Abstract
Matrix (M) protein mutants of vesicular stomatitis virus have recently been used as oncolytic viruses for tumor therapies and are being developed as vaccine vectors for heterologous antigens. Because dendritic cell (DC) maturation is an important correlate of tumor immunosurveillance and vaccine efficacy, we sought to determine the ability of a recombinant M protein mutant virus (rM51R-M virus) to mature DC in vitro. We have previously shown that rM51R-M virus is defective at inhibiting host gene expression in several cell lines compared to its recombinant wild-type counterpart, rwt virus. Therefore, rM51R-M virus allows the expression of genes involved in antiviral responses, such as the type I interferon (IFN) gene. Our results demonstrate that, in contrast to the rwt virus, rM51R-M virus induced the maturation of myeloid DC (mDC) populations, as indicated by an increase in the surface expression of CD40, CD80, and CD86 as well as the secretion of interleukin-12 (IL-12), IL-6, and type I IFN. In addition, mDC infected with rM51R-M virus effectively activated naïve T cells in vitro, whereas rwt virus-infected mDC were defective in antigen presentation. The inability of rwt virus to induce mDC maturation was correlated with the inhibition of host gene expression in rwt virus-infected cells. Our studies also indicated that the production of costimulatory molecules on mDC by rM51R-M virus was dependent on the type I IFN receptor, while maturation induced by this virus was largely independent of MyD88. These data indicate that rM51R-M virus effectively stimulates the maturation of mDC and has the potential to promote effective T-cell responses to vector-expressed antigens, activate DC at tumor sites during therapy, and aid in tumor immunosurveillance and destruction.
Vesicular stomatitis virus (VSV) is an enveloped, negative-strand RNA virus that serves as the prototypic member of the family Rhabdoviridae. Recent studies have shown that VSV is an attractive candidate as a vaccine vector for heterologous antigens (16, 17, 41). VSV is also being developed as an oncolytic agent due to its potent ability to induce apoptosis in infected cancer cells (1, 5, 6, 8, 13, 25, 40, 44, 45). In both cases, the activation of dendritic cells (DC) in response to VSV would be a desired outcome.
DC, the most potent antigen-presenting cells, play a key role in profiling immune reactivity (reviewed in reference 19). They link the innate and adaptive arms of the immune system and thus orchestrate the immune response to a variety of different pathogens. Specifically, they are able to process and present pathogen-derived antigens to naïve T cells, initiating antigen-specific immune responses. In the case of VSV vaccine vectors, the activation of DC would promote more effective T-cell responses to vector-expressed antigens, whereas in the case of VSV-based oncolytic agents, the activation of DC would elicit T cells that aid in tumor immunosurveillance and destruction.
DC differentiate into several subsets with distinct functional capacities (33). Myeloid DC (mDC) are particularly potent activators of naïve T cells (9). However, in order to fulfill this role, mDC must mature through exposure to cytokines, pathogen-derived stimuli, or CD40 ligation. Mature mDC express high levels of costimulatory molecules, such as CD40, CD80, and CD86, and cytokines, such as interleukin-6 (IL-6) and IL-12. Plasmacytoid DC (pDC) are generally less effective than mDC in the activation of T cells (10). However, pDC often play important roles in the innate response to virus infection. For example, pDC are the major interferon (IFN)-producing cell type in mice infected with wild-type (wt) VSV (37).
It has been established that DC recognize the presence of many invading organisms via receptors belonging to the Toll-like receptor (TLR) family (reviewed in references 30 and 48). TLR7 is required for responsiveness to VSV as well as another negative-strand RNA virus, influenza virus, by pDC (12, 37). However, mDC express little, if any, TLR7 and are usually unresponsive to TLR7 ligands (27). This raises the question of whether mDC are capable of responding to VSV infection.
Previous studies with VSV have shown that wt VSV strains induce the maturation of pDC accompanied by the production of type I (alpha and beta) IFN (37). In fact, studies have demonstrated that IFN signaling is important for DC maturation induced by several viruses (18, 22, 24, 34, 42). Thus, induction of the IFN pathway by VSV could provide an activating signal for mDC. However, most wt strains of VSV suppress the type I IFN response in infected cells (2, 39). We have shown that this is due to the inhibition of host gene expression by the viral matrix (M) protein (2). The VSV M protein has multiple functions during the life cycle of the virus, including the inhibition of host gene expression (reviewed in reference 38). This inhibition serves to suppress the production of IFN and other antiviral proteins in infected cells (2, 45). However, an M protein mutant of VSV, rM51R-M virus, is defective in the ability to inhibit host gene expression and therefore induces the expression of genes involved in antiviral responses, including the type I IFN response, in infected cells (2). We have recently shown that rM51R-M virus is a superior candidate as an oncolytic virus for prostate tumor therapies due to its ability to specifically target and kill prostate tumor cells that are unable to mount IFN responses (1). Similar results have been obtained with other M protein mutant viruses in other tumor systems (45). Furthermore, rM51R-M virus causes no overt disease in surrounding tissues due to the activation of normal antiviral responses (1). Our hypothesis is that rM51R-M virus would likely induce the maturation of mDC populations better than viruses containing wt M protein due to its ability to induce an effective antiviral IFN response in normal cells. Thus, tumor therapies using such viruses may have the potential to overcome the immunosuppressive environment created by tumors as well as to induce effective T-cell responses as vaccine vectors.
In the experiments presented here, we tested the ability of rM51R-M virus to induce the maturation of bone marrow-derived mDC compared to its isogenic recombinant wt counterpart, rwt virus. We found that rM51R-M virus induced the maturation of mDC, as indicated by an increase in the expression of the DC costimulatory molecules CD40, CD80, and CD86, accompanied by the secretion of IL-12, IL-6, and type I IFN. The induction of mDC maturation by rM51R-M virus correlated with its inability to shut off host gene expression. Furthermore, the expression of costimulatory molecules induced by rM51R-M virus was dependent on the type I IFN receptor, but maturation was independent of the TLR7 adaptor molecule, MyD88. In contrast to rM51R-M virus, rwt virus did not stimulate the maturation of mDC. This was due to the suppression of host gene expression by rwt virus in mDC. Studies indicated that mDC were susceptible to the cytopathic effect induced by rwt virus, which correlated with an increased expression of viral G protein as well as higher viral titers in cells infected with rwt virus than in those infected with rM51R-M virus. Therefore, these data suggest that rM51R-M virus has the potential to be a superior agent for antitumor therapies and as a vaccine vector due to its ability to induce the maturation of mDC.
MATERIALS AND METHODS
Mice.
C57/BL6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and OT-1 Rag1−/− T-cell-receptor (TCR)-transgenic mice specific for OVA(257-264) presented by Kb were purchased from Taconic Labs (Germantown, NY). MyD88−/− mice were a kind gift of D. Golenbock (University of Massachusetts), with the permission of S. Akira. IFN receptor-negative (IFNR−/−) mice were a generous gift of S. Mizel (Wake Forest University), with the permission of C. Schindler (Columbia University, New York). All mice were maintained and bred in the animal facility at Wake Forest University School of Medicine.
