Abstract
Escherichia coli zupT (ygiE), encoding a ZIP family member, mediated zinc uptake. Growth of cells disrupted in both zupT and the znuABC operon was inhibited by EDTA at a much lower concentration than a single mutant or the wild type. Cells expressing ZupT from a plasmid exhibited increased uptake of 65Zn2+.
Zinc is an essential transition metal for all organisms and serves as a cofactor in members of all six major functional classes of enzymes (18). It also serves as a structural cofactor for many proteins. As a result, organisms have developed mechanisms for maintaining adequate concentrations of intracellular zinc while preventing metal ion overaccumulation. Cells of Escherichia coli encounter fluctuating extracellular zinc levels and maintain zinc homeostasis by transporting excess metal out of the cell and regulating zinc uptake across the cytoplasmic membrane. Prior to this report, three zinc transport systems had been characterized for E. coli. Efflux of zinc is accomplished by the P-type ATPase ZntA (2, 16) and the cation diffusion facilitator ZitB (7). Under conditions of deficiency, zinc is taken up by the high-affinity ABC transporter ZnuABC (14). In this report we show that ZupT is an additional zinc transporter responsible for zinc uptake. ZupT is the first characterized bacterial member of the ZIP family of proteins, previously only reported to be present in eukaryotes. The ZIP family derived its name from the first identified members (ZRT, IRT-like protein) (9). These transporters were initially identified as iron or zinc transporters but were subsequently shown to be able to also transport manganese and cadmium. However, the specificity and affinity to different metals are specific for each ZIP transporter (6, 9).
Either ZupT (YgiE) or ZnuABC is necessary for growth under zinc-limited conditions.
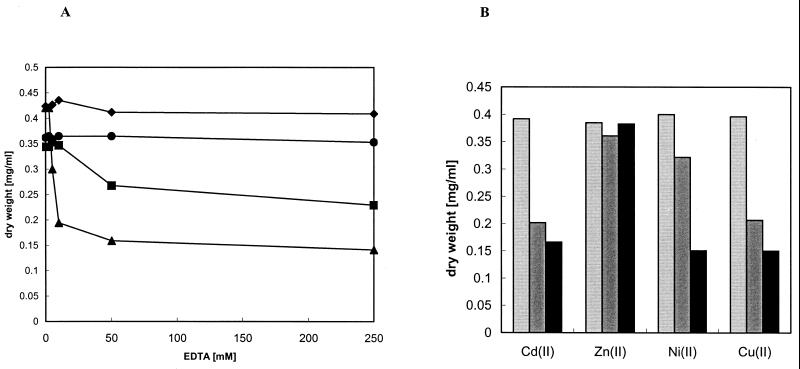
ZnuABC had been shown to be responsible for high-affinity zinc uptake (14). However, addition of EDTA to mineral salts medium did not completely abolish growth of E. coli strain GR352 ΔznuABC::cam, indicating the presence of other transporters. Recently, a review by Gaither and Eide (6) suggested that the ZIP family is not restricted to eukaryotes but is also present in bacteria. In E. coli the ygiE gene encodes a putative ZIP protein (6). To assess the physiological role, ygiE was deleted as described previously (4). Deletion of ygiE in E. coli GR316 (ΔygiE::cam) only slightly affected growth under the conditions tested (Fig. 1A). However, growth of the double mutant E. coli GR354 (ΔznuABC ΔygiE::cam) with both ygiE and znuABC deleted was severely inhibited by the presence of EDTA. This inhibition was more pronounced in the double mutant than in the single mutants E. coli GR352 (ΔznuABC::cam) and GR316 (ΔygiE::cam) (Fig. 1A). Addition of zinc but not of nickel, copper, or cadmium alleviated this inhibition (Fig. 1B), indicating that ygiE is responsible for zinc uptake. We therefore renamed ygiE as zupT to indicate its role as a zinc uptake transporter. ZnuABC appears to have a higher affinity for zinc than ZupT, since a strain with a deletion of zupT is less inhibited in growth by the addition of high concentrations of EDTA than a strain with a deletion of znuABC. E. coli GR352 (ΔznuABC) showed improvement of growth after addition of zinc but also a slight increase after nickel addition (Fig. 1B). This could be caused by slight impurities in the nickel salt used. However, zinc clearly is the growth-limiting factor in the E. coli GR352 (ΔznuABC) single mutant and the E. coli GR354 (ΔznuABC ΔygiE::cam) double mutant.
