose is metabolized to lactic acid via the glycolytic, Embden-Meyerhof-Parnas pathway, whereas in most strains galactose cannot be metabolized and is expelled into the external medium (11, 14). The organization of the galactose operon coding for the Leloir pathway enzymes in S. thermophilus has recently been elucidated (5, 24, 36), indicating that the inability of S. thermophilus to metabolize galactose is not caused by the absence of the genetic information required for the synthesis of suitable metabolic pathways. Moreover, the activities of the enzymes involved in the Leloir pathway (galactokinase, galactose-1-P uridylyltransferase, and UDP-glucose 4-epimerase) have been detected in S. thermophilus (15, 30). This pathway catalyzes the transformation of galactose into glucose-1-P, which is further transformed into glucose-6-P by the enzyme phosphoglucomutase (22). Vaughan et al. (36) have shown that the structural gal genes of galactose-negative S. thermophilus CNRZ 302 are weakly transcribed. They proposed that this poor expression is caused by naturally occurring down-mutations in the promoter of the gal operon.
Unlike S. thermophilus, S. salivarius grows well on galactose (10), which is metabolized via the Leloir pathway as indicated by the finding that galactose-grown cells possess galactokinase, galactose-1-P uridylyltransferase, and UDP-glucose 4-epimerase activities (18). However, no information on the S. salivarius gal-lac gene cluster is yet available. In this work we present a characterization of this gene cluster in terms of organization and nucleotide sequence, a study of its expression via transcriptional analysis, and a measurement of gal gene product activities. The same experiments were carried out with S. thermophilus SMQ-301, a galactose-negative strain.
MATERIALS AND METHODS
Strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. S. salivarius was grown at 37°C and S. thermophilus was grown at 42°C in M17 broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.15, 0.2, or 0.5% glucose, lactose, or galactose as necessary. For DNA isolation, S. salivarius was grown at 37°C in a medium containing 10 g of tryptone and 5 g of yeast extract (Difco Laboratories), 2.5 g of NaCl, 2.5 g of disodium phosphate, and 5 g of glucose per liter. Growth was monitored by tracking the optical density at 660 nm. For the determination of sugar concentrations in the media of growing cells, samples (0.25 to 0.5 ml) were taken at intervals, heated at 100°C for 10 min, and centrifuged to remove cells and the supernatants were stored at −20°C until used for sugar assays.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu7697) galU rpsL endAl nupG | Invitrogen |
| S. salivarius ATCC 25975 | Wild type, Lac+ Glu+ Gal+ | 12 |
| S. thermophilus SMQ-301 | Wild type, Lac+ Glu− Gal− | 32 |
| Plasmids | ||
| pUC18 | Cloning vector, AprlacZ′ (lacZα) | 37 |
| pCR-Blunt | Cloning vector | Invitrogen |
| PCR 2.1 TOPO | Cloning vector, Apr KarlacZα | Invitrogen |
| pGAL1 | Contains the last 1,040 nucleotides of galT and the first 526 nucleotides of galE from S. salivarius cloned into pCR-Blunt | This work |
| pGAL3 | Contains the last 650 nucleotides of galK and the first 1,202 nucleotides of galT from S. salivarius cloned into pCR-Blunt | This work |
| pGAL29 | Contains the first 882 nucleotides of galK and the first 853 nucleotides of galR from S. salivarius cloned into PCR 2.1 TOPO | This work |
| pGAL73 | Contains the last 1,422 nucleotides of dexB, galR, and the first 729 nucleotides of galK from S. salivarius cloned into pUC18 | This work |
| pGAL123 | Contains the last 104 nucleotides of galT, galE, and the first 928 nucleotides of galM from S. salivarius cloned into pUC18 | This work |
DNA purification and manipulations.
Chromosomal DNA was isolated from streptococci as described previously (9). Unless otherwise mentioned, DNA manipulations were performed using standard procedures (3). Transformation in Escherichia coli TOP10 was carried out as described by the supplier using One Shot Top 10-competent cells (Invitrogen, Carlsbad, Calif.). Unless otherwise specified, DNA fragments used for sequencing, subcloning, and probes were recovered from agarose gels with an Elu-Quik DNA purification kit (Schleicher & Schuell, Keene, N.H.). The PCRs were performed using a DNA Thermal Cycler 480 (Perkin-Elmer) in a total volume of 100 μl containing 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 200 to 400 ng of DNA, 0.24 or 1.2 μM primers, and 200 μM (each) of the four deoxynucleotide triphosphates. The reactions were carried out for 25 cycles in the presence of 0.04 U of Taq DNA polymerase (Sigma Diagnostic, Mississauga, Ontario, Canada) or 0.03 U of AmpliTaq (Perkin-Elmer) with the following temperature-time profile: 94°C for 1 min, 40°C for 1 min, and 72°C for 2 min. At the end of the amplification process, the samples were incubated at 72°C for 3 min.
Cloning and sequencing the gal-lac gene cluster.