Cells and viruses. (i) Dendritic cell propagation.
mDC were generated as previously described (26). Briefly, bone marrow was removed from the tibias and femurs of 8- to 10-week-old mice. Following red cell lysis and washing, progenitor cells (5 × 105/ml) were resuspended and plated in RPMI containing 10% fetal calf serum supplemented with 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (without antibiotics). DC were cultured for 6 days at 37°C in 5% CO2 and were gently washed and fed with fresh medium and cytokine on days 2 and 4. On day 6, the mDC were routinely 95% (±5%) CD11c+ and displayed low levels of CD40, CD80, CD86, and major histocompatibility complex class II molecules, characteristic of immature DC (11).
(ii) Virus growth and preparation.
The recombinant viruses, rwt and rM51R-M, were isolated from infectious VSV cDNA clones, and virus stocks were prepared as described previously (29). Briefly, viruses were grown on BHK cell monolayers. Supernatants containing progeny virions were harvested, titrated, and stored at −80°C. To rule out the possibility of endotoxin contamination in the stocks, DC were treated with supernatants from parallel cultures of uninfected BHK monolayers, and costimulatory molecule expression and cytokine levels were measured. Results from these tests indicated that there were no changes in costimulatory molecule expression and cytokine levels compared to untreated DC (data not shown).
Reagents.
Recombinant mouse GM-CSF was purchased from Biosource International (Camarillo, CA). The synthetic OVA peptide 257-264 (SIINFEKL) was purchased from the Emory University School of Medicine Peptide Synthesis Facility. Fluorescently labeled antibodies against mouse cell surface antigens (CD40-phycoerythrin [CD40-PE; clone 3/23], CD80-PE [clone 16-10A1], CD86-PE [clone GL1], CD11c-allophycocyanin [CD11c-APC; clone HL3], recombinant immunoglobulin G-PE [rIgG-PE], and rIgG-APC) were purchased from BD Biosciences/Pharmingen (San Diego, CA). Fluorescently tagged anti-IFN-γ-APC and tumor necrosis factor alpha (TNF-α)-PE-Cy7, used for intracellular cytokine staining, were also purchased from BD Pharmingen. The VSV G protein-specific mouse antibody I1 was described by Lefrancois and Lyles (32). For use as TLR agonists, lipopolysaccharide (LPS) from Salmonella enterica serovar Minnesota was purchased from Sigma (St. Louis, MO) and used at a concentration of 100 to 300 ng/ml, and poly(I-C) (used at 25 μg/ml) and loxoribine (used at 200 μM) were purchased from InvivoGen (San Diego, CA).
CFSE (5 [and 6-]-carboxyfluorescein diacetate succinimidyl ester), Alexa fluor 488-labeled anti-rabbit secondary antibodies, and DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride) were purchased from Molecular Probes (Eugene, OR).
Viral infection of DC.
mDC, cultured as described above, were harvested, plated in 48-well plates at a density of 5 × 105/well, and infected with either rwt or rM51R-M virus at the indicated multiplicity of infection (MOI) for 1 h in a small volume. The volume was then restored to 1 ml, and the cells were cultured for an additional 23 h to allow time for maturation.
G protein surface expression.
The efficiency of G protein cell surface expression during VSV infection of mDC was determined by flow cytometric analysis of infected cells. Cells were infected with wt and mutant viruses at an MOI of 1.0 PFU/cell for 24 h in RPMI containing 10% fetal bovine serum (FBS). Following infection, cells were washed, and surface-expressed G protein was labeled with the anti-G protein antibody I1 (32), used at a 1:500 dilution, for 1 h. Cells were then incubated with an anti-mouse, Alexa 488-conjugated antibody (Molecular Probes, Eugene, OR) at a dilution of 1:200 for 1 h at 4°C, fixed, and stored in 2% formaldehyde at 4°C. G protein cell surface fluorescence was quantitated using a BD FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
Growth curves.
mDC were infected with rwt or rM51R-M virus at the indicated MOI in RPMI containing 10% FBS. At 24 h postinfection, 100 μl of medium was removed from each dish and stored at −70°C. The yields of virus were determined by plaque assays on BHK cells and were expressed as PFU/ml.
35S radiolabeling of infected cells.
To analyze protein synthesis during virus infections, mDC (5 × 105 cells) were infected with rwt or rM51R-M virus at an MOI of 10 or 1 PFU/cell in RPMI containing 10% FBS. At 8 and 24 h postinfection, cells were labeled with a 15-minute pulse of [35S]methionine (100 μCi/ml) in a total volume of 0.3 ml of methionine-free medium. Cells were washed with phosphate-buffered saline and harvested in RIPA buffer (0.15 M NaCl, 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 10 mM Tris, pH 7.4). Extracts normalized for protein levels (by the Lowry protein assay) were electrophoresed by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and were analyzed by phosphorescence imaging as described previously (29).
Cell viability assay.
mDC were infected with wt and mutant viruses at an MOI of 0.01, 0.1, or 1.0 PFU/cell. At 24 h postinfection, live cells were measured by an MTT assay (Cell Proliferation kit 1; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
Measurement of costimulatory molecule and cytokine expression by DC following viral infection.
mDC were plated in 48-well plates at a density of 5 × 105 DC/ml in antibiotic-free medium. The cells were then infected with virus at the indicated MOI or treated with the indicated TLR agonist and were incubated for a total of 24 h. Culture supernatants were then removed, and the presence of cytokines (IL-6, IL-12 p40, and TNF-α) was measured by enzyme-linked immunosorbent assays (ELISAs) (OptiEIA kits; BD Pharmingen) or a bioassay (for type I IFN). The cells were then harvested and stained with PE-conjugated antibodies to murine CD40, CD80, and CD86. Nonspecific background staining was assessed using isotype control antibodies (rat IgG-PE for CD40, CD80, and CD86). mDC were costained with antibodies to CD11c to enable analysis of costimulatory molecules expressed by the distinct DC populations. In addition, dead cells were excluded from analysis based on forward and side scatter gating.
Measurement of cell death following virus infection.
mDC were infected with rwt or rM51R-M virus at different MOIs. At 24 h postinfection, cells were labeled with antibodies to CD11c to detect DC and incubated for 15 min with 7-amino-actinomycin D (7AAD) at room temperature to measure cell death. Results were obtained with a BD FACSCalibur flow cytometer (BD Biosciences, San Diego, CA).
Interferon bioassay.
