Abstract
Under anaerobic conditions, the yeast Saccharomyces bulderi rapidly ferments δ-gluconolactone to ethanol and carbon dioxide. We propose that a novel pathway for δ-gluconolactone fermentation operates in this yeast. In this pathway, δ-gluconolactone is first reduced to glucose via an NADPH-dependent glucose dehydrogenase (EC 1.1.1.47). After phosphorylation, half of the glucose is metabolized via the pentose phosphate pathway, yielding the NADPH required for the glucose-dehydrogenase reaction. The remaining half of the glucose is dissimilated via glycolysis. Involvement of this novel pathway in δ-gluconolactone fermentation in S. bulderi is supported by several experimental observations. (i) Fermentation of δ-gluconolactone and gluconate occurred only at low pH values, at which a substantial fraction of the substrate is present as δ-gluconolactone. Unlike gluconate, the latter compound is a substrate for glucose dehydrogenase. (ii) High activities of an NADP+-dependent glucose dehydrogenase were detected in cell extracts of anaerobic, δ-gluconolactone-grown cultures, but activity of this enzyme was not detected in glucose-grown cells. Gluconate kinase activity in cell extracts was negligible. (iii) During anaerobic growth on δ-gluconolactone, CO2 production exceeded ethanol production by 35%, indicating that pyruvate decarboxylation was not the sole source of CO2. (iv) Levels of the pentose phosphate pathway enzymes were 10-fold higher in δ-gluconolactone-grown anaerobic cultures than in glucose-grown cultures, consistent with the proposed involvement of this pathway as a primary dissimilatory route in δ-gluconolactone metabolism.
Biological oxidation of glucose at the C1 position by dehydrogenases or oxidases does not directly yield gluconic acid. Instead, the initial product of these incomplete oxidations is δ-gluconolactone (12, 14). The extent to which δ-gluconolactone is hydrolyzed to nondissociated gluconic acid and gluconate is strongly pH dependent. At near-neutral and alkaline pH values, the equilibrium favors δ-gluconolactone hydrolysis (13, 15, 18). At low pH values, however, the lactone is much more stable. δ-Gluconolactone is therefore a naturally occurring compound in many acidic environments from which yeasts can be isolated. Incomplete oxidation of sugars in fruits and nectar by acetic acid bacteria and fungi may lead to acidification and the accumulation of δ-gluconolactone (12, 14). It is therefore not surprising that many yeasts can use δ-gluconolactone as a growth substrate (2) (Table 1).
TABLE 1.
Occurrence of the ability of yeast species to grow on δ-gluconolactone or sodium gluconate according to the standard taxonomic assimilation tests
| Group | Growth phenotype in assimilation test
|
No. of strainsa | Example | |
|---|---|---|---|---|
| δ-Gluconolactone | Sodium gluconate | |||
| I | Negative | Negative | 90 | Kluyveromyces wickerhamii |
| II | Negative | Positive | 13 | Candida curiosa |
| III | Positive | Negative | 48 | Saccharomyces bulderi (this study) |
| IV | Positive | Positive | 147 | Yarrowia lipolytica |
Data were obtained from a taxonomic handbook (2). Species that exhibit a weak, variable, or delayed response in assimilation tests for these two substrates are not included in this table.
In the standard tests employed for the identification and classification of yeasts, the ability to perform alcoholic fermentation is only tested with sugars and sugar oligomers. Hitherto, substrates with a different degree of reduction have been assumed to be nonfermentable by yeasts. This assumption was recently disproved by Middelhoven and coworkers, who reported that δ-gluconolactone could be fermented to ethanol and CO2 by a Saccharomyces bulderi strain isolated from corn silage (11).
The aim of the present study was to investigate the pathway for δ-gluconolactone fermentation by S. bulderi. To this end, the yeast was grown in glucose- and δ-gluconolactone-limited, anaerobic chemostat cultures. Mass balances were constructed over the cultures to quantify all of the relevant fermentation products. Levels of potential key enzymes in δ-gluconolactone metabolism were assayed in cell extracts of glucose- and δ-gluconolactone-grown cultures. The results indicate that δ-gluconolactone is metabolized anaerobically via an initial reduction to glucose via NADPH-linked glucose dehydrogenase, followed by the concerted action of glycolysis and the pentose phosphate pathway.
MATERIALS AND METHODS
Yeast strain and maintenance.
S. bulderi CBS 8638, originally isolated from anaerobic corn silage (11), was obtained from the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. Stock cultures pregrown on YPD medium (1% yeast extract, 2% peptone, 2% glucose) were stored at −80°C with 20% (vol/vol) glycerol. Working stocks were maintained on YPD-agar plates.
Shake flask cultivation.
Studies of growth in shake flasks were performed with 500-ml flasks containing 100 ml of Difco yeast nitrogen base without amino acids that was prepared and filter sterilized in accordance with the manufacturer’s instructions. A carbon source (glucose, δ-gluconolactone, or sodium gluconate) was added to the medium prior to filter sterilization to a concentration of 20 g · liter−1. Cultures were incubated at 30°C and 200 rpm in a rotatory incubator. Inocula (1%, vol/vol) were prepared by growing cells overnight on glucose under the same conditions. Specific growth rates were calculated from measurements of optical density at 660 nm (26). The pH of the medium (with a carbon source) was adjusted to the indicated value prior to filter sterilization by using 2 M H2SO4 or 2 M KOH.