The procedure for cloning S. salivarius galRKTE and the 5′ end of galM is schematically shown in Fig. 1. On the basis of conserved regions found by sequence comparison of the galK, galT, galE, and galR gene products from S. thermophilus (24) (GenBank accession no. U61402), Streptococcus mutans (2), Streptococcus pneumoniae (http://www.tigr.org/tdb/mdb/bdb.html), and Lactobacillus helveticus (20), the degenerate oligonucleotides listed in Table 2 were designed and used for PCR amplification of S. salivarius gal genes. This allowed the isolation of plasmids pGAL1, pGAL3, and pGAL29, which contained, respectively, the 3′ end of galT through the 5′ end of galE, the 3′ end of galK through the 5′ region of galT, and the 5′ end of galR and the 5′ end of galK. The DNA regions upstream from galR and downstream from galE were cloned as two EcoRV DNA fragments, giving plasmid pGAL73, which contained the 3′ end of dexB, the entire galR gene, and the 5′ end of galK, and plasmid pGAL123, which contained the 3′ end of galT, the entire galE gene, and the 5′ end of galM. Using primers galM5311 and lacZ1129R, we amplified a 6.5-kb DNA fragment that encompassed the 3′ ends of galM, lacS, and lacZ. Using the nucleotide sequences of the gal gene cluster of S. thermophilus CNRZ 302 (GenBank accession no. U61402) and of the lac gene cluster of S. thermophilus strains A054 and A147 (GenBank accession no. M63636 and M23009), primers were designed and used to amplify by PCR and to sequence the gal-lac gene cluster of S. thermophilus SMQ-301.
FIG. 1.
Scheme for cloning galR, galK, galT, galE, and the 5′ end of galM of S. salivarius. The procedure is detailed in Materials and Methods. MCS, multiple cloning site.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| galK517 | ATGGAYCARTTYGCNATHGG |
| galT2357R | CCCATNACYTCDATIARNCC |
| galT1616 | AARCCIGARAARGAYCCNAA |
| galE3310R | GCNCCIGCNACRTTRAARTA |
| galR166 | GGWGGRTAIACYTGYTTNG |
| galK865R | CTTCCAAATCTCCTGCTTC |
| galM5311 | CTCACAACAGGTGCCACACTTCA |
| lacZ1129R | TAACGTCTTGGGAGTGGTTTAGTCC |
The DNA sequences on both strands were determined by the dideoxy chain-termination method of Sanger et al. (27). The nucleotide sequences of pGAL1, pGAL3, and pGAL29 inserts were confirmed by sequencing directly PCR products. DNA sequencing was carried out by the DNA sequencing service of Université Laval using an ABI Prism 3100 apparatus. Sequence analyses were performed using the GelStart, GelEnter, GelMerge, GelAssemble, GelView, Frames, Assemble, Translate, BestFit, Pileup, Pretty, Gap, FastA, TfastA, and FoldRNA programs in the Wisconsin Package software (version 9.0) of the Genetics Computer Group (GCG). The pIs and molecular masses of the gene products were calculated using the GCG Isoelectic and PeptideSort programs.
RNA isolation and Northern blot analysis.
Cells were grown in 25 ml of M17 medium supplemented with 0.5% concentrations of the different sugars to optical densities at 660 nm (OD660) of 0.45 and 0.75. Cells were collected by centrifugation and suspended in 600 μl of the RLT buffer provided with the RNeasy kit used for RNA isolation (Qiagen) supplemented with 6 μl of 14.3 M 2-mercaptoethanol. Glass beads (0.35 g, 150 to 212 μm in diameter) were added, and the cells were broken by shaking the cell suspension three times for 1 min with a Vortex set at maximum. Between treatments, the cell suspensions were chilled on ice for 1 min. After the glass beads and cellular debris were removed by centrifugation (2 min at 13,000 × g), RNA was isolated according to the procedure recommended by the manufacturer of the RNeasy kit and stored at −80°C. The RNA was fractionated on a 0.8% agarose gel as described by Ausubel et al. (3), transferred to positively charged nylon membranes using a Turboblotter apparatus (Schleicher & Schuell), and fixed by UV cross-linking for 3 min. PCR products labeled by random priming with [α-32P]ATP (Pharmacia, Baie d’Urfé, Québec, Canada) were used as hybridization probes. These included galK-, galE-, galM-, and lacS-specific probes. Prior to radiolabeling, probes were purified as described previously (6).
Primer extension analyses.
To determine the transcriptional start points of galK, galE, galM, and lacS, total RNA was isolated from lactose-grown cells as described above. Primer extension experiments were carried out as described previously (8) with synthetic oligonucleotides covering the following positions relative to the adenine of the ATG initiation codon: 90 to 112 for galK (5′-TGTAGTCGTATGCTCACCAATC-3′), 24 to 45 for galE (5′-GTGAGAACCGATATAACCAGCT-3′), 82 to 104 for galM (5′-CCTGTTGTGAGTGTGGAAATGAC-3′), and 46 to 68 for lacS (5′-ACGTCGTTACCAAAAGCACCAGC-3′).
Enzyme assays.
Strains were grown in 500 ml of M17 broth containing 0.5% lactose to an OD660 of 0.9. Cell extracts were prepared by grinding with alumina as described previously (33). The extracts were kept on ice, and enzyme assays were performed within 5 h. Galactose-1-P uridylyltransferase and UDP-glucose 4-epimerase activities were assayed as described by Maxwell et al. (19). Galactokinase activity was assayed by measuring the rate of phosphorylation of [14C]galactose at the expense of ATP as described previously (34).
Sugar and protein assays.
Glucose concentrations were measured using a peroxidase-glucose oxidase assay (Sigma). Galactose was determined using a peroxidase-galactose oxidase assay (4). Lactose was assayed by measuring the concentration of glucose or galactose in samples before and after hydrolysis with β-galactosidase for 1 h at 37°C in 233 mM citrate buffer (pH 6.6) containing 60 mM MgSO4 and 0.05 U of β-galactosidase (Worthington, Freehold, N.J.) per μl. Protein concentrations were measured using the method of Peterson (21) with bovine serum albumin as the standard.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the nucleotide sequences of the gal-lac gene clusters comprising galR, galK, galT, galE, galM, lacS, and lacZ of S. salivarius ATCC 25975 and S. thermophilus SMQ-301 are AF389474 and AF389475, respectively.