To determine the IFN activity produced by cells infected with wt and mutant viruses, supernatants (500 μl) were collected from mDC infected with rwt or rM51R-M virus or treated with poly(I-C) or loxoribine for 24 h. Infectious virus was inactivated by acid treatment (pH 2), the acid was neutralized, and serial dilutions were incubated overnight with L929 cells in 96-well plates at 37°C. To construct a standard dose-response curve, cells were incubated with serial fivefold dilutions of IFN (universal type I IFN; PBL Biomedical Laboratories, New Brunswick, NJ). The samples were aspirated, and cells were challenged with wt VSV at an MOI of 5 PFU/cell in 100 μl of medium. Controls included cells infected with VSV alone and cells that were not challenged with VSV. Cells were incubated overnight at 37°C, the medium was aspirated, and the cells were fixed with 95% ethanol. Cells were then stained with a 0.1% crystal violet solution in methanol. The absorbance was read at 550 nm on an ELISA reader. IFN levels of >800 IU/ml were detectable by this bioassay. The data shown are the averages of two independent experiments.
T-cell priming assay.
mDC (2 × 104/ml, 100 μl/well) were plated in 96-well plates and treated with LPS (300 ng/ml), left untreated, or infected with VSV (rM51R-M virus, MOI of 1 PFU/cell). To examine specifically the effects of viral infection on the costimulatory activities of DC while minimizing the contribution of antigen dose as a variable, DC were loaded with defined doses (0.01, 0.1, or 1 ng/ml) of preprocessed OVA(257-264) peptide. The cells were cultured for 24 total hours before the addition of T cells.
Naïve, OVA-specific CD8+ T cells were isolated from the spleens of OT-1 TCR-transgenic mice. T cells were stained with CFSE according to the manufacturer's instructions and were added to the DC at a ratio of 10 T cells per DC. T cells were harvested 72 h later, and the production of IFN-γ and TNF-α in response to antigen was then measured by incubation with the OVA(257-264) peptide (1 μg/ml) in the presence of Golgi Plug reagent for 5 hours. The cells were then stained, fixed, and permeabilized for intracellular cytokine staining using a Cytofix/Cytoperm kit from BD Biosciences/Pharmingen (San Diego, CA) according to the manufacturer's instructions. Flow cytometric data were acquired using a BD FACSCalibur instrument (BD Biosciences, San Diego, CA). CFSE proliferation data were analyzed as previously described (15), using the FLOJO program for flow cytometric data analysis (Tree Star, Inc., Ashland, OR). The division index represents the mean number of divisions that a cell present in the starting population undergoes. Statistical significance was determined using a two-tailed, paired Student t test. P values of <0.05 were considered significant.
RESULTS
mDC gene expression is inhibited more effectively by rwt virus than by rM51R-M virus.
Previous studies have shown that the M protein of VSV suppresses type I IFN gene expression through the general inhibition of host RNA and protein synthesis (1). Therefore, wt strains of VSV are effective suppressors of the host antiviral response. In contrast, the M protein mutant virus, rM51R-M virus, is defective at inhibiting host gene expression in several cell lines, including the expression of genes involved in antiviral responses. To determine the relative abilities of the rwt and rM51R-M viruses to inhibit host gene expression in mDC, mDC were generated by culturing bone marrow-derived DC progenitors in the presence of GM-CSF. Cells were infected at multiplicities of 1 and 10 PFU/ml and pulse labeled with [35S]methionine for 15 min at 8 and 24 h postinfection. The time of pulse labeling was short compared to the turnover rates of viral and host proteins, such that labeling primarily reflected rates of protein synthesis. Proteins were solubilized, and equivalent amounts of protein were subjected to SDS-PAGE and phosphorescence imaging. A representative image is shown in Fig. 1A. The positions of the viral proteins are indicated to the left of the image. Cells infected with rwt virus at both multiplicities effectively inhibited host translation compared to mock-infected cells. This is clearly seen in the regions of the gel that are devoid of viral proteins, such as the regions between the L and G proteins. However, rM51R-M virus was less effective at shutting off host protein synthesis. It is also apparent from this figure that low levels of viral proteins were synthesized in mDC infected with each of the viruses.
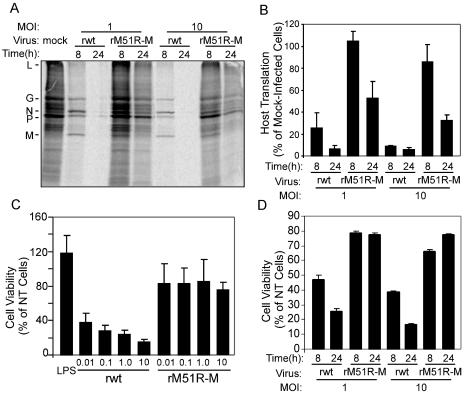
FIG. 1.
rM51R-M virus is defective at inhibiting host gene expression in mDC. mDC were infected with rwt or rM51R-M virus at a multiplicity of infection of 10 or 1 PFU/cell or were mock infected as a control. At 8 and 24 h postinfection, cells were labeled with a 15-min pulse of [35S]methionine (100 μCi/ml) and harvested. Lysates were subjected to SDS-PAGE, and labeled proteins were quantitated by phosphorimaging. (A) Representative image from analysis of rwt and rM51R-M virus-infected mDC. (B) Host protein synthesis was determined from images similar to that shown in panel A for regions of the gel devoid of viral proteins between the L and G proteins. The results are shown as percentages of the mock-infected control value and are the means ± standard errors of three independent experiments. (C) Cells were infected with the rwt and rM51R-M viruses at multiplicities of 0.01, 0.1, 1, and 10 PFU/cell or were treated with LPS, and live cells were measured by an MTT assay at 24 h postinfection. Data are expressed as percentages of the cell viability of untreated cells and are the means ± standard errors of four experiments. (D) Viability of mDC infected with rwt or rM51R-M virus at a multiplicity of 1 or 10 PFU/cell for 8 or 24 h. Data are means ± standard errors of three experiments.
Host protein synthesis in infected cells at 8 and 24 h postinfection was determined from images similar to that shown in Fig. 1A by quantitating the radioactivity in regions of the gels devoid of viral proteins. Data are shown in Fig. 1B as percentages of the values for a mock-infected control. rwt virus effectively inhibited mDC translation at both multiplicities (1 and 10 PFU/cell) so that by 24 h postinfection, host protein synthesis levels were <10% those of the mock-infected control. In contrast, host protein synthesis in cells infected with rM51R-M virus was not markedly inhibited at 8 h postinfection and was still 30 to 50% that of the control at 24 h postinfection.