Chemostat cultivation.
Anaerobic, carbon- and energy-limited chemostat cultivation was performed at 30°C in a 2-liter laboratory fermentor (Applikon, Schiedam, The Netherlands). Chemostats were operated at a dilution rate of 0.05 h−1 and at a stirrer speed of 800 rpm. The culture pH was controlled by automatic addition of 2.0 M KOH via an Applikon ADI 1030 biocontroller. The working volume was kept at 1.0 liter by removal of effluent from the middle of the culture via an electrical level controller. This ensured that the biomass concentrations in the effluent differed by less than 2% from those taken directly from the culture. The fermentor and the medium reservoir were flushed with nitrogen gas at a flow rate of 0.5 liter · min−1. To minimize oxygen diffusion, fermentors and medium reservoirs were fitted with Norprene tubing. The dissolved-oxygen concentration, which was measured with an Ingold polarographic O2 electrode, remained below 0.1% of air saturation. A defined mineral medium was used that was supplemented with vitamins and the anaerobic growth factors Tween 80 (420 mg · liter−1) and ergosterol (10 mg · liter−1) (23). A carbon source (glucose or δ-gluconolactone) was filter sterilized separately and added to the sterile medium at a concentration of 25 g · liter−1. The pH of reservoir media after δ-gluconolactone addition decreased from 4.5 to 2.7.
Estimation of carbon balances.
Culture dry weights were determined as previously described (26). A carbon content of 48% was used for the calculation of carbon balances. Substrates and metabolites were determined via high-performance liquid chromatography and enzymatic analysis (23). These methods do not discriminate between δ-gluconolactone and its hydrolysis products gluconic acid and gluconate. Therefore, the data presented on the concentrations of δ-gluconolactone in media and cultures refer to the sum of the lactone and its hydrolysis products gluconic acid and gluconate. For the construction of carbon balances, the concentrations of ethanol in the culture were corrected for the concentrations of ethanol in the reservoir media (approximately 10 mM ethanol, originating from the addition of ethanolic stock solutions of ergosterol). A correction was also made for ethanol evaporation from the bioreactors. The amount of ethanol evaporating from the chemostat cultures was determined in separate experiments by feeding a medium to the fermentor that contained the appropriate concentration of ethanol (i.e., the approximate concentration encountered in steady-state cultures). Conditions in the reactor vessel (volume, temperature, nitrogen flow, and stirrer speed) were maintained as in inoculated fermentors. After five volume changes, the ethanol concentration was determined in the reservoir and in the fermentor for 3 consecutive days. At an ethanol concentration of 260 mM, its rate of evaporation was 0.9 mmol · liter−1 · h−1.
For gas analysis, the exhaust gas of chemostat cultures was cooled in a condenser (2°C) and dried with a Perma Pure dryer (type PD-625-12P). Carbon dioxide concentrations were determined with a Beckman model 864 analyzer. The exhaust gas flow rate was measured as described previously (25). Specific rates of carbon dioxide production were calculated as previously described (23).
Preparation of cell extracts.
Cells from batch or chemostat cultures (ca. 80 mg of dry weight) were harvested by centrifugation at 1,950 × g for 10 min, washed once, and resuspended in 5 ml of 10 mM potassium phosphate buffer (pH 7.5) containing 2 mM EDTA. After storage at −37°C, cells were thawed on ice, washed once, and resuspended in 4 ml of Tris buffer (50 mM Tris-HCl [pH 7.5] containing 1 mM dithiothreitol and 2 mM MgCl2). Cells were then disrupted by sonication with 0.7-mm-diameter acid-washed glass beads (Sigma) at 0°C for 3 min (30-s intervals) with an MSE sonicator (150-W output, 8-μm peak-to-peak amplitude). Whole cells and debris were removed by centrifugation at 31,000 × g (20 min, 4°C). The clear supernatant was used as cell extract. Protein in cell extracts was assayed by the Lowry method. Bovine serum albumin (fatty acid free; Sigma) was used as a standard.
Enzyme assays.
