Abstract
Foot-and-mouth disease virus (FMDV) is the causative agent of a highly contagious vesicular disease of cloven-hoofed animals. In the present study we use FMDV serotype C infection of swine to determine, by analytical techniques, the direct ex vivo visualization of virus-infected immune cells during the first 17 days of infection. We report, for the first time, that FMDV C-S8c1 can infect T and B cells at short periods of time postinoculation, corresponding with the peak of the viremia. There is a significant lymphopenia that involves CD3+ CD4− CD8+/−, CD3+ CD4− CD8+Tc, and CD3+ CD4+ CD8+ memory Th but not CD3+ CD4+ CD8− naïve Th lymphocytes. In addition, a profound depletion of the vast majority of peripheral T cells in lymph nodes and spleen is observed. This selective depletion of T cells is not due mainly to in situ death via apoptosis as visualized by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique. Thus, early infection of T cells by FMDV may be the main cause of the observed T-cell depletion. Importantly, this lack of T cells is reflected in a reduced response to mitogen activation, which in many cases is totally eliminated. These data suggest a mechanism by which the virus causes a transient immunosuppression, subvert the immune systems, and spreads. These results have important implications for our understanding of early events in the development of a robust immune response against FMDV.
Foot-and-mouth disease virus (FMDV), member of the Picornaviridae family, and the only member of the genus Aphthovirus, is the causative agent of the highly contagious disease of cloven-hoofed animals foot-and-mouth disease (FMD). Serologically, FMDV can be classified into seven antigenically distinct serotypes, O, A, C, South African Territories 1 (SAT1), SAT2, and SAT3, and Asia 1, and innumerable subtypes (20). Immunologically, there is no cross protection between serotypes. Infection with FMDV occurs commonly via the respiratory tract following inhalation of air-borne virus, with an incubation period of 2 to 8 days.
Ruminants can become carriers of the virus, presenting a potential reservoir of infection, while pigs are particularly important in virus dissemination, due to the large quantities of infectious virus excreted per day (24). Thus, rapid spread of the virus can even occur before clinical signs become apparent. Immunity against FMDV is documented as being primarily mediated by neutralizing antibodies (reviewed in reference 14), and vaccines consisting of inactivated, purified virus induce the production of neutralizing antibodies (14, 27). The induction of an effective long-lasting immune response is a difficult task when inactivated virus is used as the immunogen, even when utilizing immunomodulators in the formulation of the vaccine (3, 4). On the other hand, the duration of protection, the neutralizing antibody titer, and the average affinity of vaccine-induced responses are lower than those following natural infection. This, and the fact that neutralizing antibodies alone are not always sufficient for control of FMDV infection (2), indicates that cell-mediated immunity may play a role in the elimination of the virus. Thus, given that exposure to the virus results in production of T-cell-dependent neutralizing immunoglobulin (IgG) antibodies, and subsequent T-cell-dependent memory, it is clear that CD4+ cells must be stimulated (6, 7, 12, 30). The role of CD8+ T cells is not as clear as that of CD4+ T cells, although in cattle these cells appear to be activated during response to FMDV (5).
The effects of FMDV on the immune system of the host have not been widely studied in detail. Analyses during the acute phase of FMDV serotype O infection in swine showed a transient lymphopenia involving T cells (1), although B cells have not been evaluated. Also, the function of T cells during the acute phase of infection was significantly impaired. Secondary immunosuppression is a well-known consequence of some viral infections in animals and humans. Viruses such as feline leukemia virus, feline immunodeficiency virus, and human immunodeficiency virus induce profound, long-lasting immunosuppression in their hosts (8, 11). Other viruses as canine and feline parvoviruses may suppress immune responses temporarily (29). In pigs, several bacteria (Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae) and viruses such as porcine reproductive and respiratory syndrome virus, Aujeszky disease virus, and African swine fever virus are capable of causing immunosuppression, and render the animal more susceptible to secondary infections. Furthermore, these and some other swine pathogens (porcine parvovirus, swine influenza virus, African swine fever virus, and Salmonella spp.) are able to replicate in a variety of immune cells and impair their function (17).