In some cell types, the inhibition of host gene expression by wt M protein leads to the induction of apoptosis. From the previous experiments, we observed that mDC infected with rwt virus showed signs of apoptosis typical of advanced VSV infection, such as chromatin condensation and nuclear fragmentation (data not shown). However, cells infected with rM51R-M virus remained healthy. To quantitate the ability of wt and M protein mutant viruses to kill mDC, cells were infected with the rwt and rM51R-M viruses at different multiplicities, and cell viability was measured at 24 h by the MTT assay (Fig. 1C). The results indicated that mDC were resistant to killing by rM51R-M virus, with approximately 80% of cells remaining viable at each MOI by 24 h. In contrast, mDC were sensitive to the cytopathic effect induced by rwt virus, as indicated by a titratable decrease in cell viability. In addition, a comparison of cell viability (Fig. 1D) and host translation in mDC infected with the rwt and rM51R-M viruses (Fig. 1B) revealed that the shutoff of host protein synthesis occurred prior to cell death. For example, at 8 h postinfection, during which time host translation in cells infected with 1 PFU/cell of rwt virus was 20% that of the control (Fig. 1B), approximately 50% of the total cells were viable (Fig. 1D). Similarly, when cells were infected with rwt virus at an MOI of 10 PFU/cell, cell viability remained close to 40% of total cells even though host translation was <10% that of mock-infected cells. Therefore, these data suggest that the inhibition of host gene expression by wt VSV leads to cell killing in mDC.
mDC are less permissive for rM51R-M virus than for rwt virus.
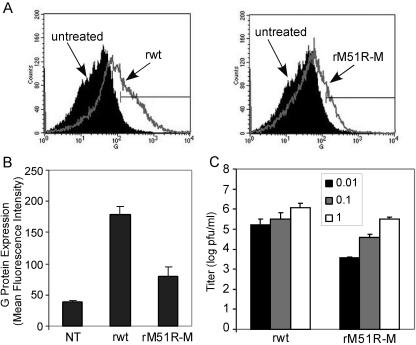
To determine whether mDC were differentially permissive to infection with rwt virus versus rM51R-M virus, the expression of the viral G protein surface antigen was determined by flow cytometry at 24 h postinfection, and yields of progeny virus were determined by a plaque assay. As depicted by the histograms in Fig. 2A, mDC infected with rwt virus displayed a small but reproducible increase in fluorescence due to expression of the G protein compared to uninfected cells. In addition, G protein expression in cells infected with rwt virus was higher than that in cells infected with rM51R-M virus. The differences in G protein expression in cells infected with the rwt and rM51R-M viruses are evident by the differences in their mean fluorescence intensities (Fig. 2B). Cells infected with rwt virus produced progeny virus at approximately 106 PFU/ml (Fig. 2C). These titers are approximately 2 log lower than those obtained with more permissive cell lines such as HeLa and BHK cells (1, 2, 29). Viral titers from cells infected with rM51R-M virus were consistently 1 to 2 log lower than those in mDC infected with rwt virus. Therefore, we can conclude that mDC are semipermissive for VSV infection and are more susceptible to infection with rwt virus than with rM51R-M virus.
FIG. 2.
mDC are semipermissive for rwt and rM51R-M virus infections. The efficiency of G protein surface expression on mDC during VSV infection was determined by flow cytometry analysis of mDC infected with the rwt and rM51R-M viruses at a multiplicity of 1 PFU/cell for 24 h. (A) Representative histograms depicting increases in G protein expression in rwt and rM51R-M virus-infected cells. (B) The geometric mean fluorescence intensity of each sample was determined. Data are expressed in arbitrary values and are the means ± standard errors of four or five experiments. (C) Viral growth analysis in mDC. Cells were infected with rwt or rM51R-M virus at multiplicities of 0.01, 0.1, and 1 PFU/cell. At 24 h postinfection, supernatants were collected to determine the amounts of progeny virus by a plaque assay. Data are the means ± standard errors of three experiments.
M protein mutant virus effectively induces maturation of mDC.
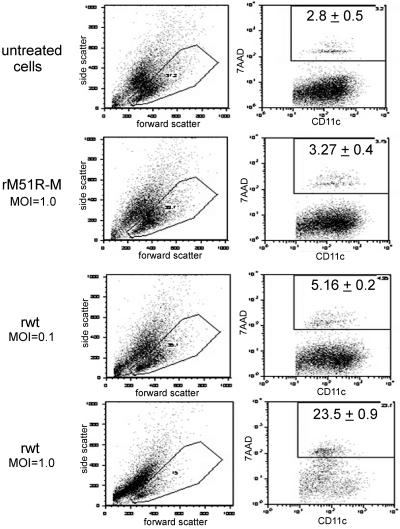
The results shown in Fig. 1 and 2 suggest that mDC respond effectively to infection with rM51R-M virus by attenuating viral replication and spread. In contrast, mDC are unable to induce an effective antiviral response against rwt virus, leading to an rwt virus-induced inhibition of host gene expression and cell death. However, previous studies have shown that ex vivo-isolated splenic pDC are responsive to wt VSV (37). Infection of these cells with VSV induced the expression of surface costimulatory molecules and the production of type I IFN. To determine the effect of VSV infection on the maturation of the mDC population, we first wanted to ensure that costimulatory molecules were measured on live mDC. Therefore, we selected cells with low side scatter and measured the percentage of these cells that became positive for 7AAD staining as an indicator of cell death (Fig. 3). Twenty-four hours following infection of mDC with rwt or rM51R-M virus, cells were harvested and stained with antibodies to CD11c, a cell surface marker for DC, and incubated with 7AAD to label dead cells. The results showed that infection of mDC with rwt virus, but not rM51R-M virus, increased the side scatter of many of the cells (an indicator of apoptosis). This was most apparent when cells were infected with rwt virus at a multiplicity of 1 PFU/cell. Although 23.5% of the cells that remained within the low-side-scatter gate were positive for 7AAD, most of the cells remained alive. When cells were infected with 0.1 PFU/cell of rwt virus, the percentage of 7AAD-positive cells was close to the levels detected in untreated and rM51R-M virus-infected cells. Because a major percentage of cells in this gate were live, this gate was used for all subsequent fluorescence-activated cell sorting analysis.
FIG. 3.
Quantitation of cell death and CD11c expression following infection with rwt and rM51R-M viruses. mDC were infected with rwt and rM51R-M viruses at multiplicities of 0.1 PFU/cell (rwt virus) and 1 PFU/cell (rwt and rM51R-M viruses). At 24 h postinfection, cells were labeled with antibodies to CD11c and incubated with 7AAD. The images shown are representative dot plots depicting the forward and side scatter of each population and CD11c versus 7AAD staining within the low-side-scatter gate. The percentage of 7AAD-positive cells in the gated CD11c+ population was determined. Data represent the means ± standard deviations of three experiments.