Enzyme assays were performed at 30°C and at 340 nm in a Hitachi model 100-60 spectrophotometer. The volume of the assay mixtures was 1 ml. The glucose dehydrogenase assay mixture contained 50 μmol of Tris-HCl (pH 8.0), 5 μmol of MgCl2, 0.4 μmol of NADP+, and cell extract. The reaction was started by the addition of 50 μmol of glucose. The glucose-6-phosphate dehydrogenase assay mixture contained 50 μmol of Tris-HCl (pH 8.0), 5 μmol of MgCl2, 0.4 μmol of NADP+, and cell extract. The reaction was started by the addition of 5 μmol of glucose-6-phosphate. The 6-phosphogluconate dehydrogenase assay mixture contained 50 μmol of Tris-HCl (pH 8.0), 5 μmol of MgCl2, 0.4 μmol of NADP+, and cell extract. The reaction was started by the addition of 2 μmol of 6-phosphogluconate. The hexokinase assay mixture contained 50 μmol of imidazole-HCl (pH 7.6), 10 μmol of MgCl2, 1 μmol of NADP+, 1.8 U of glucose 6-phosphate dehydrogenase, 10 μmol of glucose, and cell extract. The reaction was started by the addition of 1 μmol of ATP. The increase in the reaction rate after addition of ATP was taken as hexokinase activity, thus correcting for the interfering activity of glucose dehydrogenase. The gluconate kinase assay mixture contained 100 μmol of Tris-HCl (pH 8.0), 10 μmol of MgCl2, 1 μmol of NADP+, 2.5 U of 6-phosphogluconate dehydrogenase, 1 μmol of ATP, and cell extract. The reaction was started by the addition of 20 μmol of gluconate. When either this assay mixture or a mixture in which Tris-HCl was replaced with potassium phosphate (pH 7.0 or 8.0) was used, no or only very low activity was detected. Addition of 6-phosphogluconate resulted in an immediate rapid increase in absorbance, indicating that the coupling enzyme was active. Specific activities were calculated based on an NAD(P)H extinction coefficient of 6.3 mM−1 · cm−1.
Chemicals.
Enzyme kits for the determination of glucose, glycerol, acetate, gluconate, pyruvate, and lactate were obtained from Boehringer Mannheim. Alcohol oxidase for the colorimetric analysis of ethanol (23) was a gift from Bird Engineering, Rotterdam, The Netherlands.
RESULTS
Effect of culture pH on δ-gluconolactone metabolism.
Culture pH can be expected to have a strong effect on δ-gluconolactone utilization, as this compound hydrolyzes to gluconic acid, which in turn dissociates to gluconate and H+.
The pKa of the latter reaction is 3.8. Thus, if δ-gluconolactone is the only species that is metabolized by a microorganism, its utilization may be facilitated by a low culture pH. Assimilation of carbon compounds by yeasts is tested in routine taxonomic procedures by adding these compounds in a concentration of 1 to 2% (wt/vol) to a commercial mineral medium (2). In such assimilation tests, the pH is not readjusted after addition of the carbon source. Dissolving δ-gluconolactone to 1 or 2% in commercial mineral media causes the pH to drop below 3. Thus, when a yeast species is found to be unable to utilize δ-gluconolactone but able to grow on sodium gluconate (Table 1, group II), this also may reflect its inability to grow at low pHs. In S. bulderi, the reverse situation exists: the organism was reported to grow on δ-gluconolactone but not on sodium gluconate (11). This property is also found in various other yeast species (Table 1, group III).
In order to assess the effect of culture pH on δ-gluconolactone and sodium gluconate utilization by S. bulderi, the organism was grown in shake flask cultures in which the pH was adjusted before inoculation. Growth on glucose was not affected by culture pHs between 3.0 and 6.0. In contrast to glucose, δ-gluconolactone and gluconate did not support growth at pH 5.0 but were good carbon sources at an initial pH of 3.0 (Table 2). It therefore seems probable that, rather than gluconate or gluconic acid, δ-gluconolactone is the species metabolized by S. bulderi. The absence of gluconate utilization at pH 5.0 probably results from the fact that, at this pH, the chemical equilibrium favors gluconate formation, leading to negligible concentrations of the lactone.
TABLE 2.
Specific growth rates of S. bulderi as a function of culture pHa
| Substrate | pH | Growth rate (h−1) |
|---|---|---|
| Glucose | 6.0 | 0.24 |
| 3.0 | 0.24 | |
| δ-Gluconolactone | 5.0 | <0.01 |
| 2.7 | 0.22 | |
| Sodium gluconate | 5.0 | <0.01 |
| 3.0 | 0.22 |
Initial pH in shake flask cultures. Data were calculated from two independent experiments, and duplicate experiments varied less than 5%.
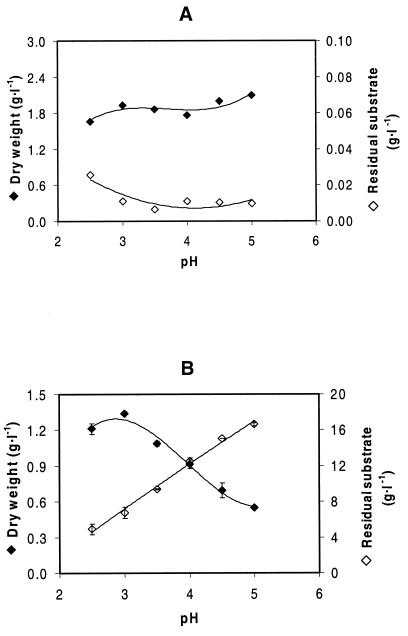
Effects of cultivation pH on δ-gluconolactone utilization were further investigated in anaerobic carbon-limited chemostat cultures. In such cultures, growing at a fixed dilution rate of 0.05 h−1 on glucose, the reservoir concentration of 25 g · liter−1 was completely consumed; the residual glucose concentration in the culture was less than 0.02 g · liter−1 at all of the culture pHs tested (Fig. 1A). With δ-gluconolactone as a carbon source, however, a high residual substrate concentration was present. The degree of utilization and, therefore, the biomass concentration were strongly dependent on the culture pH. At the highest pH tested, only one-third of the δ-gluconolactone supplied to the culture was consumed (Fig. 1B). The analytical methods used to quantify substrate concentrations (see Materials and Methods) could not discriminate among the lactone, gluconic acid, and gluconate. It is likely, however, that the residual substrate encountered in the δ-gluconolactone cultures consisted of a mixture of gluconic acid and gluconate, which are apparently nonmetabolizable, and that the actual δ-gluconolactone concentration in these cultures was negligible. Only this assumption can explain the incomplete utilization of the limiting carbon source, the degree of which depended on the culture pH (Fig. 1B).