RESULTS
Growth on lactose and galactose.
The growth of S. salivarius and S. thermophilus on 0.2% galactose is illustrated in Fig. 2A. Unlike S. thermophilus, which was unable to grow under these conditions, S. salivarius grew rapidly, with a generation time of approximately 33 min, and reached an OD660 slightly higher than 0.7 at the end of the exponential phase. Growth of S. salivarius on 0.15% lactose is illustrated in Fig. 2B. The cells had a generation time of approximately 33 min, reached an OD660 of 0.7, and consumed all the lactose. A small amount of galactose was released into the medium at the beginning but was rapidly metabolized thereafter. Under the same conditions, S. thermophilus grew to an OD660 slightly lower than 0.6, with a generation time of about 31 min, and excreted galactose, which remained in the medium until the lactose was almost completely consumed (Fig. 2C). After the disappearance of the lactose, the galactose was consumed and cells continued to grow, albeit at slower rate.
FIG. 2.
Growth of S. salivarius and S. thermophilus on galactose or lactose. (A) Tubes containing 10 ml of M17 culture medium supplemented with 0.1% galactose were inoculated with one colony of either S. salivarius or S. thermophilus and incubated overnight at either 37°C for S. salivarius or 42°C for S. thermophilus. These cultures (100 μl) were used to inoculate 10 ml of fresh media containing 0.2% galactose. Open circles, growth of S. salivarius at 37°C; solid circles, growth of S. thermophilus at 42°C. (B and C) Cells were grown overnight in the presence of 0.1% lactose. Tubes containing M17 medium supplemented with 0.15% lactose were then inoculated with 100 μl of the overnight cultures. Growth of S. salivarius at 37°C (B; open circles) and of S. thermophilus at 42°C (C; solid circles) is shown. Solid diamonds, concentration of lactose in the medium; open diamonds, concentration of galactose in the medium.
Analysis of the S. salivarius gal-lac gene cluster.
The genes of the S. salivarius gal-lac cluster were located on the chromosome in the following order: galR, galK, galT, galE, galM, lacS, and lacZ, with galR oriented in the opposite direction to the other genes (Fig. 3). Sequence analysis revealed the presence of four putative promoters upstream from four genes, namely, galR, galK, galM, and lacS (Fig. 3). Inverted sequences able to form a stem-loop structure were found downstream from galE and from lacZ (Fig. 3). The presence of these putative promoters and terminators suggested that galK, galT, and galE constituted a single transcriptional unit and that galM was transcribed independently. As no putative terminator sequence could be identified downstream from galM, transcription initiated from the galM promoter should give rise to a transcript covering galM, lacS, and lacZ (Fig. 3). Finally, lacS and lacZ should form an operon and should be expressed independently from galK, galT, galE, and galM.
FIG. 3.
The gal-lac gene cluster of S. salivarius. Shaded boxes, genes (the direction of transcription is indicated). The genes code for the following proteins: galR, putative transcriptional regulator; galK, galactokinase; galT, galactose-1-P uridylyltransferase; galE, UDP-glucose 4-epimerase; galM, galactose mutarotase; lacS, lactose transporter; lacZ, β-galactosidase. Terminators are indicated by stem-loop structures. Arrows, promoters. The sequences of the terminators, promoters (−10 and −35 boxes), and ribosome binding sites (SD) are indicated. The promoter upstream from galM is indicated within the sequence of the terminator located downstream from galE.
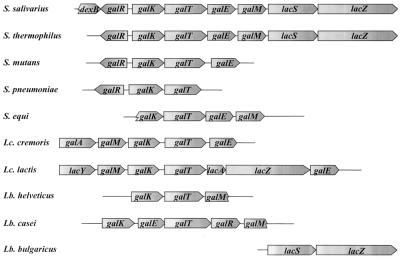
Comparison between the S. salivarius and S. thermophilus gal-lac gene clusters.
The gal-lac gene cluster of S. thermophilus SMQ-301 had the same organization as that of S. salivarius. The organization of the galactose and lactose genes in several other gram-positive bacteria is illustrated in Fig. 4. The order of the genes coding for the enzymes of the Leloir pathway is well conserved within streptococci but is variable in other gram-positive bacteria of low G+C content. Sequence comparison of S. salivarius ATCC 25975 and S. thermophilus SMQ-301 orthologues revealed high levels of identity, 75% for GalR, 95% for GalK, 96% for GalT, over 99% for GalE, 94% for GalM, 95% for LacS, and 96% for LacZ, for the first 888 amino acids (lacZ from S. thermophilus SMQ-301 has not been entirely sequenced).
FIG. 4.
Organization of the galactose and lactose genes in various gram-positive bacteria. Abbreviations: S., Streptococcus; Lc. cremoris, Lactococcus lactis subsp. cremoris; Lc. lactis, Lactococcus lactis subsp. lactis; Lb., Lactobacillus. The genes code for the following proteins: dexB, dextran glucosidase; galR, putative transcriptional regulator; galK, galactokinase; galT, galactose-1-P uridylyltransferase; galE, UDP-glucose 4-epimerase; galM, galactose mutarotase; lacS, lactose transporter; lacZ, β-galactosidase; galA, galactose permease; lacY, lactose permease; lacA, galactoside acetyltransferase.