FIG. 1.
Effect of zinc depletion on growth of E. coli W3110, GR352 (ΔznuABC::cam), and GR354 (ΔzupT::cam ΔznuABC). Growth yields with different EDTA concentrations are shown. (A) Overnight cultures grown in LB medium were diluted 1:400 into fresh mineral salts medium (12) with the indicated concentrations of EDTA. Cell growth was monitored by measuring the OD600 after 6 h of incubation at 37°C with shaking and converted to dry weight. Results are shown for E. coli W3110 (⧫), E. coli GR316 (ΔzupT::cam) (•), E. coli GR352 (ΔznuABC::cam) (▪), and E. coli GR354 (ΔzupT::cam ΔznuABC) (▴). Experiments were performed in triplicate, and the average was calculated. (B) Cells were treated as for panel A except that equimolar amounts of EDTA and metals were added (both at 250 μM). Results are shown for E. coli W3110 (light grey bars), E. coli GR352 (ΔznuABC::cam) (dark grey bars), and E. coli GR354 (ΔzupT::cam ΔznuABC) (black bars).
Expression of ZupT makes cells zinc hypersensitive.
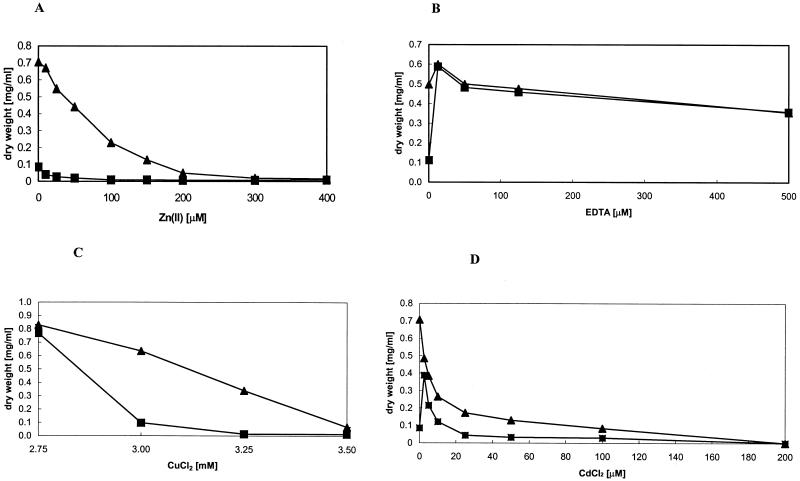
To further characterize ZupT-mediated metal transport, ZupT was cloned into the inducible expression vector pASK-IBA3 (IBA, Göttingen, Germany), creating pZUPT. Expression of zupT was induced by the addition of 200 ng of anhydrotetracycline (AHT) (Sigma-Genosys)/ml, which made cells hypersensitive to zinc (Fig. 2A). This effect was most pronounced in strain E. coli GG48, in which the zntA and zitB genes, encoding zinc efflux pumps, had been disrupted. Expression of zupT in E. coli GG48 (zntA::kan ΔzitB::cam) even led to reduced viability in Luria-Bertani (LB) broth without added zinc due to residual zinc present in the medium (Fig. 2A). However, addition of EDTA alleviated the toxic effects of expression of zupT (Fig. 2B). This indicated that zinc hypersensitivity is caused by overaccumulation of cytosolic zinc and is not due to possible toxic effects of protein overproduction. Furthermore, a slight increase in copper sensitivity in wild-type cells expressing zupT was observed, indicating that ZupT transports copper in addition to zinc (Fig. 2C). In contrast, addition of cadmium to cells expressing zupT did not lead to a pronounced increase in sensitivity as observed with zinc (Fig. 2D). On the contrary, the addition of 5 μM Cd(II) led to an increase in viability over that for cells in LB broth without added metal, indicating that Cd(II) might inhibit uptake of zinc by ZupT. This is in agreement with previously reported metal specificities of ZIP proteins from Arabidopsis thaliana (8).