To determine the effect of VSV on the expression of costimulatory molecules, mDC were infected with either rwt or rM51R-M virus at different multiplicities of infection. mDC treated with LPS served as a positive control, while untreated, immature DC served as a negative control. Cells treated with the TLR7 agonist loxoribine served as controls for the presence of pDC, since mDC lack TLR7. At 24 h, cells were harvested and stained with antibodies to the costimulatory molecules CD40, CD80, and CD86. To specifically examine DC, cells were costained with the DC cell surface marker CD11c, and flow cytometry was performed to determine the cell surface expression of CD40, CD80, and CD86. Histograms depicting changes in costimulatory molecule expression by mDC infected with VSV are shown in Fig. 4A. The results show that mDC infected with rwt virus expressed lower levels of CD40, CD80, and CD86 than untreated DC. The failure of rwt virus to up-regulate costimulatory molecules was not due to the lack of live cells in the cultures but can most likely be attributed to the shutoff of host gene expression by rwt virus and the turnover of cell surface markers. In support of this idea, treatment of rwt virus-infected mDC with LPS did not result in an increase in the expression of maturation markers (data not shown). In contrast, the levels of these molecules increased in mDC infected with rM51R-M virus. This was especially evident in changes in CD40 and CD86 expression. These data were quantitated by determining the increase in costimulatory molecule expression in the CD11c-positive population compared to untreated, immature DC. Our results demonstrate that there was an approximately threefold increase in cell surface CD40 expression, a twofold increase in CD80 expression, and a fourfold increase in CD86 expression in mDC upon infection with 1 PFU/cell of rM51R-M virus (Fig. 4B, C, and D, respectively). The increase in CD40 stimulated by rM51R-M virus was similar to that induced by the positive control, LPS. However, rM51R-M virus induced less CD80 expression than that induced by LPS and more CD86 expression than that induced by LPS. As expected, the TLR7 agonist loxoribine did not induce the expression of costimulatory molecules in mDC while inducing high levels of expression in pDC (data not shown).
FIG. 4.
rM51R-M virus induces expression of costimulatory molecules on mDC. mDC were infected with rwt or rM51R-M virus at multiplicities of 0.01, 0.1, and 1 PFU/cell or were treated with LPS or loxoribine. At 24 h postinfection or posttreatment, the cell surface expression of CD40, CD80, and CD86 was measured by flow cytometry. (A) Representative histograms depicting expression of costimulatory molecules by rwt and rM51R-M viruses (1 PFU/cell). The geometric mean fluorescence of each sample was determined and used to quantitate the increase in CD40 (B), CD80 (C), and CD86 (D) expression over that in untreated cells (NT). Data are the means ± standard errors of three or four independent experiments.
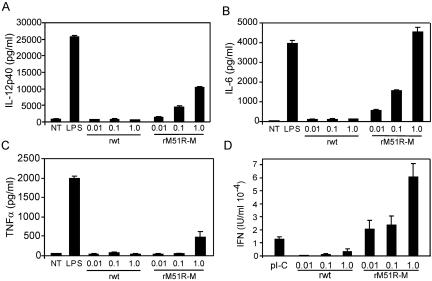
To determine how the infection of mDC with VSV affects the production of cytokines, supernatants from VSV-infected mDC were collected 24 h after treatment. The secretion of IL-12 p40, IL-6, and TNF-α was measured by ELISA, and the production of type I IFN was determined by an IFN bioassay. Data were normalized to the percentage of viable cells in the culture. Our results indicate that IL-12 p40, IL-6, and IFN (Fig. 5A, B, and D, respectively) were induced efficiently with rM51R-M virus and that production correlated with the MOI. However, little TNF-α was produced by cells infected with rM51R-M virus, and it was only detectable at the highest MOI (Fig. 5C). In contrast to cells infected with the M protein mutant virus, mDC infected with rwt virus did not produce significant amounts of IL-12 p40, IL-6, TNF-α, and type I IFN compared to background levels, even when normalized based on the percentage of viable cells. This result is consistent with the low levels of costimulatory molecules expressed by mDC infected with this virus. Thus, our conclusion based on these data is that in contrast to wt VSV, the M protein mutant virus rM51R-M virus effectively stimulates the maturation of mDC, most likely due to its inability to shut off host gene expression in these cells and the subsequent induction of type I IFN. These results are also consistent with work done with IFN-inducing strains of influenza virus and Sendai virus (34), which induce mDC maturation more effectively than their wt counterparts. Furthermore, these data indicate that the rwt virus-induced inhibition of host gene expression and subsequent loss of cellular viability were responsible for preventing the maturation of rwt virus-infected mDC.
FIG. 5.
Cytokine expression induced by rM51R-M virus. mDC were infected with rwt and rM51R-M viruses at different multiplicities or were treated with the indicated TLR agonist [LPS or poly(I-C)] for a total of 24 h. Culture supernatants were removed, and the presence of IL-12 p40 (A), IL-6 (B), and TNF-α (C) was measured by ELISA. Data are expressed in pg/ml of cytokine secreted into the supernatant. Type I IFN in the supernatant was measured by a bioassay based on the reduction of VSV cytopathic effects (D). The IFN concentration (in IU/ml) was quantitated by comparing the results to those in cells incubated with serial dilutions of an IFN standard. Data were normalized to the percentage of viable cells and are the means ± standard errors for three or four experiments.
Infection with rM51R-M mutant VSV enhances the antigen-presenting potential of DC.
To determine whether rM51R-M virus-infected mDC were fully functional at presenting antigens and activating T cells, we performed a series of in vitro T-cell priming assays. The two main facets of T-cell activation are clonal expansion (proliferation) and the acquisition of effector function (cytokine production). Therefore, for these analyses, T-cell priming was measured by dilution of CFSE (to assess proliferation) as well as intracellular cytokine staining (to detect IFN-γ and TNF-α production). mDC were infected with rM51R-M virus, treated with LPS, or left untreated and then pulsed with the indicated doses of OVA peptide. Twenty-four hours later, naïve CD8+, OVA-specific TCR-transgenic T cells (OT-1) were stained with CFSE and added to the cultures for 72 h.
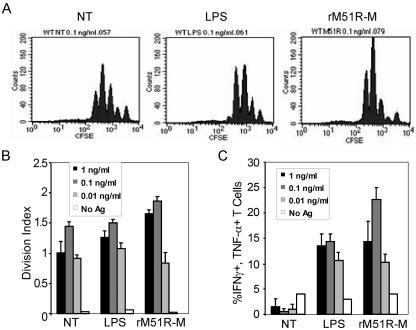
The histograms in Fig. 6A depict the proliferation (as measured by dilution of CFSE) of naïve OT-1 T cells following culture with mDC at the intermediate antigen dose, 0.1 ng/ml. These profiles illustrate that the strongest proliferative response was elicited by DC infected with the rM51R-M virus. LPS-treated mDC and untreated cells stimulated robust proliferation as well, but smaller proportions of the cells had completed four rounds of division than for the rM51R-M virus-treated mDC. The division index provides a quantitative indicator of the overall proliferative outcome of T-cell priming, accounting for both the percentage of T cells entering division and the average number of divisions per cell (Fig. 6B). We noted that for each mDC population, the division index increased in an antigen dose-dependent manner, with the optimal dose being 0.1 ng/ml OVA peptide. However, the overall magnitude of the response depended on the type of stimulus administered to the mDC. The strongest proliferative response was stimulated by mDC infected with rM51R-M virus, but LPS-treated and untreated, peptide-pulsed mDC also induced strong proliferation. As expected, rwt virus-infected mDC stimulated little, if any, T-cell proliferation (data not shown).