The present work analyzes the immune response of pigs to FMDV serotype C over a period of 17 days postinoculation. We report, for the first time, that FMDV replicates in lymphocytes in vivo, more likely kills the cells and causes an immunosuppressive stage. This is characterized by profound lymphocyte depletion, affecting T and B cells, and an inhibition of T-cell response to mitogen. Moreover, we demonstrate that the levels of apoptotic cells in lymphoid tissues are not significant enough to explain the lymphoid depletion observed. Understanding the details of these effects as well as the immune response to the viral agent will allow more efficient and potentially more rapid vaccine approaches.
MATERIALS AND METHODS
Animals, virus, and experimental design.
Twenty Large White × Landrace pigs female 9 weeks old, clinically healthy and free of antibodies against African swine fever virus, classical swine fever virus, Aujeszky disease virus, FMDV, swine vesicular disease virus, and porcine reproductive and respiratory syndrome virus were used for this study. The animals were housed in isolation at the Centro de Investigación en Sanidad Animal in Valdeolmos, Spain. Sixteen animals were inoculated by the intradermal route in the coronary band of the right front limb with 105 PFU of FMDV C-S8c1 in 1 ml of phosphate-buffered saline (PBS). FMDV C-S8c1 is a plaque-purified derivative of natural isolate C1-Sta Pau-Spain 70, a representative of the European subtype C1 FMDV (26). The animals were slaughtered in batches of two animals at 1, 2, 3, 5, 7, 10, 14, and 17 days postinoculation. Four pigs were used as uninfected controls, housed in different boxes, and killed at the end of the experiment: two were noninoculated controls and two received an injection of 1 ml sterile PBS in the coronary band of the right front limb. All experiments with live animals were performed under the guidelines of the European Community (Directive 86/609/EEC) and were approved by the site ethical review committee.
Cell infection and infectious center assay.
Monolayers of BHK cells were infected with FMDV in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. Infection of purified swine peripheral blood lymphocytes (PBLs) with FMDV C-S8c1 was done in complete RPMI medium supplemented with 10% fetal bovine serum. The infectious center assay was done as follows. PBLs isolated from FMDV C-S8c1-infected pigs at different times postinoculation were serially diluted in PBS, and each diluted cell suspension was added to 35-mm plates containing BHK-21 cells at 90% confluence and incubated at 37°C for 2 h in 7% CO2. Then semisolid agar medium was added, and the cells were incubated for another 24 h before staining for plaque counting.
Isolation of peripheral blood lymphocytes.
PBLs were prepared from whole blood of C-S8c1-infected and control pigs. Briefly, fresh heparinized peripheral blood was mixed with an equal volume of phosphate-buffered saline (PBS). Ficoll-Hypaque (density 1.007 g/liter) was layered underneath the blood/PBS mixture and centrifuged 30 min at 900 × g. The mononuclear cell layer was transferred to another tube, washed and counted with trypan blue to determine viability. PBLs purified in this way were used directly for flow cytometry analysis or lymphoproliferation assays.
Cell staining and flow cytometry analysis.
Single cells were then obtained by mechanical disruption of lymphoid organs or by isolation of PBLs as described above. Cells were pelleted by centrifugation and resuspended in staining buffer (PBS containing 2% fetal bovine serum and 0.2% NaN3) for flow cytometry. To analyze the expression of cell surface molecules, we used monospecific antibodies, fluorochrome dyes, and flow cytometry, as described (25). The antibodies used were mouse anti-pig CD8-R-phycoerythrin and mouse anti-pig CD4-fluorescein isothiocyanate (all from BD Pharmingen, San Jose, CA). For B-cell staining, primary mouse anti-human CD21 antibody (clone B-ly4, BD Pharmingen, San Jose, CA) was used, followed by secondary antibody, anti-mouse conjugated to Alexa-488. After staining, cells were fixed in PBS/1% fetal bovine serum/4% (wt/vol) paraformaldehyde. Affinity-purified antibody to FMDV-3D (3H11) or FMDV-3A (2C2) was conjugated to Alexa-647 according to the protocol recommended by the manufacturer (Molecular Probes) and used for intracellular detection of FMDV 3D or FMDV 3A. Cells were acquired using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Dead cells were excluded on the basis of forward and side light scatter. Data were analyzed with FlowJo (Tree Star, San Francisco, CA) and CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Lymphoproliferation assay.