Promoters as well as terminators identified in the S. salivarius gal-lac gene cluster were also found in the S. thermophilus cluster. The sequence alignment of the presumptive promoter regions is shown in Fig. 5. The −35 and −10 boxes of galR were identical and separated in both species by 18 nucleotides. The apparent promoters upstream from galK were also very similar. The sequences of the −35 boxes were identical, and both were separated from the −10 box by 17 nucleotides. The −10 boxes were different by two nucleotides (TACACT for S. salivarius versus TACGAT for S. thermophilus; differences underlined). Apart from three nucleotides (AAC) located nine nucleotides downstream from the −10 box of S. thermophilus, the nucleotide sequences of the −10 boxes and the initiation codons of galK were almost the same in both organisms. A palindromic nucleotide sequence characteristic of the GalR binding site identified in S. mutans by DNase I footprinting (1) was found in the galR-galK intergenic region. The apparent GalR binding sites in S. salivarius and S. thermophilus were found at the same position and differed by only two nucleotides. Intriguingly, inactivation of GalR in S. mutans results in constitutive expression of galK, indicating that S. mutans GalR is a repressor (2), whereas inactivation of GalR in S. thermophilus prevents transcription of the galactose genes, suggesting that GalR is a transcriptional activator in S. thermophilus (36). Further studies are required to determine the role of GalR in S. salivarius. Virtually identical putative CRE sequences (CcpA binding site) overlapped the −10 boxes of the promoters located upstream from galK in both organisms.
FIG. 5.
Nucleotide sequence alignment of the promoter regions of the S. salivarius and S. thermophilus gal-lac gene clusters. The ribosome binding sites (SD); the −10 and −35 boxes of the promoters; the initiation codons of galR, galK, and lacS; and the stop codons of galE and galM are in boldface. +1, transcriptional start sites determined by primer extension. The CRE sequences are boxed. Two putative GalR binding sites are underlined. Arrows, terminators.
In both species, the promoter upstream from galM consisted of a −35 box with the sequence TAAACA located nine nucleotides downstream from the stop codon of galE. Their −10 boxes were identical (TATAAT) and were separated from the −35 box by 17 nucleotides. The apparent promoter upstream from lacS consisted of a −35 box, whose sequence in S. salivarius differed by only one nucleotide from that of the box in S. thermophilus (TTGACG for S. salivarius and TTGACT for S. thermophilus; difference underlined), separated by 17 nucleotides from the −10 box, which was identical in the two species (TATAAT). CRE sequences that differed by only one nucleotide were found proximal to the −10 boxes in both species. Following the CRE sequence, a stretch of seven nucleotides (AAACAAC) was found in S. salivarius but not in S. thermophilus. Finally, second putative GalR binding sites were identified upstream from the −35 boxes in both organisms. They were almost identical, being different by only 3 nucleotides out of 28.
A nucleotide sequence that could generate a stem-loop structure was identified downstream from galE in both species (Fig. 5). The calculated free energy of formation was −16.3 kcal/mol for the S. thermophilus structure and −11.3 kcal/mol for S. salivarius. Another potential terminator with a free energy of formation of −9.8 kcal/mol was found 38 nucleotides downstream from the lacZ stop codon in S. salivarius. We did not sequence the corresponding chromosomal region of S. thermophilus SMQ-301, but a terminator with almost the same sequence and a free energy of formation of −13.8 kcal/mol was found 42 nucleotides downstream from the lacZ stop codon of S. thermophilus A147 (24).
In summary, the sequences of the putative promoters and terminators in S. salivarius 25975 and S. thermophilus SMQ-301 were virtually identical, suggesting that transcription of the gal-lac gene cluster produces the same transcripts in both species.
mRNA analysis of the gal-lac gene cluster.
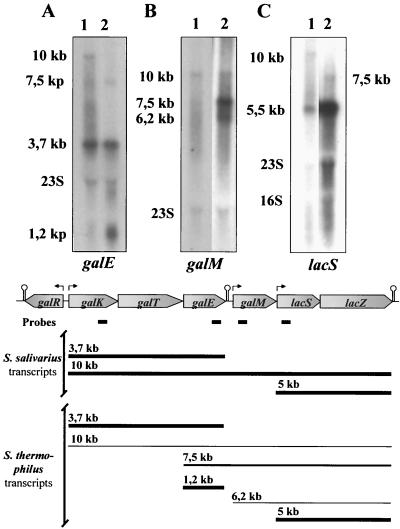
We carried out Northern blot transcriptional analyses of the S. salivarius and S. thermophilus chromosomal regions encompassing the gal and lac genes using probes specific for galK, galE, galM, and lacS. Hybridizations carried out with lactose-grown S. salivarius cells harvested at an OD660 of 0.75 gave no or weak signals with the four probes, whereas strong signals were obtained with cells harvested at an OD660 of 0.45. Conversely, very weak signals were observed with lactose-grown S. thermophilus cells harvested at an OD660 of 0.45 but conclusive signals were detected in cells harvested at an OD660 of 0.75. We do not have any meaningful explanation at this time for this phenomenon. The results summarized in Fig. 6 and described below were obtained with lactose-grown S. salivarius cells harvested at an OD660 of 0.45 and lactose-grown S. thermophilus cells harvested at an OD660 of 0.75. In S. salivarius, hybridizations with the galK probe revealed a dominant transcript of 3.7 kb and a less intensive signal corresponding to a 10-kb transcript (not shown). Similar results were obtained with the galE probe (Fig. 6A). The sizes of the observed transcripts were in agreement with the expected lengths of a galKTE mRNA (3.7 kb) and a galKTEM-lacSZ mRNA (10 kb). A similar signal pattern was observed with S. thermophilus when hybridizations were carried out with the galK probe except that the 10-kb transcript was very weak (not shown). Additional transcripts were detected in S. thermophilus with the galE probe. In addition to signals corresponding to the 3.7- and 10-kb transcripts, two other signals, which corresponded to 1.2- and 7.5-kb transcripts, were observed (Fig. 6A). The 1.2-kb transcript had the expected size of a galE monocistronic mRNA, whereas the 7.5-kb transcript would be a galEM-lacSZ polycistronic mRNA. The presence of the 1.2- and the 7.5-kb transcripts was enigmatic, as no recognizable promoter could be identified upstream from galE.
