Abstract
Infected cells recognize viral replication as a DNA damage stress and elicit the ataxia telangiectasia-mutated (ATM)/p53-mediated DNA damage response signal transduction pathway as part of the host surveillance mechanisms, which ultimately induces the irreversible cell cycle arrest and apoptosis. Viruses have evolved a variety of mechanisms to counteract this host intracellular innate immunity. Kaposi's sarcoma-associated herpesvirus (KSHV) viral interferon regulatory factor 1 (vIRF1) interacts with the cellular p53 tumor suppressor through its central DNA binding domain, and this interaction inhibits transcriptional activation of p53. Here, we further demonstrate that KSHV vIRF1 downregulates the total p53 protein level by facilitating its proteasome-mediated degradation. Detailed biochemical study showed that vIRF1 interacted with cellular ATM kinase through its carboxyl-terminal transactivation domain and that this interaction blocked the activation of ATM kinase activity induced by DNA damage stress. As a consequence, vIRF1 expression greatly reduced the level of serine 15 phosphorylation of p53, resulting in an increase of p53 ubiquitination and thereby a decrease of its protein stability. These results indicate that KSHV vIRF1 comprehensively compromises an ATM/p53-mediated DNA damage response checkpoint by targeting both upstream ATM kinase and downstream p53 tumor suppressor, which might circumvent host growth surveillance and facilitate viral replication in infected cells.
To prevent the fixation of mutations from one cell generation to the next and also maintain genomic stability, cells have evolved regulatory mechanisms that ensure the order and fidelity of cell cycle events, such as DNA replication and cell division. This regulatory mechanism becomes activated when cells are exposed to genotoxic agents or other adverse environmental conditions such as viral infection (reviewed in reference 38). The ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia- and Rad3-related (ATR) kinases, both of which are phosphoinositide 3-kinase-related kinases, are critical for transducing DNA damage signals to checkpoint control proteins (51). ATM is essential for mediating cell cycle checkpoint control in cells exposed to ionizing radiation and other agents that produce double-strand breaks in DNA (25). In contrast, ATR is activated by stalled replication forks and agents that produce bulky adducts, such as UV irradiation (45). Upon DNA damage signaling, activation of both kinases results in the direct or indirect phosphorylation or activation of a number of downstream checkpoint controls, DNA repair, or apoptosis-promoting targets, including p53, Chk1, and Chk2 (3, 8, 18, 37, 53). Chk1 and Chk2 become phosphorylated at serine 345 (34) and threonine 68 (37), respectively, following genotoxic exposure. The roles of Chk1 and Chk2 in response to DNA damage are essential, as inhibition of the ATM/Chk2 pathway results in a loss of the G2/M checkpoint and increased sensitivity to ionizing radiation, whereas Chk2 mutation has been implicated in the cancer Li-Fraumeni syndrome (59). Thus, activation of the Chk1 and Chk2 checkpoint proteins is necessary for maintaining genome stability and overall cellular viability following genotoxic stress.
The tumor suppressor protein p53 maintains the genomic integrity of cells by arresting the cell cycle or inducing apoptosis in response to various sources of environmental stress, including oncogene activation and DNA damage (reviewed in references 14 and 39). Therefore, p53 should be tightly regulated below a certain level for a normal cell growth. Often, failure of the p53 level correlates with uncontrolled cell growth and tumorigenesis (reviewed in reference 22). The protein level of p53 is usually regulated by mdm2-mediated ubiquitination (20, 23), followed by 26S proteasome-mediated degradation (15, 36) (reviewed in reference 62), and in this process, posttranslational modifications of p53 such as phosphorylation and acetylation (reviewed in references 1 and 5) play important roles in the stability of p53, possibly through modulating ubiquitination by mdm2. The N-terminal transactivation domain of p53 has several key phosphorylation sites. Phosphorylation near the mdm2 binding sites has been believed to reduce the affinity between p53 and mdm2 and therefore cause the stabilization of p53. In fact, activated ATM/ATR and Chk1/2 phosphorylate the serine 15 and 20 residues, respectively, which lie right under the binding pocket of mdm2, and these phosphorylations disrupt the binding with mdm2 (11, 12), resulting stabilization of p53 (13, 50, 58). This event results in either apoptosis or cell cycle arrest, which is dependent on the milieu of each cell type.
The irreversible cell cycle arrest and apoptosis are part of the host surveillance mechanisms for viral infection and tumor induction. Viruses have evolved a variety of mechanisms to counteract host innate immune controls. Kaposi's sarcoma-associated herpesvirus (KSHV) is the most recently discovered human tumor virus and is associated with the pathogenesis of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (9, 10, 54). The K9 open reading frame of KSHV exhibits significant sequence homology with cellular interferon (IFN) regulatory factors (IRFs) and is thus designated viral interferon regulatory protein 1 (vIRF1) (40, 47). We and others have demonstrated that expression of vIRF1 dramatically represses transcriptional activation induced by alpha and beta IFNs (17, 30, 63) and also leads to transformation of rodent fibroblast cells, resulting in morphological change, focus formation, growth at reduced serum concentration, and tumor induction in nude mice (17, 30). Further studies have demonstrated that these functional activities of vIRF1 appear to be attributed in part to an interaction with and inhibition of p300 (7, 29, 33). Interaction of vIRF1 with p300 inhibits the histone acetyltransferase (HAT) activity of p300 in vitro and induces a dramatic hypoacetylation of nucleosomal histone H3 and H4 in vivo, resulting in global alteration of nucleosomal chromatin structure and inhibition of IFN-mediated gene expression (29). Thus, the modulation of p300 HAT activity is likely part of the mechanisms which vIRF1 employs to block cellular IFN-mediated antiviral activity. Finally, we (41) and others (48) have demonstrated that KSHV vIRF1 interacts with the cellular p53 tumor suppressor through the putative DNA binding region of vIRF1 and the central region of p53. This interaction suppresses the level of acetylation of p53 and inhibits transcriptional activation of p53. As a consequence, vIRF1 efficiently prevents p53-mediated apoptosis. These results suggest that KSHV vIRF1 interacts with and inhibits the p53 tumor suppressor to circumvent host growth surveillance and to facilitate uncontrolled cell proliferation.