FIG. 6.
T-cell-activating capacity of rM51R-M virus-infected mDC. mDC were either infected with rM51R-M virus (1 PFU/cell), treated with LPS, or left untreated (NT) and then were pulsed with the indicated doses of OVA peptide antigen for 24 h. CFSE-labeled OT-1 cells (naïve, OVA-specific transgenic T cells) were then cultured with the DC for an additional 72 h. T cells were harvested and stained intracellularly for IFN-γ and TNF-α and analyzed by flow cytometry. (A) Histograms of CFSE fluorescence for the indicated DC treatments at the intermediate antigen dose, 0.1 ng/ml OVA peptide. (B) Division indices for each treatment at three antigen doses. The data shown are means and standard deviations for triplicate cultures. (C) Percentage of live, CD8+ T cells that produce both IFN-γ and TNF-α. Data are means and standard deviations for triplicate cultures and are representative of three independent experiments.
A hallmark of full T-cell effector function is the expression of both TNF-α and IFN-γ upon exposure to antigen. The percentage of T cells expressing both IFN-γ and TNF-α (as detected by intracellular cytokine staining) was highest for T cells primed by mDC infected with the rM51R-M virus and was even higher than that for LPS-stimulated mDC (Fig. 6C). The smallest proportion of cytokine-producing T cells was primed by untreated, immature DC, which was a predicted outcome. These findings suggest that rM51R-M virus induces potent T-cell priming potential in mDC that is even greater than that induced by LPS.
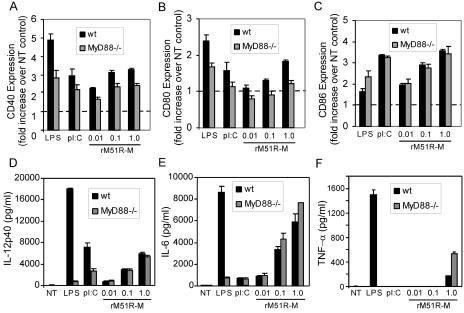
Stimulation of mDC by rM51R-M virus is largely independent of MyD88.
In order to determine whether the TLR adapter molecule MyD88 contributes to the maturation of mDC induced by rM51R-M virus, we compared the response of mDC lacking this molecule to that of wt mDC to infection with VSV. As previously reported (28), the expression of CD86 induced by LPS was not dependent on MyD88, nor was that induced by poly(I-C) (Fig. 7C). However, the expression of CD40 and CD80 induced by LPS and poly(I-C) was modestly diminished in the absence of MyD88 (Fig. 7A and B). Similar to results obtained with LPS and poly(I-C), the expression of CD86 induced by rM51R-M virus remained the same regardless of the presence or absence of MyD88, indicating that the expression of this molecule induced by rM51R-M virus is independent of MyD88. As expected, rwt virus did not induce costimulatory molecule expression in either the presence or absence of MyD88 (data not shown). Interestingly, the modest reduction in CD80 induced by rM51R-M virus in MyD88−/− DC was statistically significant (P > 0.05). Only a slight (not significant) reduction was noted for CD40 expression induced by rM51R-M virus in cells lacking this molecule.
FIG. 7.
Maturation of mDC by rM51R-M virus occurs independently of MyD88. mDC derived from wt or MyD88−/− mice were infected with rM51R-M virus and treated with LPS or poly(I-C) for 24 h. The cell surface expression of CD40 (A), CD80 (B), and CD86 (C) was determined by flow cytometry. Data represent the means ± standard errors of three to five experiments. The production of IL-12 p40 (D), IL-6 (E), and TNF-α (F) in the supernatants was determined by ELISA. The data shown are representative of three experiments.
When we measured the production of IL-12 p40, IL-6, and TNF-α, we observed differential patterns of MyD88 dependence which were based on the stimulus. LPS induced robust production of all of these cytokines (Fig. 7D, E, and F) in wt DC, but this production was ablated in cells lacking MyD88. We also detected a decrease in the expression of IL-12 p40 induced by poly(I-C) in the absence of MyD88. However, unlike LPS, poly(I-C) did not stimulate the production of high levels of IL-6 or TNF-α, regardless of the presence or absence of MyD88. In contrast to the cytokines produced in response to LPS or poly(I-C), the production of these cytokines in response to the rM51R-M virus was not diminished in cells lacking MyD88. These findings suggest that MyD88 is not a major mediator of mDC costimulatory molecule or cytokine expression induced by rM51R-M virus. Taken together, our data also indicate that rM51R-M virus stimulates mDC in a manner that is distinct from that of both TLR3 and TLR4 agonists.
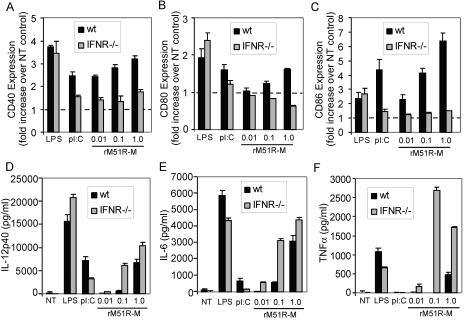
Differential effects of type I IFN signaling on maturation versus cytokine production by rM51R-M virus-infected mDC.
Studies by several groups have shown that IFN signaling contributes to DC maturation induced by several viruses (22, 24, 34, 42). Thus, the difference in activation of IFN between the rM51R-M and rwt viruses in mDC (Fig. 5) could account for their differential induction of DC maturation markers (Fig. 4). To test this possibility, we generated mDC from mice lacking the receptor for type I IFN (IFNR−/−). The expression of the costimulatory molecules CD40, CD80, and CD86 induced by LPS in IFNR−/− mDC was similar to that in wt mDC, while the expression of CD40 and CD86 induced by poly(I-C) was reduced in the absence of the IFN receptor, as expected (Fig. 8A, B, and C). Importantly, a significant reduction in the expression of costimulatory molecules (particularly CD40 and CD86) stimulated by rM51R-M virus was observed in IFNR−/− mDC. These results indicate that the IFN response is necessary for rM51R-M virus-induced expression of costimulatory molecules on mDC.
FIG. 8.
Maturation of IFNR−/− mDC by rM51R-M virus. mDC derived from wt or IFNR−/− mice were infected with rM51R-M virus and treated with LPS or poly(I-C) for 24 h. The cell surface expression of CD40 (A), CD80 (B), and CD86 (C) was determined by flow cytometry. Data represent the means ± standard errors of three to five experiments. The production of IL-12 p40 (D), IL-6 (E), and TNF-α (F) in the supernatants was determined by ELISA. The data shown are representative of three experiments.