FIG. 1.
Biomass dry weight and residual substrate concentration in carbon- and energy-limited anaerobic chemostat cultures of S. bulderi. Steady-state cultures were maintained at a dilution rate of 0.05 h−1 and at different pHs. A, glucose-grown cultures; B, δ-gluconolactone-grown cultures.
Growth on δ-gluconolactone at pH 5.0 in chemostat cultures seems to be in conflict with the absence of growth at this pH in shake flask cultures (Table 2). However, it should be realized that the chemostat cultures were fed continuously with a δ-gluconolactone-containing medium with a low pH (see Materials and Methods). The hydrolysis of δ-gluconolactone is rather slow, with a half-life of 10 min at pH 6.6 and of 60 min at pH 4 (13, 18). Thus, although the chemostat cultures were kept at pH 5.0, metabolism of δ-gluconolactone could apparently compete with chemical hydrolysis. This is in contrast to the situation in batch cultures, in which chemical equilibrium is already established before inoculation.
Stoichiometry of δ-gluconolactone fermentation.
The stoichiometry of growth was investigated in detail by using carbon-limited anaerobic chemostat cultures. In view of the profound effect of pH on growth (Table 2; Fig. 1B), cultures were grown at a range of pHs at a fixed dilution rate of 0.05 h−1.
Glucose-limited cultures grown under identical conditions served as a reference. The results listed in Tables 3 and 4 confirm the observations of Middelhoven et al. (11) with respect to the stoichiometry of δ-gluconolactone fermentation: CO2, ethanol, and glycerol were the major fermentation products. Also, small amounts of acetate and lactate were detected in both glucose- and δ-gluconolactone-grown cultures. At pHs greater than 3.0, the biomass yield (grams of biomass formed per gram of substrate utilized) was nearly independent of the culture pH for both substrates.
TABLE 3.
Physiology of S. bulderi in anaerobic glucose-limited chemostat cultures at a dilution rate of 0.05 h−1 and at different culture pHsa
| pH | Ysx | CO2/ethanol ratio | qs | qCO2 | qethanol | qglycerol | qpyruvate | qacetate | qlactate | C recovery (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 0.067 | 0.97 | 4.56 | 8.0 | 8.2 | 0.55 | <0.01 | <0.01 | <0.01 | 103.3 |
| 3.0 | 0.078 | 1.01 | 3.92 | 6.7 | 6.7 | 0.54 | <0.01 | 0.02 | 0.08 | 102.7 |
| 3.5 | 0.075 | 1.03 | 4.10 | 7.2 | 7.0 | 0.61 | <0.01 | 0.02 | 0.07 | 103.8 |
| 4.0 | 0.071 | 1.03 | 4.32 | 7.6 | 7.3 | 0.64 | <0.01 | 0.03 | 0.08 | 103.1 |
| 4.5 | 0.082 | 1.02 | 3.74 | 6.3 | 6.2 | 0.61 | <0.01 | 0.02 | 0.09 | 103.1 |
| 5.0 | 0.085 | 1.00 | 3.59 | 5.9 | 6.0 | 0.59 | <0.01 | 0.02 | 0.10 | 102.7 |
| Avg | 0.076 | 1.01 | 4.04 | 7.0 | 6.9 | 0.59 | <0.01 | 0.02 | 0.07 | 103.1 |
Specific rates of substrate consumption (qs) and metabolite production (q) are presented as millimoles per gram of biomass per hour. The CO2 to-ethanol ratio equals the quotient of the specific rates of production of these metabolites. The biomass yield on a substrate (Ysx) is given as grams of biomass per gram of substrate utilized (i.e., the reservoir substrate concentration minus the residual concentration in the culture). Data were calculated as described in Materials and Methods and are the result of one cultivation at each pH.
TABLE 4.