To further delineate the role of vIRF1 in the viral immune evasion strategy, we examined the potential effect of vIRF1 on ATM/p53 DNA damage response signal transduction. We reported here that, in addition to the abrogation of p53 transcriptional activity, vIRF1 also suppressed p53 protein stability. The latter activity of vIRF1 was derived from its interaction with and inhibition of upstream ATM kinase activity, which resulted in the reduction of Ser15 phosphorylation and the enhanced degradation of p53. These results indicate that KSHV vIRF1 comprehensively inhibits p53 tumor suppressor function by downregulating its transcriptional activity as well as its protein stability. These results also emphasize the important role of ATM/p53-mediated irreversible cell cycle arrest and apoptosis as part of the host surveillance mechanisms for viral infection and tumor induction.
MATERIALS AND METHODS
Plasmids, cell culture and transfection.
DNA fragments containing wild-type (wt) p53, the p53(14/19) mutant, or the p53 [22/23] mutant were cloned into pCMV-Tag2B vector (Stratagene), and DNA fragments containing full-length vIRF1 or its truncated mutants (see Fig. 4B) were cloned into pEF-IRES-Puro vector (21). Plasmids were purified by standard CsCl2 ultracentrifugation. HCT116 cells (kindly provided by B. Vogelstein, Johns Hopkins University) and p53−/− and p53−/− mdm2−/− mouse embryo fibroblast cells (MEF) (kindly provided by S. Jones, University of Massachusetts Medical School) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin-streptomycin and were transfected using Lipofectamine 2000 (Invitrogen). For transient expression, 293T cells were maintained in the same medium and transfected using the CalPhos mammalian transfection kit (BD Biosciences).
FIG. 4.
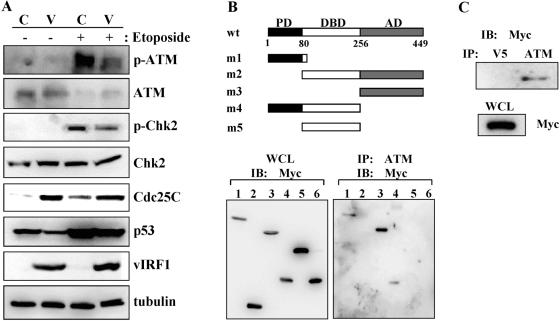
vIRF1 interacts with and inhibits ATM kinase. (A) vIRF1 suppresses ATM and Chk2 activation. TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells were incubated with doxycycline for 24 h, followed by treatment with or without etoposide for 4 h. Cell lysates were then used for immunoblotting with anti-Ser1981 phospho-specific ATM, anti-ATM, anti-phospho-specific Chk2, anti-Chk2, anti-Cdc25C, anti-p53, anti-vIRF1 (myc), and antitubulin antibodies. (B) The carboxyl-terminal transcriptional domain of vIRF1 interacts with ATM. pEF-IRES-vIRF1 wt or pEF-IRES-vIRF1 mutants were transfected into 293T cells. Cells were lysed at 48 h posttransfection with lysis buffer, and whole-cell lysates (WCL) were used for immunoprecipitation (IP) with an anti-ATM antibody, followed by immunoblotting (IB) with anti-Myc antibody (bottom right panel). WCL were also used for immunoblotting with an anti-myc antibody to demonstrate the expression of vIRF1 wt and mutant proteins (bottom left panel). (C) Interaction of full-length vIRF1 with ATM in KSHV-infected BABCL1 cells. TRExBCBL1-vIRF1 cells were treated with doxycycline for 48 h and lysed with lysis buffer. WCL were then used for immunoprecipitation with anti-ATM and anti-V5 antibodies, followed by immunoblotting with an anti-myc antibody. WCL were also used for immunoblotting with an anti-myc antibody to demonstrate the myc-tagged vIRF1 expression.
Construction of cell lines.
Tetracycline-inducible TRExBJAB and TRExBCBL-1 cells were constructed as described previously (42) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin-streptomycin. HCT116 cells (wild type) and p53−/− and p53−/− mdm2−/− MEF were transfected with empty pEF-IRES-Puro plasmids or pEF-IRES-vIRF1, followed by the selection with 2 μg/ml of puromycin (Sigma).
Reagents and chemicals.
Cells were treated with 50 μM of MG132 (Calbiochem), 50 nM of leptomycin B (Sigma), 1 μg/ml of doxycycline (Sigma), 50 μg/ml of cycloheximide (Sigma), or 40 μg/ml of etoposide (Sigma) for the indicated periods.
RPA.
TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells were treated with doxycycline (1 μg/ml) to induce protein expression and harvested at the indicated time points. Cellular total RNA was extracted with TRIzol reagent (Invitrogen). A [32P]UTP-labeled antisense RNA probe transcribed from the hCC-2 multiprobe template set (BD Bioscience) was hybridized with 30 μg of total RNA at 90°C for 3 min and subsequently at 56°C for 16 h. RNase protection reactions were carried out using an RNase protection assay (RPA) kit (BD PharMingen) according to the manufacturer's instructions, and the samples were separated on a 5% denaturing acrylamide gel and analyzed using a Phosphorimager (BAS 2000; Fuji Photo film Co., Tokyo, Japan).
Determination of half-life of p53.
Exponentially growing cells were treated with cycloheximide, harvested at the indicated time points, and subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis followed by immunoblotting with an anti-p53 antibody (DO-1; Santa Cruz Biotech). The protein amount loaded in each lane was normalized with an anti-β-tubulin antibody (Santa Cruz Biotech). Signal intensities were determined using the ImageGauge program, and the half-life (t1/2) of p53 was calculated using the formula log2(Q0/Qt) = t/t1/2, in which Q0 and Qt represent the intensity of p53 at time points 0 and t, respectively, after cycloheximide treatments.
Immunoblotting and immunoprecipitation.
Transfected cells were harvested and resuspended with lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.5% NP-40, and protease inhibitor cocktail [Roche]), followed by centrifugation at 12,000 rpm for 5 min, and the supernatants were collected. Cell lysates were precleared with protein A/G-agarose (Santa Cruz Biotech) and incubated with specific antibody. Immune complexes were resuspended with SDS sample buffer (Sigma), separated by SDS-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane (Roche). The membrane was blocked with phosphate-buffered saline (PBS) containing 5% skim milk for 20 min, incubated with primary antibody for 1 h, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. Specific signals were detected with an enhanced chemiluminescence system. The primary antibodies were purchased from the following sources: p53 (DO-1 and DO-1-HRP), β-tubulin (D-10), and cdc25C (H-150) antibodies from Santa Cruz Biotechnology; ATM (SYR6D4) and FLAG (M2) antibodies from Sigma; c-myc (9E10) antibody from Covance; Chk2 antibody and phospho-specific antibodies for p53 (Ser15 and Ser-20) and Chk2 from Cell Signaling; and phospho-ATM (Ser 1981) and γH2AX antibodies from Upstate Biotech.
