Abstract
Immunogold electron microscopy was used to determine whether the tegument proteins VP13/14, VP22, and VP16 of herpes simplex virus type 1 (HSV1) are components of primary enveloped virions. Whereas VP13/14 and VP22 were not detected in virus particles in the perinuclear space and were present in only mature extracellular virions, VP16 was acquired prior to primary envelopment of the virus at the inner nuclear membrane. This finding highlights potential similarities and differences between HSV1 and the related alphaherpesvirus, pseudorabies virus, in which the homologues of all three of these tegument proteins are not incorporated into the virion until secondary envelopment.
All herpesviruses share a common virion morphology; icosahedral capsids containing the viral genome are surrounded by an amorphous layer of at least 15 proteins termed the tegument, and this is encased in a lipid bilayer containing about a dozen different viral glycoproteins. A number of recent studies have begun to elucidate the pathway of herpesvirus morphogenesis and assembly. The process begins in the nucleus with the packaging of the viral genome into capsids. This is followed by a budding event at the inner nuclear membrane resulting in the formation of primary enveloped virions in the perinuclear space. Fusion of these virions with the outer nuclear membrane releases capsids into the cytoplasm, and final envelopment and formation of the mature virus particle is believed to take place at the trans-Golgi network (3, 16, 20, 22). Although this route is widely accepted, alternative models have been proposed (14). The complexity of this assembly route clearly raises the issue that different tegument proteins and glycoproteins may be incorporated into herpesvirus particles at different stages of egress and that the composition of virions in the perinuclear space may not be the same as that of the mature extracellular virus. This view is supported by electron microscopic analyses of the ultrastructure of primary enveloped virions which show that both the primary envelope and primary tegument appear morphologically different from the tegument and envelope structures in mature virus particles (8, 9). Furthermore, recent immunogold electron microscopy studies on the morphogenesis of the alphaherpesvirus pseudorabies virus (PRV) have demonstrated that primary and mature virions do indeed contain different complements of tegument and membrane proteins. The UL31 and UL34 gene products of PRV are present in virus particles in the perinuclear space but are absent from virions at later stages of assembly (7). In constrast, the UL36, UL37, UL49, UL46, UL47, and UL48 tegument proteins are found in only cytoplasmic enveloped virions and mature PRV particles and are therefore presumably not recruited into the virion during primary envelopment (6, 10-12). The PRV US3 protein kinase, on the other hand, is a component of both primary and mature enveloped virions (9).
Less is known about the tegument composition of herpes simplex virus (HSV) particles at different stages of virion morphogenesis. However, it is clear that there are some similarities between HSV type 1 (HSV1) and PRV in this regard, in that herpes simplex virus homologues of UL31 and UL34 are not present in extracellular virions and an HSV1 mutant lacking US3 fails to undergo efficient egress from the nucleus into the cytoplasm, suggesting that it is a component of primary virions (18).
To extend these findings, we used immunogold electron microscopy to determine whether three of the major HSV1 tegument proteins, VP22, VP16, and VP13/14 (the homologues of the PRV UL49, UL48, and UL47 gene products, respectively), are components of primary enveloped virions. The viruses used for this study had all been engineered to express GFP or YFP-tagged fusion proteins of VP16 (13), VP22 (5), or VP13/14 (4) and were chosen so that each tegument protein could be detected with an antibody to GFP rather than with antibodies specific for each individual protein. This approach results in relatively weak gold decoration but has the advantage that a single set of detection procedures can be used regardless of the target protein. Furthermore, since these proteins are known to exist in multiple phosphorylated forms, this strategy eliminates the possibility that specific antibodies might detect different phosphorylated forms with different efficiencies. A potential weakness of this approach is instability of the GFP fusion proteins, since this would result in the failure to detect the tegument protein or in the detection of free GFP. However, the stability of all three tagged tegument proteins examined in this study had previously been demonstrated by the authors who generated the recombinant viruses which we used (4, 5, 13), and we have confirmed their findings.