FIG. 6.
Northern blot analysis of S. salivarius and S. thermophilus. The lanes contained total RNA isolated from cells grown on 0.5% lactose. S. salivarius was harvested at an OD660 of 0.45, and S. thermophilus was harvested at an OD660 of 0.75. Lane 1, S. salivarius; lane 2, S. thermophilus. (A) Experiments performed with a probe specific to galE. (B) Experiments performed with a probe specific to galM. Both lanes contain total RNA isolated from S. thermophilus. (C) Experiments performed with a probe specific to lacS. RNA molecular weight markers were used to determine the sizes of the transcripts. (Bottom) Map of the S. salivarius and S. thermophilus gal-lac gene clusters. The symbols are the same as those described in the legend of Fig. 3. Black boxes, probes used in Northern blot experiments; thick lines, major transcripts; thin lines, transcripts found at low levels.
A single 10-kb transcript was observed in S. salivarius with the galM probe, suggesting that the promoter identified upstream from galM was not functional under the experimental conditions tested (Fig. 6B). This transcript would correspond to the 10-kb mRNA observed with the galK and galE probes. A different signal pattern was observed with S. thermophilus and the galM probe (Fig. 6B). We detected two weak transcripts of 6.2 and 10 kb as well as an intensive signal corresponding to a 7.5-kb transcript. The 10-kb transcript was probably the counterpart of the 10-kb transcript detected in S. salivarius, while the 7.5-kb transcript corresponds to the transcript of identical size detected with the galE probe. The 6.2-kb transcript had the expected size of an mRNA initiated from the putative promoter upstream from galM and stopped at the terminator located downstream from lacZ.
Two signals corresponding to 10- and 5-kb transcripts were detected in S. salivarius with the lacS probe (Fig. 6C). The sizes of these transcripts corresponded to the expected size of a mRNA initiated from the promoter upstream from galK and stopped at the terminator downstream from lacZ and a polycistronic mRNA initiated from the promoter located upstream from lacS. In addition to a weak 10-kb transcript and an intense 5-kb transcript, a 7.5-kb transcript was detected in S. thermophilus with the lacS probe. This transcript had the expected size of an mRNA covering galE, galM, lacS, and lacZ. The transcripts detected in lactose-grown cells were not detected in S. salivarius after growth on glucose or in S. thermophilus after growth on sucrose (S. thermophilus SMQ-301 did not grow on glucose) except for the 5-kb transcript detected with the lacS probe, suggesting that the lactose genes were constitutively expressed in S. thermophilus. A summary of the mRNAs covering the galactose and lactose genes after growth of S. salivarius and S. thermophilus on lactose is presented in the lower part of Fig. 6.
Identification of transcriptional start sites.
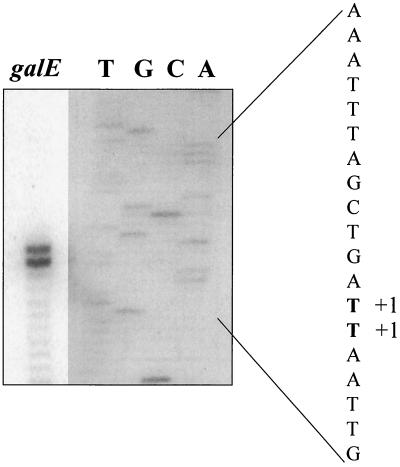
The transcriptional start points of the S. salivarius and S. thermophilus 3.7- (galKTE), 5- (lacSZ), and 10-kb (galKTEM lacSZ) transcripts as well as the transcriptional start points of the S. thermophilus 7.5- (galEM lacSZ) and 1.2-kb (galE) transcripts were determined by primer extension analyses. For both organisms, there was a strong signal that corresponded to a T residue located eight nucleotides downstream from the last nucleotide (T) of the inferred −10 box of the promoter found upstream from galK (TACACT for S. salivarius and TACGAT for S. thermophilus; Fig. 5) (not shown). We also observed a major transcriptional start point corresponding to a G located seven nucleotides downstream from the last nucleotide (T) of the −10 box upstream from lacS (TATAAT in both organisms; Fig. 5) (not shown). For the galE and galEM lacSZ transcripts found in S. thermophilus, two strongly labeled extension products were detected (Fig. 7). They corresponded to 2 contiguous T nucleotides located 32 and 33 nucleotides upstream from the ATG initiation codon of galE. The second T constituted the first nucleotide of the galT stop codon (TAA) (Fig. 5).
FIG. 7.
Mapping of the 5′ ends of the galE and the galEM lacSZ transcripts by primer extension analysis. For lane galE, oligonucleotide 5′-GTGAGAACCGATATAACCAGCT-3′ was annealed to 20 μg of S. thermophilus RNA and extended using murine leukemia virus reverse transcriptase. The nucleotide sequence was determined with an amplicon encompassing the DNA region of interest by using the same oligonucleotide as a primer (lanes T, G, C, and A). Samples of both reaction mixtures were loaded on the same gel, and the final figure is a composite. The exposure time for the primer extension reaction was 7.5 h, and that for the sequence reaction was 21.5 h. +1, position of the primer extension products.