FIG. 2.
Growth of E. coli strains expressing zupT in the presence of metals or EDTA. Overnight cultures grown in LB medium were diluted 1:400 into fresh LB medium with the indicated concentrations of zinc (A), EDTA (B), copper (C), or cadmium (D), and expression of zupT was induced with 200 ng of AHT/ml. Growth was monitored after 6 h by measuring the OD600 and converted to dry weight (mg/ml). (A, B, D) ▪, E. coli GG48 (ΔzntA::kan ΔzitB::cam)(pZUPT); ▴, E. coli GG48 (ΔzntA::kan ΔzitB::cam) vector control. (C) ▪, E. coli W3110(pZUPT); ▴, E. coli W3110 vector control. Results of representative experiments are shown.
ZupT mediates uptake of 65Zn.
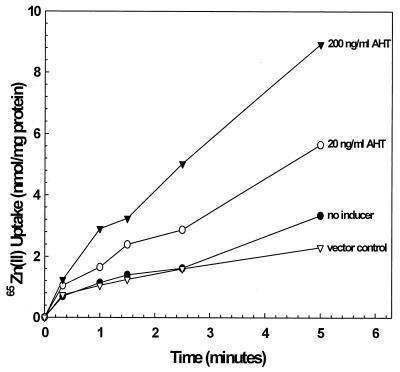
To measure uptake of 65Zn2+ by ZupT, all known zinc transport systems in E. coli were disrupted (4). The zntA and zitB genes both encode efflux systems (7, 16), whereas znuABC and zupT are responsible for zinc uptake (14; this report). In addition, the zntB gene, encoding a putative zinc efflux system, was deleted (data not shown). This strain, E. coli GR362 (zntA::kan ΔzitB ΔzupT ΔznuABC ΔzntB::cam), showed normal growth in LB medium and was transformed with the plasmid pZUPT. Expression of zupT was induced with different concentrations of AHT for 10 min. Cells with the largest amount of inducer added (200 ng/ml) showed the largest increase in zinc uptake compared to the vector control, E. coli GR362(pASK-IBA3) (Fig. 3). Addition of smaller amounts of inducer still led to a significantly higher level of accumulation of 65Zn2+ than was found with control cells (Fig. 3).
FIG. 3.
Uptake of 65Zn(II) by cells of E. coli GR362 (ΔznuABC ΔzupT ΔzntB::cam ΔzitB zntA::kan) expressing zupT. Cells were grown overnight in LB medium, diluted 50-fold into fresh prewarmed LB medium, and grown to an OD600 of 0.5, and expression of zupT was induced with 200 or 20 ng of AHT/ml for 10 min or not induced. The cells were washed with buffer A (10 mM Tris-HCl [pH 7.0], 2 g of glucose/liter, 10 mM Na2HPO4) and resuspended in the same buffer. 65ZnSO4 was added to a final concentration of 10 μM. The cells were incubated at 37°C, and 0.1-ml aliquots were filtered through nitrocellulose membranes (pore size, 0.45 μm) at various times and immediately washed with 10 ml of buffer B (10 mM Tris-HCl [pH 7.0], 10 mM MgCl2). The membranes were dried, and radioactivity was measured using a liquid scintillation counter. The protein concentration was determined using the method of Lowry et al. (11), and the amount of Zn(II) per milligram of protein was calculated. •, E. coli GR362 (ΔznuABC ΔzupT ΔzntB::cam ΔzitB zntA::kan)(pZUPT) (no inducer); (○), E. coli GR362 (ΔznuABC ΔzupT ΔzntB::cam ΔzitB zntA::kan)(pZUPT) (20-ng/ml AHT); ▾, E. coli GR362 (ΔznuABC ΔzupT ΔzntB::camΔzitB zntA::kan)(pZUPT) (200 ng/ml); ▿, E. coli GR362 (ΔznuABC ΔzupT ΔzntB::cam ΔzitB zntA::kan)(pASK-IBA3).