HaCaT cells (immortalized human keratinocytes) were infected at a multiplicity of infection of 0.1 and cultured for 16 h. This infection protocol was chosen because in order to make valid comparisons, it was important that gold labeling of virions at different stages of assembly (i.e., perinuclear and extracellular virus) could be examined in the same specimens and therefore that individual grids would have cells at different stages of infection. Cells were fixed in 4% formaldehyde-0.1% glutaraldehyde for 2 h, followed by cryoprotection, freezing, freeze substitution, and low-temperature embedding as described in Skepper (19). Sections (50 nM) were incubated with rabbit polyclonal antibody to GFP (Abcam ab6556), followed by incubation with 10 nM gold-conjugated goat anti-rabbit immunoglobulin G (British Biocell International). Sections were examined with a Philips CM100 transmission electron microscope.
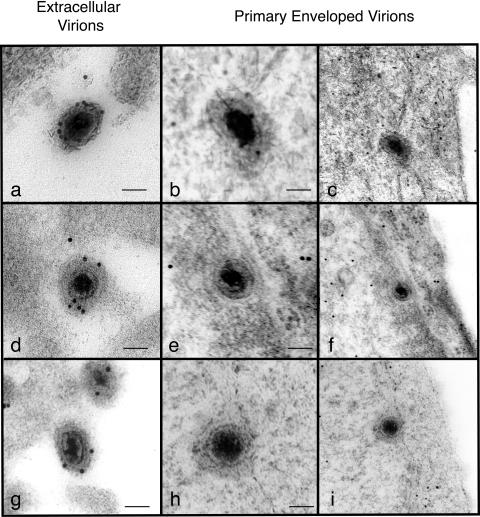
Examples of extracellular and perinuclear virus particles from grids treated with anti-GFP are shown in Fig. 1. To quantify these observations, multiple grids were examined and the number of gold particles associated with each extracellular and perinuclear virion was recorded. Sections of cells infected with wild-type virus were also examined to determine the nonspecific background binding of anti-GFP antibody to virions that contained no GFP. The results are summarized in Table 1. It is apparent that extracellular virions from cells infected with recombinants expressing VP22gfp or VP13/14yfp were labeled specifically, whereas the corresponding perinuclear virions were labeled at or below background levels. This is consistent with the finding that the PRV homologues of VP22 and VP13/14 are absent from primary enveloped virions and are incorporated into mature virions during secondary envelopment (10). The results using cells infected with a recombinant virus expressing VP16gfp were different. In these cells, both extracellular virions and perinuclear virions were labeled specifically with anti-GFP. Mature virions were labeled more heavily than perinuclear virions observed on the same grid, and this difference was highly significant (P < 0.01). Since primary enveloped virions and extracellular virions have different tegument compositions and structures, it is possible that the observed difference in labeling reflects different accessibility to antibody, and this is perhaps the simplest explanation. We cannot, however, rule out the possibility that VP16 is incorporated into virions during both primary and secondary envelopment such that the mature virion contains higher levels than perinuclear virions. The detection of VP16 in perinuclear HSV1 particles conflicts with the findings reported for PRV. The UL48 gene product (the PRV homologue of HSV1 VP16) was found to be absent from primary enveloped virions (6), although it is complexed with capsids during anterograde transport in axons (15). It appears that despite their genetic similarities, different alphaherpesviruses may differ in the details of virion assembly and in the functions of conserved tegument proteins, although we accept that PRV may have VP16 in perinuclear particles that was not detectable with the antibody used in the previous study. We also accept the possibility that the HSV VP16gfp fusion protein might behave differently from authentic VP16. However, the recombinant virus expressing VP16gfp has a wild-type phenotype (13), so this seems an extremely unlikely explanation for our findings. We note that mutants of PRV lacking the UL48 gene accumulate large numbers of naked nucleocapsids in the cytoplasm, implying a defect in secondary envelopment (6). HSV1 VP16-null mutants also show an assembly deficit (1, 2, 21), and Mossman et al. (17) observed that cells infected with the VP16-null virus, 8MA, contained large numbers of enveloped particles between the inner and outer nuclear membranes, suggesting that VP16 is required for one or more steps in the egress pathway downstream of primary envelopment. The proposal that VP16 is acquired by HSV1, but not by PRV, during primary envelopment is therefore consistent with the phenotypes of VP16 and UL48-null mutants of these viruses and supports the view that this tegument protein may have different functions in different alphaherpesviruses.