Confocal microscopy.
TRExBCBL-1 and TRExBCBL1-vIRF1 cells were loaded on Superfrost-plus microscope slide (Fisher) by cytospin, fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.2% Triton X-100 in PBS for 20 min. Anti-p53 antibody (no. 9282; Cell Signaling), anti-myc antibody (9E10; Covance), phospho-ATM antibody, and rH2AX antibody were diluted 1:500 with PBS containing 3% bovine serum albumin and reacted for 30 min, followed by washing with PBS. Alexa-conjugated anti-mouse and anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, CA) were diluted with PBS (1:1,000) and reacted for 30 min. After washing three times with PBS, nuclei were stained with Topro-3 (Molecular Probes; 1:5,000 dilution) for 5 min, followed by washing with PBS. Confocal microscopy was performed according to the procedures described previously (41).
RESULTS
Downregulation of p53 protein level by vIRF1.
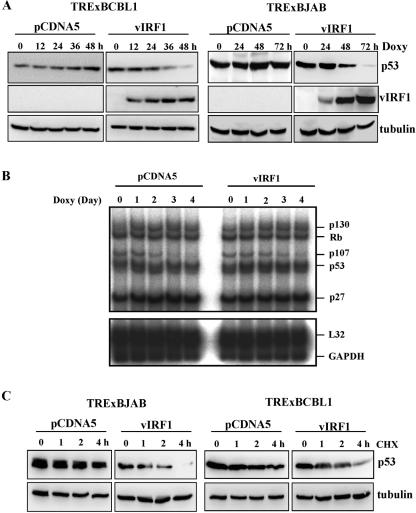
Since the p53 tumor suppressor plays a major role in host surveillance upon viral infection, we examined the potential effect of vIRF1 on p53 stability in addition to its transcriptional activity. To test this hypothesis, the carboxyl-terminal myc-tagged vIRF1 was ectopically expressed in virus-free BJAB cells and KSHV-infected BCBL1 cells in a tetracycline-inducible manner aspreviously described (42). These cells were designated TRExBJAB-vIRF1 and TRExBCBL1-vIRF1, respectively. Immunoblot assay showed that vIRF1 protein was induced in both TRExBCBL1-vIRF1 and TRExBJAB-vIRF1 cells upon doxycycline treatment, and its expression was detected within 12 h of doxycycline treatment (Fig. 1A). Under these conditions, the endogenous p53 level was examined by immunoblotting with an anti-p53 DO-1 antibody. This showed that the p53 protein amount was considerably decreased in TRExBCBL1-vIRF1 and TRExBJAB-vIRF1 cells when the vIRF1 protein amount was increased (Fig. 1A). In contrast, the reduction of p53 protein amount was not observed in control TRExBCBL1-cDNA5 and TRExBJAB-cDNA5 cells under the same conditions (Fig. 1A). To further delineate the effect of vIRF1 on p53 expression, RPA was performed to determine the transcript levels of p53 and other related cellular genes, including p130, Rb, p107, and p27. L32 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were also included as loading controls. This showed that vIRF1 expression did not affect mRNA levels of p53 and other related cellular genes in TRExBCBL1 cells (Fig. 1B). To further elucidate downregulation of p53 induced by vIRF1, the half-life of p53 protein was measured upon treatment with cycloheximide, which blocks the de novo protein synthesis. This showed that vIRF1 expression greatly reduced the half-life of p53 in both TRExBJAB-vIRF1 and TRExBCBL1-vIRF1 cells compared to control TRExBCBL1-cDNA5 and TRExBJAB-cDNA5 cells: the half-lives were 5.1 h in TRExBJAB-cDNA5 cells versus 0.9 h in TRExBJAB-vIRF1 cells and 3.9 h in TRExBCBL1-cDNA5 cells versus 1.7 h in TRExBCBL1-vIRF1 cells (Fig. 1C). These results demonstrate that vIRF1 expression significantly downregulates p53 expression at the posttranslational level.
FIG. 1.
Downregulation of p53 protein amount by vIRF1. (A) vIRF1 expression deceases the endogenous p53 protein amount. TRExBJAB-cDNA5, TRExBJAB-vIRF1, TRExBCBL1-cDNA5, and TRExBCBL1-vIRF1 cells were treated with doxycycline (Doxy) for the indicated times, and their cell lysates were used for immunoblotting with anti-p53, anti-vIRF (myc), and antitubulin antibodies. (B) RNase protection assay. TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells were treated with doxycycline for the indicated times, their total RNA was isolated by phenol-chloroform extraction, and contaminated DNA was removed by DNase I treatment. Thirty micrograms of total RNA was then subjected to RPA using an RPA kit from BD PharMingen (San Diego, CA) according to the manufacturer's recommendation. (C) Reduction of p53 protein stability by vIRF1 expression. TRExBJAB-cDNA5, TRExBJAB-vIRF1, TRExBCBL1-cDNA5, and TRExBCBL1-vIRF1 cells were stimulated with doxycycline for 24 h, followed by treatment with cycloheximide (50 μg/ml) for the indicated times. Whole-cell lysates were used for immunoblotting with anti-p53 and antitubulin antibodies. The half-life of p53 was calculated by the formula described in Materials and Methods.
Enhanced ubiquitination of p53 induced by vIRF1.
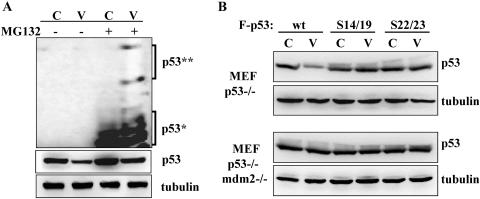
It has been extensively shown that p53 undergoes mdm2-mediated ubiquitination, which leads to 26S proteasome-mediated degradation (15, 36). To test whether vIRF1 affected this process to facilitate p53 degradation, TRExBJAB-cDNA5 and TRExBJAB-vIRF1 cells were treated with MG132, a potent inhibitor of 26S proteasomes (46), and then examined for p53 protein amount by immunoblotting. MG132 treatment detectably reversed p53 downregulation induced by vIRF1 (Fig. 2A). Furthermore, while the ubiquitination of p53 was readily detected in both TRExBJAB-cDNA5 and TRExBJAB-vIRF1 cells upon MG132 treatment, it was significantly higher in TRExBJAB-vIRF1 cells than in TRExBJAB-cDNA5 control cells (Fig. 2A). These results showed that vIRF1 expression enhanced p53 ubiquitination.