Conclusions.
In this report we show that ZupT is responsible for zinc uptake in E. coli. ZupT represents the first bacterial member of the ZIP family (5, 6, 9). Therefore, the transporters involved in zinc homeostasis in E. coli constitute a mixture of uniquely bacterial systems, such as ZnuABC and transporters that belong to ubiquitously distributed protein families, such as the CDF member ZitB (13, 15) and the ZIP protein ZupT. P1-type ATPases such as ZntA have also been identified but not yet characterized in plants (1), indicating that this family is also present in all three kingdoms. In addition to these well-characterized metal transporters, zinc might also be able to enter as a metal phosphate via the Pit phosphate uptake system (3), but this appears to be adventitious. Since zinc is such an important nutrient, it is not surprising to observe redundancy in transport systems. However, the number and families of putative zinc transporters are not conserved in different bacteria and might reflect different physiological needs. In addition, metal transporters sometimes seem to be acquired by horizontal gene transfer, since the sequences of transporters often do not follow the overall phylogenetic patterns of the particular microbes as determined by sequence comparison of small-subunit rRNAs (10, 17, 17a).
Finally, it appears likely that ZupT is a broad-range metal ion transporter in E. coli, perhaps mediating transport of other divalent cations including Cd(II) and Cu(II). It demonstrably transports Zn(II); Cd(II) antagonizes the effect of Zn(II) in a zupT-overexpressing strain; and cells expressing ZupT exhibit a slight increase in Cu(II) sensitivity.
Acknowledgments
This work was supported by Hatch project 136713 and NIEHS grant ESO4940 with funds from the EPA to C.R. and U.S. Public Health Grant GM 55425 to B.P.R.
REFERENCES
- 1.Axelsen, K. B., and M. G. Palmgren. 2001. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 126:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard, S. J., R. Hashim, J. Membrillo-Hernandez, M. N. Hughes, and R. K. Poole. 1997. Zinc(II) tolerance in Escherichia coli K12; evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol. Microbiol. 25:883–891. [DOI] [PubMed] [Google Scholar]
- 3.Beard, S. J., R. Hashim, G. Wu, M. R. B. Binet, M. N. Hughes, and R. K. Poole. 2000. Evidence for the transport of zinc(II) ions via the Pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184:231–235. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eide, D. J. 1998. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu. Rev. Nutr. 18:441–469. [DOI] [PubMed] [Google Scholar]
- 6.Gaither, L. A., and D. J. Eide. 2001. Eukaryotic zinc transporters and their regulation. Biometals 14:251–270. [DOI] [PubMed] [Google Scholar]
- 7.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB, (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grotz, N., T. Fox, E. Connolly, W. Park, M. L. Guerinot, and D. Eide. 1998. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 95:7220–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerinot, M. L. 2000. The ZIP family of metal transporters. Biochim. Biophys. Acta 1465:190–198. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence, J. G., and H. Ochman. 1998. Molecular archaeology of the Escherichia coli genome. Proc. Natl. Acad. Sci. USA 95:9413–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- 12.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. van Gijsegem. 1985. Alcaligenes eutrophus is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nies, D. H., and S. Silver. 1995. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14:186–199. [DOI] [PubMed] [Google Scholar]
- 14.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199–1210. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen, I. T., and M. J. Saier. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99–103. [DOI] [PubMed] [Google Scholar]
- 16.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 94:14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rensing, C., M. Ghosh, and B. P. Rosen. 1999. Families of soft-metal-ion-transporting ATPases. J. Bacteriol. 181:5891–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Rensing, C., D. T. Newby, and I. L. Pepper. The role of selective pressure and selfish DNA in horizontal gene transfer and soil microbial community adaptation. Soil Biol. Biochem., in press.
- 18.Vallee, B. L., and D. S. Auld. 1990. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 29:5647–5659. [DOI] [PubMed] [Google Scholar]