FIG. 1.
Immunoelectron microscopy of extracellular and primary enveloped virions observed in cells infected with recombinant viruses expressing VP16gpf (a to c), VP22gfp (d to f), or VP13/14yfp (g to i). Panels c, f, and i represent lower-magnification images of the primary enveloped virions shown in panels b, e, and h respectively. Scale bars, 100 nm.
TABLE 1.
Mean numbers of gold particles associated with extracellular and perinuclear virions
| Virusa | Extracellular
|
Perinuclearb
|
||
|---|---|---|---|---|
| No. of virions | Mean no. of gold particles/virion | No. of virions | Mean no. of gold particles/virion | |
| Wild type (SC16) (2) | 209 | 0.10 | ND | ND |
| VP22gfp (4) | 417 | 1.47 | 25 | 0.08 |
| VP13/14yfp (8) | 817 | 1.00 | 32 | 0.06 |
| VP16gfp (12) | 883 | 1.56 | 49 | 0.82 |
Numbers in parentheses are the number of grids examined. In each case, grids were derived from at least two different experiments.
ND, not done.
Acknowledgments
This work was supported by the Wellcome Trust UK and an MRC Cooperative Group grant. R.N.-S. was supported by the Fundação para a Ciência e Tecnologia (grant no. SFRH/BD/9632/2002) through the GABBA Programme, University of Porto, Porto, Portugal.
We thank Jeremy Skepper and Janet Powell, Multi-Imaging Centre, Department of Anatomy, University of Cambridge, for assistance with electron microscopy and Gill Elliot and Peter O'Hare, Marie Curie Institute, Oxted, United Kingdom, for recombinant viruses.
REFERENCES
- 1.Ace, C. I., M. A. Dalrymple, F. H. Ramsay, V. G. Preston, and C. M. Preston. 1988. Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J. Gen. Virol. 69:2595-2605. [DOI] [PubMed] [Google Scholar]
- 2.Ace, C. I., T. A. McKee, J. M. Ryan, J. M. Cameron, and C. M. Preston. 1989. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 63:2260-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly, M., and G. Elliott. 2001. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J. Virol. 75:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott, G., and P. O'Hare. 1999. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J. Virol. 73:4110-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J. Virol. 76:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2004. The pseudorabies virus US3 protein is a component of primary and of mature virions. J. Virol. 78:1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp, M., B. G. Klupp, H. Granzow, W. Fuchs, and T. C. Mettenleiter. 2002. Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: role for UL47 in virion morphogenesis in the cytoplasm. J. Virol. 76:8820-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Boissiere, S., A. Izeta, S. Malcomber, and P. O'Hare. 2004. Compartmentalization of VP16 in cells infected with recombinant herpes simplex virus expressing VP16-green fluorescent protein fusion proteins. J. Virol. 78:8002-8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuzinger, H., U. Ziegler, E. M. Schraner, C. Fraefel, D. L. Glauser, I. Heid, M. Ackermann, M. Mueller, and P. Wild. 2005. Herpes simplex virus 1 envelopment follows two diverse pathways. J. Virol. 79:13047-13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 17.Mossman, K. L., R. Sherburne, C. Lavery, J. Duncan, and J. R. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skepper, J. N. 2000. Immunocytochemical strategies for electron microscopy: choice or compromise. J. Microsc. 199:1-36. [DOI] [PubMed] [Google Scholar]
- 20.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinheimer, S. P., B. A. Boyd, S. K. Durham, J. L. Resnick, and D. R. O'Boyle II. 1992. Deletion of the VP16 open reading frame of herpes simplex virus type 1. J. Virol. 66:258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. The effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]