Physiology of S. bulderi in anaerobic δ-gluconolactone-limited chemostat cultures at a dilution rate of 0.05 h−1 and at different culture pHa
| pH | Ysx | CO2/ethanol ratio | qs | qCO2 | qethanol | qglycerol | qpyruvate | qacetate | qlactate | C recovery (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 0.060 | 1.27 | 5.25 | 10.2 | 8.1 | 0.75 | <0.01 | 0.08 | 0.07 | 98.9 |
| 3.0 | 0.074 | 1.33 | 4.10 | 8.2 | 6.2 | 0.68 | 0.03 | 0.03 | 0.11 | 102.8 |
| 3.5 | 0.070 | 1.33 | 4.49 | 8.6 | 6.5 | 0.73 | <0.01 | 0.03 | 0.09 | 97.6 |
| 4.0 | 0.074 | 1.34 | 4.08 | 7.8 | 5.8 | 0.70 | <0.01 | 0.04 | 0.09 | 98.0 |
| 4.5 | 0.075 | 1.37 | 4.06 | 8.1 | 5.9 | 0.70 | <0.01 | 0.07 | 0.08 | 100.7 |
| 5.0 | 0.071 | 1.38 | 4.32 | 8.6 | 6.2 | 0.81 | <0.01 | 0.12 | 0.09 | 102.4 |
| Avg | 0.070 | 1.34 | 4.38 | 8.6 | 6.5 | 0.73 | <0.01 | 0.06 | 0.09 | 100.0 |
Specific rates of substrate consumption (qs) and metabolite production (q) are presented as millimoles per gram of biomass per hour. The CO2-to-ethanol ratio equals the quotient of the specific rates of production of these metabolites. The biomass yield on a substrate (Ysx) is given as grams of biomass per gram of substrate utilized (i.e., the reservoir substrate concentration minus the residual concentration in the culture). Data were calculated as described in Materials and Methods and are the average of two independent cultivations at each pH. Data from these independent replicate experiments differed by less than 5%.
Glucose and δ-gluconolactone-grown cultures differed with respect to the stoichiometry of alcoholic fermentation. CO2 and ethanol were produced in equimolar amounts in glucose-grown cultures, but in gluconolactone-grown cultures the average ratio of CO2 to ethanol was 1.34 (Table 4).
Enzymology of δ-gluconolactone fermentation.
During glucose fermentation, ethanol and CO2 are formed in equimolar amounts via the action of pyruvate decarboxylase and alcohol dehydrogenase. The results obtained with δ-gluconolactone-grown cultures (Table 4) therefore indicate that pyruvate decarboxylation is not the only source of CO2, as CO2 production exceeded that of ethanol by one-third. It was anticipated that the pentose phosphate pathway could contribute to the observed CO2 production. Indeed, levels of the key enzymes of this pathway, glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, were almost 10-fold higher in cell extracts prepared from δ-gluconolactone-grown cultures than in extracts of glucose-grown cultures (Table 5). Interestingly, a very high rate of NADPH formation was also observed with glucose as a substrate instead of glucose-6-phosphate, thus revealing the presence of a glucose dehydrogenase (EC 1.1.1.47). The enzyme preferred NADP+ as a coenzyme. A 10-fold lower activity was observed with NAD+ (results not shown). The NADP+-dependent glucose dehydrogenase activity was absent in glucose-grown cells, indicating that this enzyme plays a role in the metabolism of δ-gluconolactone. Gluconate-kinase activity was very low in δ-gluconolactone-grown cells. Hexokinase activities were high in both cultures (Table 5).
TABLE 5.
Enzyme activities in cell extracts of S. bulderi on different growth substratesa
| Enzyme | Sp act (μmol·min−1·mg of protein−1)
|
|
|---|---|---|
| Glucose | δ-Gluconolactone | |
| Glucose-6-phosphate dehydrogenase | 0.52 | 3.6 |
| 6-phosphogluconate dehydrogenase | 0.13 | 1.5 |
| Glucose dehydrogenase | <0.02 | 7.3 |
| Hexokinase | 9.6 | 5.2 |
| Gluconate kinase | <0.02 | <0.02 |
The yeast was grown at pH 3.0 in anaerobic glucose- or δ-gluconolactone-limited chemostat cultures at a dilution rate of 0.1 h−1.
DISCUSSION
Proposed pathway for δ-gluconolactone fermentation in S. bulderi.
The ability to assimilate δ-gluconolactone is widespread among yeasts (2; Table 1). However, only one-third of the species listed in Table 1 that are able to grow on δ-gluconolactone or sodium gluconate are facultatively fermentative. Therefore, the property of anaerobic growth on δ-gluconolactone is probably rare among the yeasts described so far. In fact, it would only be expected to occur among Saccharomyces species as these are exceptional in displaying fast growth both aerobically and anaerobically (20, 21, 24). Some Saccharomyces cerevisiae strains have been reported to grow slowly on δ-gluconolactone. However, these strains were unable to grow anaerobically with this substrate (19). The aerobic utilization of δ-gluconolactone by these strains was reported to occur via a phosphorylation of gluconate or δ-gluconolactone, followed by metabolism via 6-phosphogluconate dehydrogenase and the pentose phosphate pathway enzymes (19).