FIG. 2.
Enhanced ubiquitination of p53 induced by vIRF1. (A) TRExBJAB-cDNA5 (C) and TRExBJAB-vIRF1 (V) cells were treated with doxycycline for 24 h and further incubated in the presence (+) or absence (−) of MG132 for 4 h. Whole-cell lysates were used for immunoblotting with anti-p53 and antitubulin antibodies. The polyvinylidene difluoride nitrocellulose membrane was exposed for a short time (middle panel) to detect the unmodified p53 or for a long time (top panel) to detect the ubiquitin-modified p53. The monoubiquitinated and polyubiquitinated p53 are marked with * and **, respectively. (B) Mdm2 is necessary for vIRF1-induced degradation of p53. Expression vectors containing the Flag-tagged wild-type p53, p53(14/19) mutant, or p53(22/23) mutant were transfected into stable p53−/− MEF or p53−/− mdm2−/− MEF expressing control-puro (C) or vIRF1-puro (V). At 48 h posttransfection, cell lysates were used for immunoblotting with anti-FLAG and antitubulin antibodies.
mdm2 has been known as the principal E3 ubiquitin ligase enzyme of p53, ligating ubiquitin moieties to lysine residues of p53. To test whether mdm2 was necessary for p53 ubiquitination induced by vIRF1, p53 was exogenously expressed in MEF. p53−/− single-knockout (KO) and p53−/− mdm2−/− double-KO MEF stably expressing empty vector or vIRF1 were established as described in Materials and Methods. While vIRF1-mediated downregulation of exogenous p53 was readily observed in p53−/− single-KO MEF, it was not detected in p53−/− mdm2−/− double-KO MEF, indicating that mdm2 is necessary for vIRF1-mediated downregulation of p53 (Fig. 2B). The p53(14/19) and p53(22/23) mutants, which have replacements with alanines at the amino-terminal residues 14 and 19 and amino-terminal residues 22 and 23, respectively, have been described as being resistant to mdm2-mediated degradation (31, 32). These p53 mutants were also exogenously expressed in p53−/− single-KO MEF or p53−/− mdm2−/− double-KO MEF in the presence or absence of vIRF1, followed by immunoblotting with an anti-Flag antibody. This showed that vIRF1 was no longer able to downregulate these p53 mutants in both p53−/− single-KO MEF and p53−/− mdm2−/− double-KO MEF (Fig. 2B). The endogenous tubulin level was included as a loading control (Fig. 2B), and the exogenous glutathionine S-transferase was included as a transfection control (data not shown). These results suggest that vIRF1 expression enhances the mdm2-mediated degradation of p53.
vIRF1 inhibits the Ser15 phosphorylation of p53.
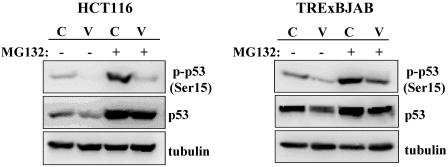
The phosphorylation of p53 has been shown to play an important role in the regulation of its stability (reviewed in reference 61). Of the many phosphorylation sites of p53, the Ser15 and Ser20 residues are the key regulatory sites, since their phosphorylation sterically hinders mdm2 interaction, which leads to the suppression of p53 ubiquitination and thereby its stabilization (11-13, 49, 58). To investigate whether vIRF1 affected the Ser15 and Ser20 phosphorylation of p53, HCT116-puro and HCT116-vIRF1 cells and TRExBJAB-cDNA5 and TRExBJAB-vIRF1 cells were treated with or without MG132 for 4 h. TRExBJAB-cDNA5 and TRExBJAB-vIRF1 cells were incubated with doxycycline for 24 h prior to MG132 treatment. Cell lysates were then reacted with the Ser15 phospho-specific and Ser20 phospho-specific p53 antibodies. A significant reduction of Ser15 phosphorylation was observed in HCT116-vIRF1 and TRExBJAB-vIRF1 cells compared to that in control HCT116-puro and TRExBJAB-cDNA5 cells (Fig. 3). Furthermore, the reduction of Ser15 phosphorylation of p53 induced by vIRF1 expression was more evident after MG132 treatment (Fig. 3). However, this reduced level of p53 Ser15 phosphorylation upon MG132 treatment was not due to a reduced level of p53 protein, since similar amounts of p53 protein were detected in control and vIRF1-expressing HCT116 and TRExBJAB cells (Fig. 3). Unlike Ser15 phosphorylation, however, we were not able to detect Ser20 phosphorylation of p53 in both control and vIRF1-expressing HCT116 and TRExBJAB cells in multiple trials with several Ser20 phospho-specific commercial antibodies (data not shown). Nevertheless, these results indicate that vIRF1 expression considerably reduces the Ser15 phosphorylation of p53.
FIG. 3.
vIRF1 inhibits the Ser15 phosphorylation of p53. HCT116 cells (HCT116-puro [C] and HCT116-vIRF1 cells [V]) and TRExBJAB cells (TRExBJAB-cDNA5 [C] and TRExBJAB-vIRF1 cells [V]) were treated with or without MG132 for 4 h. TRExBJAB cells were treated with doxycycline for 24 h prior to MG132 treatments. Whole-cell lysates were then used for immunoblotting with anti-Ser15 phospho-specific p53, anti-p53, and antitubulin antibodies.
vIRF1 interacts with and inhibits ATM kinase.