In IFNR−/− mDC, the expression of the inflammatory cytokines IL-12 p40, IL-6, and TNF-α was actually enhanced compared to that observed in wt mDC following stimulation with rM51R-M virus (Fig. 8D, E, and F). This was especially evident for the expression of TNF-α (Fig. 8F). This trend is similar to that observed following infection of IFNR−/− mice with Listeria monocytogenes (3). In contrast, the induction of cytokines by LPS changed minimally in the absence of the IFN receptor, while levels decreased upon stimulation with poly(I-C). Further examination of these cells revealed that rM51R-M virus replicated more effectively in IFNR−/− mDC than in wt mDC (data not shown). Therefore, it is possible that IFNR−/− mDC respond to rM51R-M virus infection by producing higher levels of cytokines than those detected in wt mDC. These data suggest that costimulatory molecule expression induced by the rM51R-M virus is dependent on cell responsiveness to the type I IFN receptor but that cytokine production stimulated by this virus is not dependent on this receptor.
DISCUSSION
The results presented here demonstrate that the ability of mDC to effectively mature upon VSV infection is inversely correlated with the ability of the virus to inhibit host gene expression. In mDC, the rwt virus inhibits host gene expression, including the expression of genes involved in DC maturation as well as those necessary for eliciting an effective antiviral response. In fact, this shutoff of host gene expression not only results in the prevention of mDC maturation but ultimately leads to cell death. In contrast, rM51R-M virus is defective at inhibiting host gene expression in mDC. Therefore, mDC respond to rM51R-M virus infection by inducing the expression of IFN and other cytokines. This antiviral response leads to an attenuation of viral replication and spread and to mDC maturation.
Previous work by several groups has shown that the IFN response activated by viruses or exogenously added type I IFN stimulates the maturation of DC (18, 22, 24, 34, 36, 42) and their ability to cross-prime CD8 T cells (31). In fact, viruses that are strong inducers of IFN are also the best stimulators of DC maturation, indicating a link between the type I IFN pathway and the pathways leading to DC maturation (34). In our model, not only does the IFN signaling pathway activated by rM51R-M virus regulate virus replication and spread for optimal mDC maturation, but IFN induced by the virus may mediate “bystander” maturation of the uninfected mDC. In support of our model, we observed that mDC generated from IFNR−/− animals were more susceptible to infection with rM5IR-M virus than were mDC from wt animals (data not shown). Furthermore, the greater susceptibility of IFNR−/− mDC to rM51R-M virus infection correlated with a decrease in the expression of costimulatory molecules on the surfaces of these cells (Fig. 8). Therefore, these data suggest that type I IFN regulates virus infection and spread in mDC to prevent virus-induced inhibition of host gene expression and cell killing. In addition, the notion of bystander maturation is consistent with the observation that although most of the rM51R-M virus-infected mDC did not express viral antigens (Fig. 2), they displayed high levels of costimulatory molecules (Fig. 4). Thus, it appears that cells that are not detectably infected by rM51R-M virus mature in response to secreted factors produced by the virus, such as type I IFN. Taken together, these results indicate that type I IFN induced by rM51R-M virus contributes to mDC maturation by attenuating viral spread to prevent virus-induced cytopathic effects as well as by inducing bystander activation.
Our data suggest that the differences between pDC and mDC play an important role in their sensitivities and responses to VSV infection. Previous studies have shown that pDC respond to infection with wt VSV by maturing and producing large quantities of type I IFN (37). This is likely due to their expression of TLR7, which has been identified as the putative receptor recognizing VSV (37). The recognition of wt VSV by pDC through TLR7 is also critical for survival of the cells. The expression and triggering of TLR7 by pDC likely occur to provide a rapid IFN response, as well as additional antiviral responses (23, 43, 46), to inhibit viral replication and virus-induced cytopathic effects. The lack of expression of TLR7 (as indicated by the lack of responsiveness to the TLR7 agonist loxoribine) is likely the reason that mDC succumb to wt VSV infection, fail to mature, and subsequently die as a result of virus infection. However, even in the absence of TLR7, mDC are able to respond to the M protein mutant of VSV, rM51R-M virus, and undergo maturation. Ultimately, because the mutant virus is less effective at inhibiting host cell gene expression, DC have the chance to get ahead of the viral infection by producing type I IFN and other antiviral factors, thus inhibiting viral replication. A similar failure to suppress mDC antiviral responses is also likely to account for the maturation of mDC induced by an NS1 deletion mutant of influenza virus (34). Based on these examples, we suggest that for most virus types, mutant viruses that are defective in suppressing host responses will be effective inducers of mDC maturation.
Our studies demonstrate that the maturation of mDC induced by rM51R-M virus is largely MyD88 independent, except for a modest induction of CD80 expression. In contrast to the data obtained with LPS-treated cells, the cytokines IL-12 p40, IL-6, and TNF-α were produced by mDC in response to rM51R-M virus in a MyD88-independent and perhaps TLR-independent manner (Fig. 7). Several reports have implicated intracellular pathways independent of TLR-mediated signaling as being responsible for viral triggering of DC maturation (7, 21, 35). The MyD88-independent mechanism for sensing rM51R-M virus is not known, but alternative mechanisms can be envisioned. It is possible that the stimulation of mDC by rM51R-M virus is due to the activation of alternative factors by RNA intermediates that are formed during the life cycle of the virus. One possible candidate is the RNA helicase, retinoic acid-inducible gene I (RIG-I). Studies have demonstrated that RIG-I is an essential regulator of virus-induced antiviral immunity due to the activation of IRF-3 and NF-κB and the enhanced production of type I IFN (20, 50). Further studies will involve distinguishing the pathways involved in inducing the maturation of both mDC and pDC by wt and M protein mutant viruses.
wt and mutant strains of VSV are currently being developed as oncolytic viruses for antitumor therapies (1, 5, 6, 8, 13, 40, 44, 45). Although reports have documented the presence of DC within several types of human tumors, studies have shown that the tumor microenvironment does not provide strong DC activation signals (49). In fact, tumors can escape detection by the immune system through a variety of mechanisms, including interference with DC migration and/or inhibition of activation signals (14, 47). Therefore, therapies that are able to overcome the inhibitory signals provided by tumors would have an advantage over more conventional therapies.
The M protein mutant virus rM51R-M virus offers great potential as an oncolytic virus for antitumor therapies due to its ability to directly kill and clear tumor cells. Additionally, because this mutant virus is a strong inducer of DC maturation, it offers promise for initiating tumor-specific T-cell responses, leading to memory as a means to prevent tumor reemergence. For example, while tumor cells are killed by the virus, type I IFN will be produced by normal tissue, leading to the maturation of DC in the local environment. These DC will likely ingest tumor cell debris and subsequently migrate to lymph nodes, where they can cross-present tumor antigens to CD8+ T cells.