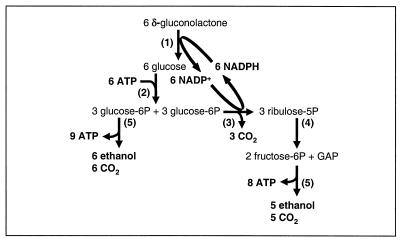
The ability of S. bulderi to ferment δ-gluconolactone to CO2, ethanol, and some glycerol under strictly anaerobic conditions excludes the metabolic routes known to date. For example, metabolism via an Entner-Doudoroff type of pathway would result in the formation of pyruvate (or other compounds with the same degree of reduction) and ethanol in a 1:1 ratio. Alternatively, complete dissimilation via the pentose phosphate pathway requires the formation of a reduced compound to dispose of the reducing equivalents generated in the 6-phosphogluconate dehydrogenase reaction under anaerobic conditions. If glycerol formation served this purpose, the glycerol formation rate should have equaled the rate of δ-gluconolactone utilization and, moreover, the CO2-to-ethanol ratio should be at least 2.5. If δ-gluconolactone metabolism proceeded via a phosphoketolase reaction rather than via rearrangement of the carbon skeleton by the pentose phosphate pathway enzymes transaldolase and transketolase, substantial acetate formation should occur. Thus, all of the biochemical routes for microbial gluconate fermentation reported to date are at variance with the observed stoichiometry of growth of S. bulderi on δ-gluconolactone (Table 4). Instead, our results (Tables 2, 3, and 4 and Fig. 1A and B) suggest that δ-gluconolactone fermentation by S. bulderi proceeds via a novel route, which is depicted in Fig. 2.
FIG. 2.
Proposed pathway for δ-gluconolactone metabolism in S. bulderi. 1, glucose dehydrogenase; 2, hexokinase; 3, glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase; 4, rearrangement reactions in the pentose phosphate pathway via transaldolase and transketolase; 5, glycolysis. GAP, glyceraldehyde-3-phosphate.
The first step in δ-gluconolactone metabolism is its transport over the cell membrane. The strong effect of culture pH on growth indicates that the lactone rather than gluconic acid or gluconate is the species that is transported. (Table 2; Fig. 1B). After transport, the lactone is reduced to glucose via glucose dehydrogenase. The reducing equivalents required for this reaction are generated in the pentose phosphate pathway via glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. Both enzymes are NADP dependent in S. cerevisiae and other yeasts (16). In order to generate sufficient NADPH, half of the glucose originating from δ-gluconolactone reduction must be channeled into the pentose phosphate pathway. The ribulose 5-phosphate thus formed can then be rearranged to hexose and triose phosphates via epimerase, isomerase, transaldolase, and transketolase reactions. Hexose phosphates and triose phosphate are further metabolized via glycolysis.
The metabolic pathway outlined above yields the following overall stoichiometry:
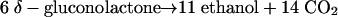 |
(1) |
The CO2-to-ethanol ratio would thus be 1.27 in the case of a resting cell suspension.
Stoichiometry and energetics of alcoholic fermentation in anaerobic cultures.
In a resting cell suspension, anaerobic alcoholic fermentation of glucose proceeds according to the well-known equation
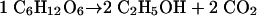 |
(2) |
However, in a growing culture, the CO2-to-ethanol ratio must be slightly higher than unity. This is due to the fact that in the metabolic network of biomass formation from glucose, decarboxylation reactions are in excess over carboxylations (5). The process of biomass formation can be presented by the following equation (25), with coefficients in millimoles:
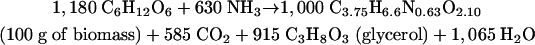 |
(3) |
During anaerobic growth on δ-gluconolactone, this effect is even more pronounced than during growth on glucose. Biomass formation from δ-gluconolactone requires additional CO2 production. For every 2 mol of gluconolactone, 1 mol of CO2 must be generated via the pentose phosphate pathway (Fig. 2 and equation 4).
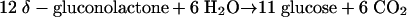 |
(4) |
Thus, the equation for the synthesis of biomass from δ-gluconolactone can be calculated by combining equations 3 and 4, resulting in the following equation:
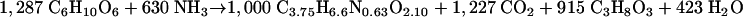 |
(5) |
From the biomass yields (Tables 3 and 4), the equations for substrate dissimilation (equations 1 and 2), and those for substrate assimilation (equations 3 and 5), theoretical values for the CO2-to-ethanol ratio in growing cultures can be calculated. This ratio should be 1.05 for growth on glucose and 1.36 for growth on δ-gluconolactone. These theoretical values, based on an assumed biomass composition (25) and observed biomass yields, are close to the experimental values listed in Tables 3 and 4.
The biomass yields of S. bulderi on glucose and δ-gluconolactone were only slightly different (Tables 3 and 4). In anaerobic cultures, the ATP yield from dissimilation is a major determinant of the biomass yield (22). This is exemplified by a comparison of S. cerevisiae and Zymomonas mobilis for anaerobic growth on glucose (3). The former has a much higher biomass yield due to the fact that in the yeast the ATP yield from glycolysis is twice as high as in the bacterium, which uses the Entner-Doudoroff route.
From a comparison of the physiology of S. bulderi during anaerobic growth on glucose and gluconolactone (Tables 3 and 4), it can be concluded that the dissimilation of the substrate yields almost the same amount of ATP. A slightly lower biomass yield on δ-gluconolactone was obtained, which is in line with the pathway depicted in Fig. 2. For every 12 mol of gluconolactone metabolized, 1 mol has to be oxidized to CO2 in the hexose monophosphate to yield the reducing equivalents for its metabolism via glucose (equation 4). Consequently, the ATP yield from dissimilation is 11/6 = 1.83 mol of ATP per mol of substrate, compared to 2.0 for glucose.