Enzyme activities.
The activities of galactokinase, galactose-1-P uridylyltransferase, and UDP-glucose 4-epimerase in lactose-grown cells of S. salivarius and S. thermophilus were determined. The results, summarized in Table 3, indicate that although the two species possess similar levels of galactose-1-P uridylyltransferase and UDP-glucose 4-epimerase, S. thermophilus has approximately 24-fold less galactokinase activity than S. salivarius.
TABLE 3.
Activity of enzymes of the Leloir pathway in S. salivarius and S. thermophilus
| Straina | Activityb (nmol of product formed/mg of protein/min) of:
|
||
|---|---|---|---|
| Galactokinase | Galactose-1-P uridylyltransferase | UDP-glucose 4-epimerase | |
| S. salivarius | 600 ± 5 | 1,258 ± 101 | 834 ± 213 |
| S. thermophilus | 26 ± 9 | 901 ± 138 | 525 ± 56 |
Cells were grown on lactose.
Values are the means ± standard deviations of at least two independent experiments.
Comparison of SD sequences.
To determine whether the differences in galactokinase activities resulted from a variation in the rate of gene translation, we compared the ribosome binding site sequences of galK, galT, and galE. The ribosome binding site sequences of S. salivarius and S. thermophilus galT and galE were identical, i.e., AAAGAAGU and AAAGGAGA, respectively. However, the galK ribosome binding site of S. thermophilus (AAGUGAGA) differed from that of S. salivarius (AAGCGAGG) by two nucleotides. Analysis of S. thermophilus and S. salivarius 16S rRNA suggested that the optimal mRNA sequence for ribosome binding would be AAAGGAGG. The Shine-Dalgarno (SD) sequence of S. salivarius galK had two mismatches with the optimal sequence, whereas the SD sequence of S. thermophilus galK had three mismatches. Calculation of the free energy of the mRNA(SD)-rRNA 16S complex formation (31) gave values of −8.6 kcal/mol for the S. salivarius complex but only −3.6 kcal/mol for the S. thermophilus complex.
DISCUSSION
The detection of galactokinase, galactose-1-P uridylyltransferase, and UDP-glucose 4-epimerase activities after growth on galactose (18) provided evidence that S. salivarius metabolizes galactose via the Leloir pathway. In this paper, we showed that the genes coding for these enzymes, galK (galactokinase), galT (galactose-1-P uridylyltransferase), and galE (UDP-glucose 4-epimerase), were transcribed in that order and organized into a gal operon. The galR gene, which coded for a putative transcriptional regulatory protein belonging to the LacI/GalR family, was found upstream from the gal operon and was divergently transcribed. The galM gene (galactose mutarotase) was found downstream from galE. Finally, downstream from galM, we identified the lacS and lacZ genes, which formed the lac operon and which coded for the lactose transporter and β-galactosidase, respectively. The gene order galR and galKTE is well conserved among streptococci but is different from those in other low-G+C-content gram-positive fermentative bacteria. Mollet and Pilloud (20) suggested that the gal gene cluster of gram-positive bacteria evolved independently from single genes into distinct transcriptional units. The data presented here suggested that the gal gene cluster within streptococci was established when the streptococcal branch of the Clostridium subphylum branched off and was then conserved during evolution.
The gal-lac gene clusters of S. salivarius ATCC 25975 and S. thermophilus SMQ-301 had the same organization. Moreover, sequence comparison revealed that the gene products of the gal and lac operons and galM possessed over 90% identity with those of S. thermophilus. These results are consistent with the close phylogenetic relationship between the two species. Analysis of S. salivarius nucleotide sequences led to the identification of four putative promoters located upstream from galR, the gal operon, galM, and the lac operon. Promoters with virtually identical nucleotide sequences were found at the same positions in the S. thermophilus gal-lac gene cluster, suggesting that these genes should be transcribed with comparable efficiencies in both species. Results from Northern blot analyses and primer extension experiments conducted with mRNA isolated from lactose-grown cells clearly indicated that the gal-lac gene cluster was efficiently transcribed in both species. These results contrast with those of Vaughan et al. (36), who were unable to detect mRNA resulting from transcription of the gal operon in lactose-grown cells of the galactose-negative strain S. thermophilus CRNZ 302. These authors proposed that the inability of S. thermophilus to grow on galactose was caused by a gal-defective promoter. Surprisingly, sequence comparison revealed that the sequence of the gal promoter of strain CRNZ 302 was identical with that of strain SMQ-301. We cannot explain the discrepancy between our results and those of Vaughan et al. (36). However, the results reported here unequivocally indicated that the gal promoter of S. thermophilus SMQ-301 is virtually identical with that of galactose-positive species S. salivarius ATCC 25975 and was able to direct transcription of the gal operon when cells were grown on lactose.
Interestingly, we observed three transcripts in S. thermophilus that were not detected in S. salivarius. A weak transcript (6.2 kb) originated from the putative galM promoter and encompassed galM, lacS, and lacZ. The two other transcripts (1.2 and 7.5 kb), which were present in higher concentrations, resulted from the transcription of galE as a monocistronic mRNA and from the transcription of galE, galM, lacS, and lacZ as a polycistronic mRNA. As examination of the nucleotide sequence of galT did not reveal the presence of sequences typical of a promoter, we cannot rule out the possibility that the 1.2- and 7.5-kb transcripts in S. thermophilus result from a processing of the 3.7- and 10-kb transcripts initiated from the promoter upstream from galK.