Several studies have demonstrated that not all DC subsets are equivalent in their abilities to present antigens and prime naïve T cells (33). The tasks of antigen presentation and T-cell priming have been largely attributed to a population of DC resembling mDC that express both CD11c and CD8α (9, 33). Therefore, with the ability to target mDC, rM51R-M virus has the potential to effectively stimulate the most potent of T-cell-stimulatory antigen-presenting cells. Future studies will therefore involve an evaluation of the cross-presenting potential of bone marrow-derived mDC in our system.
Although this study focuses on the utility of rM51R-M virus as a therapeutic agent due to its ability to induce mDC maturation in vitro and the subsequent activation of T cells, it is important to note that wt VSV can also stimulate robust T-cell responses in vivo (4, 51). Our studies suggest that wt VSV may stimulate a class of DC that are more resistant to the shutoff of host gene expression than mDC, perhaps pDC. Therefore, these alternative subsets of DC may trigger T cells in response to infection with wt VSV. It is also possible that in vivo, mDC are activated indirectly through IFN produced by VSV-responsive pDC. Future studies will therefore seek to distinguish between the mechanisms by which wt and mutant VSVs activate DC maturation and the type of T-cell responses that result from targeting distinct subsets of DC.
Acknowledgments
We thank Douglas S. Lyles for helpful advice and comments on the manuscript. We also thank Shizuo Akira for the MyD88−/− mice, Christian Schindler for the IFNR−/− mice, and Shelby Puckett for carrying out viral plaque assays.
This work was supported by Public Health Service grants AI 060642 (project leader, S. B. Mizel) and AI 32983 (to D. S. Lyles) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of M protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., T. M. Kundig, G. Freer, Y. Li, C. Y. Kang, D. H. Bishop, H. Hengarner, and R. M. Zinkernagel. 1994. Induction of protective cytotoxic T cells with viral proteins. Eur. J. Immunol. 24:2228-2236. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., and G. N. Barber. 2001. Oncloytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135-138. [DOI] [PubMed] [Google Scholar]
- 7.Barchet, W., A. Krug, M. Cella, C. Newby, J. A. Fischer, A. Dzionek, A. Pekosz, and M. Colonna. 2005. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35:236-242. [DOI] [PubMed] [Google Scholar]
- 8.Bell, J. C., B. Lichty, and D. F. Stojdl. 2003. Getting oncolytic virus therapies off the ground. Cancer Cell 4:7-11. [DOI] [PubMed] [Google Scholar]
- 9.Belz, G. T., C. M. Smith, L. Kleinert, P. Reading, A. Brooks, K. Shortman, F. R. Carbone, and W. R. Heath. 2004. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA 101:8670-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711-6719. [DOI] [PubMed] [Google Scholar]
- 11.Brzoza, K. L., A. B. Rockel, and E. M. Hiltbold. 2004. Cytoplasmic entry of Listeria monocytogenes enhances dendritic cell maturation and T cell differentiation and function. J. Immunol. 173:2641-2651. [DOI] [PubMed] [Google Scholar]
- 12.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 13.Ebert, O., K. Shinozaki, T. G. Huang, M. J. Savontaus, A. Garcia-Sastre, and S. L. Woo. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 63:3605-3611. [PubMed] [Google Scholar]
- 14.Engleman, E. G., J. Brody, and L. Soares. 2004. Using signaling pathways to overcome immune tolerance to tumors. Sci. STKE 241:28-30. [DOI] [PubMed] [Google Scholar]
- 15.Gett, A. V., and P. D. Hodgkin. 2000. A cellular calculus for signal integration by T cells. Nat. Immunol. 1:239. [DOI] [PubMed] [Google Scholar]
- 16.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8(+) T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76:7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahm, B., M. J. Trifilo, E. I. Zuniga, and M. B. A. Oldstone. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 22:247-257. [DOI] [PubMed] [Google Scholar]
- 19.Heath, W. R., G. T. Belz, G. M. Behrens, C. M. Smith, S. P. Forehan, I. A. Parish, G. M. Davey, N. S. Wilson, F. R. Carbone, and J. A. Villadangos. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199:9-26. [DOI] [PubMed] [Google Scholar]
- 20.Heim, M. H. 2005. RIG-I: an essential regulator of virus-induced interferon production. J. Hepatol. 42:431-433. [DOI] [PubMed] [Google Scholar]
- 21.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornung, V., M. Guenthner-Biller, C. Bourquin, A. Alabasser, M. Schlee, S. Uematsu, A. Noronha, M. Manoharan, S. Akira, A. de Fougerolles, S. Endres, and G. Hartmann. 2005. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11:263-270. [DOI] [PubMed] [Google Scholar]
- 24.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K.-K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 25.Huang, T. G., O. Ebert, K. Shinozaki, A. Garcia-Sastre, and S. L. Woo. 2003. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 8:434-440. [DOI] [PubMed] [Google Scholar]
- 26.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadowaki, N., S. Ho, S. Antonenko, R. de Waal Malefyt, R. A. Kastelein, F. Bazan, and Y.-J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 29.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 31.Le Bon, A., N. Etchart, C. Rossman, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 32.Lefrancois, L., and D. S. Lyles. 1982. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 121:157-167. [PubMed] [Google Scholar]
- 33.Liu, Y.-J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 34.Lopez, C. B., A. Garcia-Sastre, B. R. G. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 35.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 36.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. J. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 37.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcus, P. I., and M. J. Sekellick. 1985. Interferon induction by viruses. XIII. Detection and assay of interferon induction-suppressing particles. Virology 142:411-415. [DOI] [PubMed] [Google Scholar]
- 40.Obuchi, M., M. Fernandez, and G. N. Barber. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77:8843-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsburg, E., N. F. Rose, P. A. Marx, M. Mefford, D. F. Nixon, W. J. Moretto, D. Montefiori, P. Earl, B. Moss, and J. K. Rose. 2004. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J. Virol. 78:3930-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K.-K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenemeyer, A., B. J. Barnes, M. E. Mancl, E. Latz, N. Goutagny, P. M. Pitha, K. A. Fitzgerald, and D. T. Golenbock. 2005. The interferon regulatory factor, IRF5, is a central mediator of Toll-like receptor 7 signaling. J. Biol. Chem. 280:17005-17012. [DOI] [PubMed] [Google Scholar]
- 44.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 45.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 46.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K. J. Ishii, T. Kawai, O. Takeuchi, and S. Akira. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR) 7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201:915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicari, A. P., C. Caux, and G. Trinchieri. 2002. Tumour escape from immune surveillance through dendritic cells inactivation. Cancer Biol. 12:33-42. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, M., K. Takeda, and S. Akira. 2004. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 40:861-868. [DOI] [PubMed] [Google Scholar]
- 49.Yang, L., and D. P. Carbone. 2004. Tumor-host immune interactions and dendritic cell dysfunction. Adv. Cancer Res. 92:13-27. [DOI] [PubMed] [Google Scholar]
- 50.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagashi, K. Taira, S. Akira, and T. Fujiita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 51.Zammit, D. J., L. S. Cauley, Q.-M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]