Anaerobic chemostat cultures of S. cerevisiae transport glucose via facilitated diffusion via the HXT2, -5, and -7 gene products (7). Maltose, on the other hand, is transported via a sugar-proton symport mechanism (26). As a result, the anaerobic biomass yield of S. cerevisiae is substantially lower on maltose than on glucose since sugar proton symport in S. cerevisiae requires 1 mol of ATP per mol of sugar transported (26).
When, as in S. cerevisiae, transport of glucose in S. bulderi occurs via facilitated diffusion, the comparison of biomass yields of S. bulderi on glucose and δ-gluconolactone points to facilitated diffusion as the mechanism of entry of δ-gluconolactone into the cell. If the latter occurred via a proton symport mechanism and thus required 1 mol of ATP per mol of substrate fermented, the biomass yield on δ-gluconolactone should have been approximately half of that on glucose.
Physiological function of glucose dehydrogenase in microorganisms.
In the proposed pathway for δ-gluconolactone metabolism by S. bulderi (Fig. 2), glucose dehydrogenase acts as a δ-gluconolactone reductase (Fig. 2). The equilibrium of the reaction δ-gluconolactone + NAD(P+) ↔ glucose + NAD(P)H + H+ is in favor of this proposal (equilibrium constant, 10−6 to 10−7; 1). It is therefore plausible that not only in S. bulderi but also in some other organisms in which the enzyme is present (1, 6, 17), the physiological function of the enzyme (EC 1.1.1.47 or EC 1.1.1.119; 17) may be catalysis of the reduction of δ-gluconolactone to glucose. For example, Gluconobacter spp. are known to possess high activities of an NADP-linked glucose dehydrogenase (1, 12). As these bacteria produce δ-gluconolactone themselves by a periplasmic pyridine nucleotide-independent dehydrogenase (12), it may well be that, also in these bacteria, the NADP-linked glucose dehydrogenase functions as a δ-gluconolactone reductase in vivo.
Metabolic role of the pentose phosphate pathway in yeasts.
The pentose phosphate pathway in yeasts is generally considered to be an anabolic route. Its function is the provision of NADPH for biosynthetic processes and the generation of pentose phosphates required for nucleic-acid synthesis (16). On the basis of radiorespirometric studies, the contribution of the pentose phosphate pathway in various yeasts has been calculated to be between 7 and 11% during aerobic glucose-limited growth (4). During anaerobic, fermentative growth, its relative contribution is much smaller as relatively more glucose is metabolized via glycolysis (9).
It is evident from Fig. 2 that, during anaerobic metabolism of δ-gluconolactone, the contribution of the pentose phosphate pathway must be 50% to obtain a closed redox balance for dissimilation of δ-gluconolactone to ethanol and CO2 under anaerobic conditions. This pathway serves both an anabolic and a catabolic function in S. bulderi growing anaerobically on δ-gluconolactone. The observation that S. bulderi grown on δ-gluconolactone contains high glucose-6-phosphate and 6-phosphogluconate dehydrogenase activities is in accordance with this conclusion.
The physiological characteristics of S. bulderi make it an interesting yeast for metabolic engineering of xylose fermentation. The pentose sugar xylose is not a natural growth substrate for Saccharomyces species. To enable ethanol production from hemicellulose hydrolysates, many metabolic engineering studies have aimed at introduction of heterologous key enzymes in xylose metabolism into S. cerevisiae (8, 10). The initial conversion of xylose by these heterologous enzymes is intended to enable xylose dissimilation via the pentose phosphate pathway. The ability of S. bulderi to use the pentose phosphate pathway as an efficient, high-throughput catabolic pathway, under strictly anaerobic conditions, may be an important advantage for efficient xylose fermentation by engineered strains.
Acknowledgments
We thank Frieda von Boltog for her help and patience in the preparation of the manuscript.