In response to genotoxic insults that induce DNA double-strand breaks, ATM undergoes prompt activation through its autophosphorylation on serine 1981 (2). Activated ATM then effectively phosphorylates the serine 15 residue of p53, which disrupts the binding with mdm2, resulting in the stabilization of p53 (13, 50, 58). Since vIRF1 expression considerably reduced the Ser15 phosphorylation of p53, we examined whether vIRF1 expression affected the activation of ATM kinase activity upon treatment with etoposide, a DNA topoisomerase II inhibitor which induces DNA double-strand breaks. Upon etoposide treatment, serine 1981 phosphorylation of ATM was readily detected in control TRExBCBL1-cDNA5 cells, whereas it was significantly reduced in TRExBCBL1-vIRF1 cells (Fig. 4A). Inaddition to the augmentation of ATM phosphorylation, Chk2phosphorylation was robustly increased in TRExBCBL1-cDNA5 cells upon etoposide treatment, whereas it was only weakly increased in TRExBCBL1-vIRF1 cells (Fig. 4A). Chk2 has been shown to phosphorylate Cdc25C on serine 216, a site known to be involved in negative regulation of Cdc25C, resulting in a rapid degradation of Cdc25C (4, 16). Indeed, the reverse correlation between Chk2 phosphorylation and Cdc25C amount was observed in TRExBCBL1-vIRF1 cells (Fig. 4A). In summary, these results indicate that vIRF1 expression significantly blocks the etoposide-induced ATM activation, which leads to the attenuation of Chk2/Cdc25C downstream signal transduction pathway.
To further investigate how vIRF inhibited ATM activation, we tested whether vIRF1 specifically targeted ATM for an interaction. To test this, myc-tagged wt vIRF1 and its truncation mutants were expressed in 293T cells. The vIRF1 mutants were as follows: mutant 1 (m1), containing the amino-terminal unique region; m2, containing the central DNA binding region and the carboxyl transactivation region; m3, containing the carboxyl transactivation region; m4, containing the amino-terminal unique region and the central DNA binding region; and m5, containing the central DNA binding region. At 48 h posttransfection, cell lysates were immunoprecipitated with an anti-ATM antibody, followed by immunoblotting with an anti-myc antibody. The wt vIRF1, vIRF1 m2, and vIRF1 m3 proteins were effectively detected in the ATM immune complex (Fig. 4B, lanes 1, 3, and 4). However, the vIRF1 m1, m4, and m5 proteins were not detected in the ATM immune complex under the same conditions (Fig. 4B). vIRF1 and its mutant forms were expressed atcomparable levels (Fig. 4B). Finally, KSHV-infected TRExBCBL1-vIRF1 cells containing the myc-tagged vIRF1 were treated with doxycycline for 48 h and used for coimmunoprecipitation to detect the interaction between ATM and vIRF1. The ATM protein complex was precipitated with an anti-ATM antibody, followed by immunoblotting with an anti-myc antibody. An anti-V5 monoclonal antibody was also included as a nonspecific antibody control for immunoprecipitation. This showed that vIRF1 was readily detected in the anti-ATM immune complex but not in the anti-V5 immune complex, demonstrating the specific interaction between full-length vIRF1 and endogenous ATM (Fig. 4C). In summary, vIRF1 interacts with cellular ATM kinase through its carboxyl-terminal transactivation domain.
vIRF1 interaction with ATM is necessary to block ATM activation.
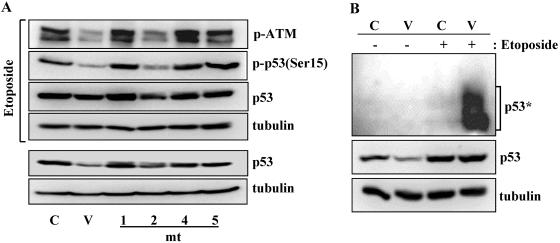
To correlate vIRF1 and ATM interaction with the suppression of ATM activation, we constructed TRExBCBL1 cell lines expressing various vIRF1 mutants. TRExBCBL1-cDNA5, TRExBCBL1-vIRF1, and TRExBCBL1-vIRF1 mutants were induced with doxycycline for 24 h and further treated with or without etoposide for 4 h, and the levels of p53, Ser15-phosphorylated p53, ATM, Ser1981-phosphorylated ATM, and tubulin were then examined by immunoblotting with their specific antibodies. A reduction of the p53 amount was detected in TRExBCBL1-vIRF1 wt and TRExBCBL1-vIRF1 mt2 cells in the absence of etoposide treatment, whereas it was not detected in TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 mt1, TRExBCBL1-vIRF1 mt4, and TRExBCBL1-vIRF1 mt5 cells (Fig. 5A). The p53 amount in TRExBCBL1-vIRF1 mt1, TRExBCBL1-vIRF1 mt4, and TRExBCBL1-vIRF1 mt5 cells was equivalent to that in control TRExBCBL1-cDNA5 cells (Fig. 5A). Since etoposide-mediated DNA damage stress strongly induces p53 transcription (44), TRExBCBL1-cDNA5, TRExBCBL1-vIRF1, and TRExBCBL1-vIRF1 mutant cells showed increased amounts of p53 (Fig. 5A). Under this condition, ATM Ser1981 phosphorylation (p-ATM) and p53 Ser15 phosphorylation were robustly increased in TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 mt1, TRExBCBL1-vIRF1 mt4, and TRExBCBL1-vIRF1 mt5 cells, whereas they were minimally increased in TRExBCBL1-vIRF1 wt and TRExBCBL1-vIRF1 mt2 cells (Fig. 5A). Equivalent amounts of tubulin were observed in all cell lysates (Fig. 5A).
FIG. 5.
vIRF1 interaction with ATM is necessary to block ATM activation. (A) Correlation between vIRF1 interaction and ATM inhibition. TRExBCBL1-cDNA5 (C), TRExBCBL1-vIRF1 (V), TRExBCBL1-vIRF1 mt1, TRExBCBL1-vIRF1 mt2, TRExBCBL1 mt4, and TRExBCBL1-vIRF1 mt5 cells were incubated with doxycycline for 24 h, followed by treatment with or without etoposide for 4 h. Each vIRF1 mutant except vIRF1 mt1 was as described in Fig. 4. vIRF1 mt1 contained amino-terminal amino acids 1 to 80. Cell lysates were used for immunoblotting with anti-Ser1981 phospho-specific ATM, anti-Ser15 phospho-specific p53, anti-p53, and antitubulin antibodies. (B) Enhanced ubiquitination of p53 in vIRF1-expressing HCT116 cells. HCT116-puro (C) and HCT116-vIRF1 (V) cells were treated with etoposide for 4 h, and their lysates were used for immunoblotting with anti-p53 and antitubulin antibodies. The monoubiquitinated p53 is marked with an asterisk.