Previous studies have indicated that β-galactosidase is coded for by an inducible gene in S. salivarius (10, 34). This was confirmed by the transcription studies reported here, which unequivocally showed that the S. salivarius lac operon was transcribed in lactose-grown but not in glucose-grown cells. Conversely, our results indicated that a transcript encompassing lacS and lacZ was synthesized in lactose-grown and, to a lesser extent, in sucrose-grown S. thermophilus cells, suggesting that the lactose genes were less stringently controlled in this species. These data are consistent with previous observations indicating that S. thermophilus possesses significant β-galactosidase activity after growth under noninducing conditions (24, 36). Surprisingly, the nucleotide sequences of the S. salivarius and S. thermophilus lac promoters and operator regions were virtually identical. The major differences were the replacement of a G by a T at the last 3′-end position of the S. thermophilus −35 box and a deletion of 7 bp downstream from the S. thermophilus CRE sequence. Whether this deletion interferes somehow with the binding of regulatory protein CcpA, which is known to prevent transcription of several genes by binding to CRE (26), remains to be demonstrated. It is noteworthy that the lactose genes are derepressed sevenfold in a CcpA-negative strain of S. thermophilus CNRZ 302 (35), indicating that the 7-bp deletion downstream from CRE does not totally prevent the binding of CcpA.
Northern blot experiments indicated that the galactose operons of S. salivarius and S. thermophilus were both inducible. Measurement of enzyme activities confirmed that lactose-grown cells of S. salivarius possessed high levels of all of the Leloir enzymes, a result consistent with the transcriptional data. In S. thermophilus, the levels of galactose-1-P uridylyltransferase and UDP-glucose 4-epimerase in lactose-grown cells were comparable to those found in S. salivarius, as expected from the transcriptional analysis. However, the levels of galactokinase activity in S. thermophilus were unexpectedly low, being approximately 24-fold lower than those measured in S. salivarius. The sequences of the ribosome binding sites of galT and galE in both species were identical, but the ribosome binding site of S. thermophilus galK differed from that of S. salivarius by two nucleotides. Moreover, calculation of the free energy of formation for the mRNA-rRNA base pairing (31) indicated that the S. thermophilus galK ribosome binding site-16S rRNA interaction was very weak. These results suggest that the inability of S. thermophilus to grow on galactose results, to some extent, from a weak interaction between the ribosome and the galK ribosome binding site, resulting in insufficient levels of galactokinase in the cell. Nevertheless, we observed that S. thermophilus SMQ-301 was able to consume the galactose released into the medium during growth on lactose but only after the lactose was almost totally consumed. This result suggested that either the low levels of galactokinase found in S. thermophilus were sufficient to metabolize galactose when cells were growing on lactose or that galactose was metabolized by an undefined metabolic pathway. Accordingly, the inability of S. thermophilus to grow on galactose would not be caused solely by low levels of galactokinase. Another possibility is that extracellular galactose is unable to induce the necessary metabolic enzymes or transport system whereas these proteins would be induced during growth on lactose. Further research to elucidate galactose metabolism in S. thermophilus is under way.
Acknowledgments
We thank the following organizations for their financial support: Conseil des Recherches en Pêche et en Agroalimentaire du Québec (CORPAQ), l’Action Concertée Fonds FCAR-NOVALAIT-MAPAQ, and the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (FCAR).
REFERENCES
- 1.Ajdic, D., and J. J. Ferretti. 1998. Transcriptional regulation of the Streptococcus mutans gal operon by the GalR repressor. J. Bacteriol. 180:5727–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdic, D., I. Sutcliffe, R. R. B. Russell, and J. J. Ferretti. 1996. Organization and nucleotide sequence of the Streptococcus mutans galactose operon. Gene 180:137–144. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1997. Current protocols in molecular biology. Greene Publishing and Wiley Interscience, New York, N.Y.
- 4.Avigad, G., D. Amaval, C. Ascension, and B. L. Horecker. 1962. The D-galactose oxydase of Polyporus circinatus. J. Biol. Chem. 237:2736–2743. [PubMed] [Google Scholar]
- 5.de Vos, W. M., and E. E. Vaughan. 1994. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 15:217–237. [DOI] [PubMed] [Google Scholar]
- 6.Émond, É., R. Lavallée, G. Drolet, S. Moineau, and G. Lapointe. 2001. Molecular characterization of a theta replication plasmid and its use for development of a two-component food-grade cloning system for Lactococcus lactis. Appl. Environ. Microbiol. 67:1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrow, J. A. E., and M. D. Collins. 1984. DNA base composition, DNA-DNA homology and long-chain fatty acid studies of Streptococcus thermophilus and Streptococcus salivarius. J. Gen. Microbiol. 130:357–362. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon, G., C. Vadeboncoeur, L. Gauthier, and M. Frenette. 1995. Regulation of ptsH and ptsI gene expression in Streptococcus salivarius ATCC 25975. Mol. Microbiol. 16:1111–1121. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier, L., S. Thomas, G. Gagnon, M. Frenette, L. Trahan, and C. Vadeboncoeur. 1994. Positive selection for resistance to 2-deoxyglucose gives rise, in Streptococcus salivarius, to seven classes of pleiotropic mutants, including ptsH and ptsI missense mutants. Mol. Microbiol. 13:1101–1109. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier, M., D. Brochu, L. D. Eltis, S. Thomas, and C. Vadeboncoeur. 1997. Replacement of isoleucine-47 by threonine in the HPr protein of Streptococcus salivarius abrogates the preferential metabolism of glucose and fructose over lactose and melibiose but does not prevent the phosphorylation of HPr on serine 46. Mol. Microbiol. 25:695–705. [DOI] [PubMed] [Google Scholar]
- 11.Gunnewijk, M. G., and B. Poolman. 2000. HPr(His∼P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34080–34085. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, I. R. 1968. Synthesis and degradation of intracellular polyglucose in Streptococcus salivarius. Can. J. Microbiol. 14:65–77. [DOI] [PubMed] [Google Scholar]
- 13.Hardie, J. M., and R. A. Whiley. 1992. The genus Streptococcus-oral, p.1421–1449. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, vol II. Springer-Verlag KG, Berlin, Germany.