REFERENCES
- 1.Avigad, G., Y. Alroy, and S. England. 1968. Purification and properties of a nicotinamide adenine dinucleotide phosphate-linked aldohexose dehydrogenase from Gluconobacter cerinus. J. Biol. Chem. 243:1936–1941. [PubMed] [Google Scholar]
- 2.Barnett, J. A., R. W. Payne, and D. Yarrow. 1990. Yeasts: characteristics and identification, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 3.Bauchop, T., and S. R. Elsden. 1960. The growth of microorganisms in relation to their energy supply. J. Gen. Microbiol. 23:457–469. [DOI] [PubMed] [Google Scholar]
- 4.Bruinenberg, P. M., G. W. Waslander, J. P. van Dijken, and W. A. Scheffers. 1986. A comparative radiorespirometric study of glucose metabolism in yeasts. Yeast 2:117–121. [DOI] [PubMed] [Google Scholar]
- 5.Bruinenberg, P. M., J. P. van Dijken, and W. A. Scheffers. 1983. A theoretical analysis of NADPH production and consumption in yeasts. J. Gen. Microbiol. 129:953–964. [Google Scholar]
- 6.Bückmann, A. F., and D. Schmid. 1991. A different acceptance of adenine-modified NADP+ by NADP+-dependent glucose dehydrogenase from Bacillus megaterium and strictly NADP+-dependent glucose dehydrogenase from Cryptococcus uniguttulatus. Biotechnol. Appl. Biochem. 14:104–113. [Google Scholar]
- 7.Diderich, J. A., M. E. Schepper, P. van Hoek, M. A. H. Luttik, J. P. van Dijken, J. T. Pronk, P. Klaassen, J. M. Teixeira de Mattos, K. van Dam, and A. L. Kruckeberg. 1999. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 274:15350–15359. [DOI] [PubMed] [Google Scholar]
- 8.Eliasson, A., C. Christensson, C. F. Wahlbom, and B. Hahn-Hägerdahl. 2000. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl. Environ. Microbiol. 66:3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gancedo, J. M., and R. Lagunas. 1973. Contribution of the pentose-phosphate pathway to glucose metabolism in Saccharomyces cerevisiae: a critical analysis on the use of labeled glucose. Plant Sci. Lett. 1:193–200. [Google Scholar]
- 10.Ho, N. W. Y., Z. Chen, and A. P. Brainard. 1998. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 64:1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middelhoven, W. J., C. P. Kurtzman, and A. Vaughan-Martini. 2000. Saccharomyces bulderi sp. nov., a yeast that ferments gluconolactone. Antonie van Leeuwenhoek 77:223–228. [DOI] [PubMed] [Google Scholar]
- 12.Pronk, J. T., P. R. Levering, W. Olijve, and J. P. van Dijken. 1989. Role of NADP-dependent and quinoprotein glucose dehydrogenases in gluconic acid production by Gluconobacter oxydans. Enzyme Microb. Technol. 11:160–164. [Google Scholar]
- 13.Rees, E. T., and M. Mandels. 1960. Gluconolactone as an inhibitor of carbohydrates. Dev. Ind. Microbiol. 1:171–179. [Google Scholar]
- 14.Roehr, M., C. P. Kubiceck, and J. Komínek. 1996. Gluconic acid, p.347–362. In H. J. Rehm and G. Reed (ed.), Bio/Technology, 2nd edition, vol. 6. VCH Verlagsgesellschaft mbH, Weinheim, Germany.
- 15.Sawyer, D. T., and J. B. Bagger. 1959. The lactone-acid-salt equilibria for d-glucono-δ-lactone and the hydrolysis kinetics for this lactone. J. Am. Chem. Soc. 81:5302–5310. [Google Scholar]
- 16.Schaaff-Gerstenschläger, I., and T. Miosga. 1997. The pentose phosphate pathway, p.271–283. In F. K. Zimmermann and K. D. Entian (ed.), Yeast sugar metabolism. Biochemistry, genetics, biotechnology and applications. Technomic Publishing Co., Inc., Lancaster, Pa.
- 17.Schomburg, D., and D. Stephan. 1995. Glucose 1-dehydrogenase. E.C. 1.1.1.47 and E.C. 1.1.1.119, p.1–6. In D. Schomburg and D. Stephan (ed.), Enzyme handbook, vol. 9. Springer-Verlag, Berlin, Germany.
- 18.Shimahara, K., and T. Takahashi. 1970. An infrared spectrophotometric study on the interconversion and hydrolysis of d-glucono-γ- and -δ-lactone in deuterium oxide. Biochim. Biophys. Acta 201:410–415. [DOI] [PubMed] [Google Scholar]
- 19.Sinha, A., and P. K. Maitra. 1992. Induction of specific enzymes of the oxidative pentose phosphate pathway by glucono-δ-lactone in Saccharomyces cerevisiae. J. Gen. Microbiol. 138:1865–1873. [DOI] [PubMed] [Google Scholar]
- 20.Van Dijken, J. P., and W. A. Scheffers. 1986. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Rev. 32:199–224. [Google Scholar]
- 21.Van Dijken, J. P., E. van den Bosch, J. J. Hermans, L. Rodrigues de Miranda, and W. A. Scheffers. 1986. Alcoholic fermentation by “non-fermentative” yeasts. Yeast 2:123–127. [DOI] [PubMed] [Google Scholar]
- 22.Verduyn, C. 1991. Physiology of yeast in relation to growth yields. Antonie van Leeuwenhoek 60:325–353. [DOI] [PubMed] [Google Scholar]
- 23.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395–403. [DOI] [PubMed] [Google Scholar]
- 24.Visser, W., W. M. Batenburg-van der Vegte, W. A. Scheffers, and J. P. van Dijken. 1990. Oxygen requirements of yeasts. Appl. Environ. Microbiol. 56:3785–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visser, W. 1995. Oxygen requirements of fermentative yeasts. Ph.D. thesis. Delft University of Technology, Delft, The Netherlands.
- 26.Weusthuis, R. A., H. Adams, W. A. Scheffers, and J. P. van Dijken. 1993. Energetics and kinetics of maltose transport in Saccharomyces cerevisiae: a continuous culture study. Appl. Environ. Microbiol. 59:3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]