Finally, we examined the level of p53 ubiquitination in the absence of MG132. HCT116-puro cells showed little or no p53 ubiquitination either before or after etoposide treatment, and HCT116-vIRF1 cells also showed no detectable level of p53 ubiquitination before etoposide treatment (Fig. 5B). In striking contrast, HCT116-vIRF1 cells demonstrated a robust increase of p53 monoubiquitination after etoposide treatment (Fig. 5B). This suggests that while etoposide treatment induced p53 expression, the lack of p53 Ser15 phosphorylation may facilitate its ubiquitination in vIRF1-expressing cells. In summary, vIRF1 interaction strongly correlates with the inhibition of ATM activation, and this interaction leads to the decrease of ATM Ser1981 phosphorylation and p53 Ser15 phosphorylation, resulting in an increase of p53 ubiquitination and degradation.
Effect of vIRF1 on H2AX1 phosphorylation and p53 nuclear export.
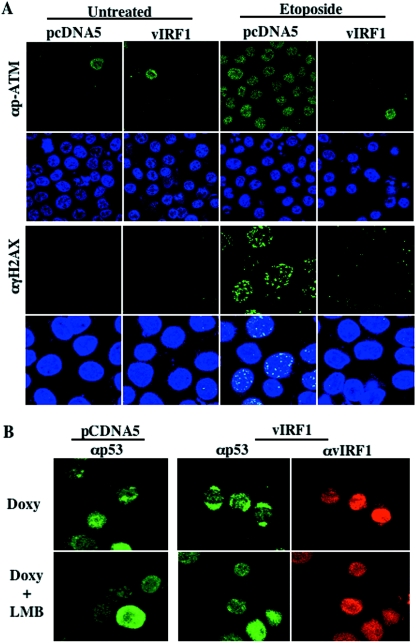
An early response to double-strand breaks is phosphorylation of a variant form of the histone H2A, designated H2AX, and this phosphorylation is mediated primarily by ATM kinase (6). Ser1981-phosphorylated ATM and phosphorylated H2AX, called γH2AX, can be visualized as foci by immunofluorescence using phospho-specific ATM and H2AX antibodies. Since vIRF1 negatively regulated ATM activity, we examined the phosphorylation of ATM and H2AX in untreated or etoposide-treated TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells, using confocal microscopy. The number and intensity of Ser1981-phosphorylated ATM and γH2AX foci dramatically increased in TRExBCBL-cDNA5 cells upon etoposide treatment, whereas they were significantly low or undetectable in TRExBCBL-vIRF1 cells under the same conditions (Fig. 6A). This result suggests that vIRF1 expression readily suppresses the ATM activation and ATM-mediated phosphorylation of H2AX.
FIG. 6.
vIRF1 inhibits ATM activation and γH2AX induction (A) and enhances p53 cytoplasmic localization (B). (A) TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells were incubated with doxycycline for 24 h, treated with or without etoposide (5 μM) for 3 h, fixed with paraformaldehyde, and reacted with anti-Ser1981 phospho-specific ATM (αp-ATM) or anti-γH2AX antibody (green). Cells were stained with Topro-3 (blue) to show the nucleus and then subjected to confocal microscopy. (B) vIRF1 facilitates the cytoplasmic localization of p53. TRExBCBL1-cDNA and TRExBCBL1-vIRF1 cells were treated with doxycycline (Doxy) for 24 h and then further incubated in the presence or absence of 50 nM leptomycin (LMB) for 3 h. These cells were fixed, reacted with anti-p53 (green) and anti-vIRF1 (red) antibodies, and subjected to confocal microscopy.
It has been shown that p53 ubiquitination is tightly coupled with rapid cytoplasmic export for degradation (19, 35). Since vIRF1 enhanced p53 ubiquitination by suppressing ATM-mediated phosphorylation, we further investigated whether vIRF1 expression affected the cytoplasmic localization of p53 in TRExBCBL1 cells. TRExBCBL1-cDNA5 and TRExBCBL1-vIRF1 cells were treated with doxycycline for 2 days, fixed with paraformaldehyde, and reacted with anti-p53 antibody or anti-vIRF1 (myc) antibody. In addition, leptomycin B, an antibiotic that inhibits CRM1-dependent nuclear export (24), was included as a control to enhance the nuclear localization of p53. Confocal microscopy showed that the majority of p53 was present in the nuclei of doxycycline-treated TRExBCBL1-cDNA5 cells, while a small amount of p53 was also detected in the cytoplasm (Fig. 6B). In contrast, p53 was present in the cytoplasmic region of doxycycline-treated TRExBCBL1-vIRF1 cells (Fig. 6B). In addition, leptomycin B treatment effectively blocked the cytoplasmic localization of p53 in TRExBCBL1-vIRF1 cells. These results showed that vIRF1 expression enhanced p53 cytoplasmic localization and that this activity was likely correlated with its ability to induce p53 ubiquitination and degradation.
DISCUSSION
Upon viral infection, interferon and the p53 tumor suppressor are major antiviral defense mechanisms employed by cells. Therefore, it is not surprising that many viruses, especially persistent herpesviruses, carry a number of genes that deregulate these host antiviral mechanisms (56; reviewed in references 28 and 43). Particularly, KSHV encodes various homologues of cellular proteins involved in host defense. These include virus-encoded interferon regulatory factors. vIRF1 has been shown to effectively block the function of its cellular counterparts, IRFs, to interfere with IFN-mediated antiviral activity. Furthermore, we (41) and others (48) have demonstrated that KSHV vIRF1 interacts with the cellular p53 tumor suppressor through its putative DNA binding region and that this interaction suppresses the level of acetylation of p53 and inhibits transcriptional activation of p53. Here, we report that vIRF1 furthermore deregulates the stability of p53 protein by inducing its ubiquitination. This activity is mediated by a novel mechanism by which vIRF1 directly inhibits the activation of ATM kinase, which is an upstream signaling molecule to stabilize p53. These results suggest that KSHV vIRF1 comprehensively inhibits host growth surveillance by targeting both upstream ATM kinase and downstream p53 tumor suppressor, which may also contribute to uncontrolled cell proliferation.