- 14.Hutkins, R. W., and H. A. Morris. 1987. Carbohydrate metabolism by Streptococcus thermophilus: a review. J. Food Prot. 50:876–884. [DOI] [PubMed] [Google Scholar]
- 15.Hutkins, R. W., H. A. Morris, and L. L. McKay. 1985. Galactokinase activity in Streptococcus thermophilus. Appl. Environ. Microbiol. 50:777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura, Y., X.-G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406–408. [DOI] [PubMed] [Google Scholar]
- 17.Kilpper-Bälz, R., G. Fischer, and K. H. Schleifer. 1982. Nucleic acid hybridization of group N and group D streptococci. Curr. Microbiol. 7:245–250. [Google Scholar]
- 18.Lapointe, R., M. Frenette, and C. Vadeboncoeur. 1993. Altered expression of several genes in IIILMan-defective mutants of Streptococcus salivarius demonstrated by two-dimensional gel electrophoresis of cytoplasmic proteins. Res. Microbiol. 144:305–316. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell, E. S., K. Kurahashi, and H. M. Kalckar. 1962. Enzymes of the Leloir pathway. Methods Enzymol. 5:174–189. [Google Scholar]
- 20.Mollet, B., and N. Pilloud. 1991. Galactose utilization in Lactobacillus helveticus: isolation and characterization of the galactokinase (galK) and galactose-1-phosphate uridyltransferase (galT) genes. J. Bacteriol. 173:4464–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson, G. L. 1983. Determination of total protein. Methods Enzymol. 91:95–119. [DOI] [PubMed] [Google Scholar]
- 22.Poolman, B. 1993. Energy transduction in lactic acid bacteria. FEMS Microbiol. Rev. 12:125–148. [DOI] [PubMed] [Google Scholar]
- 23.Poolman, B., J. Knol, C. van der Does, P. J. F. Henderson, W.-J. Liang, G. Leblanc, T. Pourcher, and I. Mus-Veteau. 1996. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol. Microbiol. 19:911–922. [DOI] [PubMed] [Google Scholar]
- 24.Poolman, B., T. J. Royer, S. E. Mainzer, and B. F. Schmidt. 1990. Carbohydrate utilization in Streptococcus thermophilus: characterization of the gene for aldose 1-epimerase (mutarotase) and UDP-glucose 4-epimerase. J. Bacteriol. 172:4037–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poyart, C., G. Quesne, S. Coulon, P. Berche, and P. Trieu-Cuot. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in gram-positive bacteria. Microbiology 142:217–230. [DOI] [PubMed] [Google Scholar]
- 27.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schleifer, K. H., M. Ehrmann, U. Krusch, and H. Neve. 1991. Revival of the species Streptococcus thermophilus (ex Orla-Jensen, 1919) nom. rev. Syst. Appl. Microbiol. 14:386–388. [Google Scholar]
- 29.Stiles, M. E., and W. H. Holzapfel. 1997. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 36:1–29. [DOI] [PubMed] [Google Scholar]
- 30.Thomas, T. D., and V. L. Crow. 1984. Selection of galactose-fermenting Streptococcus thermophilus in lactose-limited chemostat cultures. Appl. Environ. Microbiol. 48:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinoco, I., P. N. Borer, B. Dengler, M. D. Levine, O. C. Uhlenbeck, D. M. Crothers, and J. Gralla. 1973. Improved estimation of secondary structure in ribonucleic acids. Nat. New Biol. 246:40–41. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay, D. M., and S. Moineau. 1999. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology 255:63–76. [DOI] [PubMed] [Google Scholar]
- 33.Vadeboncoeur, C. 1984. Structure and properties of the phosphoenolpyruvate:glucose phosphotransferase system of oral streptococci. Can. J. Microbiol. 30:495–502. [DOI] [PubMed] [Google Scholar]
- 34.Vadeboncoeur, C., G. Bourgeau, D. Mayrand, and L. Trahan. 1983. Control of sugar utilization in the oral bacteria Streptococcus salivarius and Streptococcus sanguis by the phosphoenolpyruvate:glucose phosphotransferase system. Arch. Oral Biol. 28:123–131. [DOI] [PubMed] [Google Scholar]
- 35.van den Bogaard, P. T. C., M. Kleerebezem, O. P. Kuipers, and W. M. de Vos. 2000. Control of lactose transport, β-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar. J. Bacteriol. 182:5982–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaughan, E. E., P. T. C. van den Bogaard, P. Catzeddu, O. P. Kuipers, and W. M. de Vos. 2001. Activation of silent gal genes in the lac-gal regulon of Streptococcus thermophilusS. J. Bacteriol. 183:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vector and host strains: nucleotide sequences of the m13mp18 and pUC19 vector. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]