We showed that vIRF1 downregulated the p53 protein amount by facilitating its ubiquitination and degradation. This activity was dependent on the vIRF1-mediated inhibition of the upstream kinase ATM, which phosphorylates the Ser15 residue of p53. There are several potential mechanisms explaining how Ser15 phosphorylation contributes to the stability of p53. As a direct mechanism, Ser15 phosphorylation that lies in the mdm2 binding region may disrupt p53 binding to mdm2, resulting in suppression of its ubiquitination (49). As an indirect mechanism, Ser15 phosphorylation may facilitate the acetylation of p53 through the recruitment of CBP/p300 and PCAF (26), which ultimately blocks its ubiquitination. We demonstrated that the level of Ser15 phosphorylation was directly correlated with the level of p53 downregulation. In addition, we have previously demonstrated that vIRF1 expression suppresses the acetylation of p53 by inhibiting p300 HAT activity (29). Thus, it is likely that KSHV vIRF1 may employ both mechanisms to efficiently downregulate p53 protein stability.
vIRF1 expression effectively decreased Chk2 activation but increased the amount of Cdc25C, showing a reverse correlation between Chk2 activation and Cdc25C amount. In fact, Chk2 has been shown to phosphorylate Cdc25C on serine 216, resulting in a rapid degradation of Cdc25C (4, 16). This suggests that the decrease of Chk2 activation induced by vIRF1 leads to an increase of the Cdc25C amount. However, since the Cdc25C gene is a target for transcriptional repression induced by p53 (55), it is also possible that the increase of Cdc25C caused by vIRF1 is partly due to the downregulation of p53 transcriptional activity and protein amount. On the other hand, the increase of Cdc25C amount caused by vIRF1 was still detected in p53−/− HCT116 cells (data not shown), suggesting that the inhibition of Chk2 activation by vIRF1 primarily contributes to the increase of the Cdc25C amount.
ATM and ATR, which are both phosphoinositide 3-kinase-related kinases, are critical for transducing DNA damage signals to checkpoint control proteins (reviewed in reference 51). ATM is essential for mediating cell cycle checkpoint control in cells exposed to ionizing radiation and other agents that produce double-strand breaks in DNA (25). In contrast, ATR is activated by stalled replication forks and agents that produce bulky adducts, such as UV irradiation (45). Upon DNA damage signaling, activation of both kinases results in the direct or indirect phosphorylation or activation of a number of downstream checkpoint controls. Several viruses have been shown to control the ATM pathway for their propagation. Human immunodeficiency virus type 1 (HIV-1) integrase stimulates an ATM-dependent DNA damage response (27). A deficiency of ATM kinase sensitizes cells to retrovirus-induced cell death. In fact, a specific small-molecule inhibitor of ATM kinase activity has been shown to suppress the replication of both wild-type and drug-resistant HIV-1 (27). As seen with HIV-1, herpes simplex virus (HSV) infection has also been shown to elicit a cellular DNA damage response, with activation of the ATM signal transduction pathway (52, 57, 60). Activated ATM and the DNA damage sensor MRN complex, composed of Mre11, Rad50, and Nbs1, were recruited and retained at sites of viral DNA replication, probably recognizing newly synthesized viral DNAs as abnormal DNA structures (52). However, the detailed mechanisms of how HSV infection activates ATM checkpoint signaling and how HSV is able to complete its replication in the presence of activated an ATM checkpoint need to be further investigated. This indicates that infected cells recognize viral replication as a DNA damage stress and robustly induce ATM/p53-mediated signal transduction as a host surveillance. As seen with HIV-1 and HSV, KSHV replication likely induces a ATM/p53-mediated DNA damage response pathway. To block this host growth scrutiny and facilitate viral replication, KSHV may carry the vIRF1 gene, which targets and suppresses both the ATM upstream kinase and the p53 downstream tumor suppressor. Interestingly, these interactions are genetically separable: the central DNA binding region of vIRF1 targets p53 interaction, and the carboxyl transcriptional activation region of vIRF1 targets ATM interaction. It should be noted that vIRF1 interaction with ATM was detected by immunoprecipitation with an anti-ATM antibody, whereas it was not detected with an anti-vIRF1 antibody, suggesting that this interaction may be structurally sensitive. Nevertheless, KSHV vIRF1 is capable of comprehensively compromising an ATM/p53-regulated DNA damage response checkpoint, which might circumvent host growth surveillance and facilitate viral replication in infected cells. Finally, this further emphasizes that the irreversible cell cycle arrest and apoptosis induced by the ATM/p53 DNA damage response signal transduction pathway are an important part of the host surveillance mechanisms for viral infection and tumor induction.
Acknowledgments
We thank S. Grossman and S. Jones for helpful discussion and providing reagents and Bert Vogelstein for providing HCT116 cells.
This work was partly supported by Public Health Service grants CA106156, CA82057, CA91819, and RR00168. J. Jung is a Leukemia & Lymphoma Society Scholar.
REFERENCES
- 1.Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. [DOI] [PubMed] [Google Scholar]
- 2.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 3.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 4.Blasina, A., I. V. de Weyer, M. C. Laus, W. H. Luyten, A. E. Parker, and C. H. McGowan. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, C. L., and W. Gu. 2003. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 15:164-171. [DOI] [PubMed] [Google Scholar]
- 6.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 7.Burysek, L., W. S. Yeow, B. Lubyova, M. Kellum, S. L. Schafer, Y. Q. Huang, and P. M. Pitha. 1999. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 73:7334-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Chehab, N. H., A. Malikzay, E. S. Stavridi, and T. D. Halazonetis. 1999. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc. Natl. Acad. Sci. USA 96:13777-13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig, A. L., L. Burch, B. Vojtesek, J. Mikutowska, A. Thompson, and T. R. Hupp. 1999. Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem. J. 342:133-141. [PMC free article] [PubMed] [Google Scholar]
- 13.Dumaz, N., D. M. Milne, L. J. Jardine, and D. W. Meek. 2001. Critical roles for the serine 20, but not the serine 15, phosphorylation site and for the polyproline domain in regulating p53 turnover. Biochem. J. 359:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fei, P., and W. S. El-Deiry. 2003. P53 and radiation responses. Oncogene 22:5774-5783. [DOI] [PubMed] [Google Scholar]
- 15.Freedman, D. A., C. B. Epstein, J. C. Roth, and A. J. Levine. 1997. A genetic approach to mapping the p53 binding site in the MDM2 protein. Mol. Med. 3:248-259. [PMC free article] [PubMed] [Google Scholar]
- 16.Furnari, B., A. Blasina, M. N. Boddy, C. H. McGowan, and P. Russell. 1999. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 10:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 18.Gatei, M., K. Sloper, C. Sorensen, R. Syljuasen, J. Falck, K. Hobson, K. Savage, J. Lukas, B. B. Zhou, J. Bartek, and K. K. Khanna. 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278:14806-14811. [DOI] [PubMed] [Google Scholar]
- 19.Gu, J., L. Nie, D. Wiederschain, and Z. M. Yuan. 2001. Identification of p53 sequence elements that are required for MDM2-mediated nuclear export. Mol. Cell. Biol. 21:8533-8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs, S., S. Jitrapakdee, and J. C. Wallace. 1998. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem. Biophys. Res. Commun. 252:368-372. [DOI] [PubMed] [Google Scholar]
- 22.Hollstein, M., D. Sidransky, B. Vogelstein, and C. C. Harris. 1991. p53 mutations in human cancers. Science 253:49-53. [DOI] [PubMed] [Google Scholar]
- 23.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurz, E. U., and S. P. Lees-Miller. 2004. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amsterdam) 3:889-900. [DOI] [PubMed] [Google Scholar]
- 26.Lambert, P. F., F. Kashanchi, M. F. Radonovich, R. Shiekhattar, and J. N. Brady. 1998. Phosphorylation of p53 serine 15 increases interaction with CBP. J. Biol. Chem. 273:33048-33053. [DOI] [PubMed] [Google Scholar]
- 27.Lau, A., K. M. Swinbank, P. S. Ahmed, D. L. Taylor, S. P. Jackson, G. C. Smith, and M. J. O'Connor. 2005. Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nat. Cell Biol. 7:493-500. [DOI] [PubMed] [Google Scholar]
- 28.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., B. Damania, X. Alvarez, V. Ogryzko, K. Ozato, and J. U. Jung. 2000. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell. Biol. 20:8254-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, M., H. Lee, J. Guo, F. Neipel, B. Fleckenstein, K. Ozato, and J. U. Jung. 1998. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 72:5433-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, J., J. Chen, B. Elenbaas, and A. J. Levine. 1994. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8:1235-1246. [DOI] [PubMed] [Google Scholar]
- 32.Lin, J., X. Jin, C. Page, V. K. Sondak, G. Jiang, and R. K. Reynolds. 2000. A modified p53 overcomes mdm2-mediated oncogenic transformation: a potential cancer therapeutic agent. Cancer Res 60:5895-5901. [PubMed] [Google Scholar]
- 33.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 35.Lohrum, M. A., D. B. Woods, R. L. Ludwig, E. Balint, and K. H. Vousden. 2001. C-terminal ubiquitination of p53 contributes to nuclear export. Mol. Cell. Biol. 21:8521-8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maki, C. G., J. M. Huibregtse, and P. M. Howley. 1996. In vivo ubiquitination and proteasome-mediated degradation of p53(1). Cancer Res 56:2649-2654. [PubMed] [Google Scholar]
- 37.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc.Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGowan, C. H., and P. Russell. 2004. The DNA damage response: sensing and signaling. Curr. Opin. Cell Biol. 16:629-633. [DOI] [PubMed] [Google Scholar]
- 39.Meek, D. W. 2004. The p53 response to DNA damage. DNA Repair (Amsterdam) 3:1049-1056. [DOI] [PubMed] [Google Scholar]
- 40.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura, H., M. Li, J. Zarycki, and J. U. Jung. 2001. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 75:7572-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura, H., M. Lu, Y. Gwack, J. Souvlis, S. L. Zeichner, and J. U. Jung. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neil, J. C., E. R. Cameron, and E. W. Baxter. 1997. p53 and tumour viruses: catching the guardian off-guard. Trends Microbiol. 5:115-120. [DOI] [PubMed] [Google Scholar]
- 44.Nelson, W. G., and M. B. Kastan. 1994. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol. Cell. Biol. 14:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osborn, A. J., S. J. Elledge, and L. Zou. 2002. Checking on the fork: the DNA-replication stress-response pathway. Trends Cell Biol. 12:509-516. [DOI] [PubMed] [Google Scholar]
- 46.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78:761-771. [DOI] [PubMed] [Google Scholar]
- 47.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo, T., J. Park, D. Lee, S. G. Hwang, and J. Choe. 2001. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 75:6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325-334. [DOI] [PubMed] [Google Scholar]
- 50.Shieh, S. Y., Y. Taya, and C. Prives. 1999. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J. 18:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiloh, Y. 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11:71-77. [DOI] [PubMed] [Google Scholar]
- 52.Shirata, N., A. Kudoh, T. Daikoku, Y. Tatsumi, M. Fujita, T. Kiyono, Y. Sugaya, H. Isomura, K. Ishizaki, and T. Tsurumi. 2005. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J. Biol. Chem. 280:8156-8163. [DOI] [PubMed] [Google Scholar]
- 53.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 55.St. Clair, S., L. Giono, S. Varmeh-Ziaie, L. Resnick-Silverman, W. J. Liu, A. Padi, J. Dastidar, A. DaCosta, M. Mattia, and J. J. Manfredi. 2004. DNA damage-induced downregulation of Cdc25C is mediated by p53 via two independent mechanisms: one involves direct binding to the cdc25C promoter. Mol. Cell 16:725-736. [DOI] [PubMed] [Google Scholar]
- 56.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 424:516-523. [DOI] [PubMed] [Google Scholar]
- 57.Taylor, T. J., and D. M. Knipe. 2004. Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J. Virol. 78:5856-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unger, T., T. Juven-Gershon, E. Moallem, M. Berger, R. Vogt Sionov, G. Lozano, M. Oren, and Y. Haupt. 1999. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 18:1805-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vahteristo, P., A. Tamminen, P. Karvinen, H. Eerola, C. Eklund, L. A. Aaltonen, C. Blomqvist, K. Aittomaki, and H. Nevanlinna. 2001. p53, CHK2, and CHK1 genes in Finnish families with Li-Fraumeni syndrome: further evidence of CHK2 in inherited cancer predisposition. Cancer Res 61:5718-5722. [PubMed] [Google Scholar]
- 60.Wilkinson, D. E., and S. K. Weller. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 78:4783-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, Y. 2003. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 10:400-403. [DOI] [PubMed] [Google Scholar]
- 62.Yang, Y., C. C. Li, and A. M. Weissman. 2004. Regulating the p53 system through ubiquitination. Oncogene 23:2096-2106. [DOI] [PubMed] [Google Scholar]
- 63.Zimring, J. C., S. Goodbourn, and M. K. Offermann. 1998. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 72:701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]