Proliferation assays were carried out in 96-well flat-bottomed microtiter plates. Cells were plated at a concentration of 4 × 106/ml, 100 μl/well in RPMI/10% (vol/vol) fetal bovine serum, and 0.05 mM 2-mercaptoethanol. Triplicate cultures were stimulated with concanavalin A (Sigma, St. Louis, MO) at a final concentration of 1 μg/ml. The cultures were incubated for 3 days at 37°C, 5% (vol/vol) CO2. They were then pulsed overnight with [3H]thymidine (Amersham Pharmacia), 0.5 μCi/well. The cells were harvested on to filter mats using a cell harvester and incorporation of radioactivity into the DNA was measured by liquid scintillation in a Microbeta counter (Pharmacia, Uppsala, Sweden). Data are expressed as stimulation index, calculated by dividing the mean cpm obtained in the stimulated cultures by the mean cpm obtained in the medium control cultures.
Tissue samples and immunohistochemical techniques.
Animals were anesthetized with Tiletamina and Zolacepam (5 mg/kg) and Atropina (100 mg/kg), and painlessly killed with a lethal dose of sodium pentobarbital (Dolethal). Tissue samples were taken and fixed in buffered formalin, 10%. After fixation, samples were dehydrated through a graded series of alcohol to xylol and embedded in paraffin wax. The avidin-biotin-peroxidase complex (Vectastain ABC kit elite, Vector) technique was used for the immunohistochemical detection of T cells with a monoclonal anti-CD3 antibody (Dako, Glostrup, Demark). After deparaffination and dehydration, endogenous peroxidase activity was quenched by incubation with 3% hydrogen peroxide in methanol for 45 min at room temperature. Then, tissue sections were rinsed in PBS and incubated with 10% normal goat serum (Sigma Chemical Company, Poole, Dorset, United Kingdom) for 30 min at room temperature. Primary antibody was incubated overnight at 4°C diluted in normal goat serum, 10%. A secondary goat anti-mouse immunoglobulin G (Dako, Glostrup, Denmark) diluted 1:100 in PBS was used. An ABC kit was used and 3,3′-diaminobenzidine tetrahydrochloride (Sigma), diluted 0.035% in Tris-buffered saline, pH 7.6, with 0.01% hydrogen peroxide was applied for 1 min as the chromogen. The slides were counterstained with Mayer hematoxylin for 1 min, dehydrated, and routinely mounted. Specific primary antibodies were replaced by PBS or normal mouse serum in tissue sections used as negative controls.
In situ detection of apoptosis.
Tissue sections were deparaffinized, rehydrated, and treated for quenching endogenous peroxidase activity as described above. Sections were permeabilized by incubation in 20 mg/ml proteinase K (Roche, Basel, Switzerland) in PBS for 20 min and washed twice for 5 min in PBS. The terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) method was used for the histological detection of apoptotic cells. The cells were detected with a kit which utilizes horseradish peroxidase (In Situ Cell Death Detection kit; Roche); it was used following the manufacturer's directions. The color reaction was developed with DAB and slides were counterstained with Mayer hematoxylin. Negative controls were always included in each series of sections assayed.
RESULTS
Lymphopenia during acute infection with FMDV.
To examine the early events of lymphocyte balance during FMDV infection, we designed the following experiment. Twenty Swiss White Landrace pigs were inoculated with 105 PFU of FMDV C-S8c1 and four pigs were used as uninfected controls. Animals inoculated with FMDV developed clinical signs, including fever and vesicle formation in the tongue, snout, and coronary bands of the feet. These symptoms were resolved and animals recovered by days 6 to 10 postinoculation. Two FMDV-inoculated animals per time point were slaughtered (1, 2, 3, 5, 7, 10, 14, and 17 days postinoculation), and blood and several tissue samples (spleen, thymus, superficial inguinal lymph node, prescapular lymph node, tonsil, and skin at the coronary band) were taken.
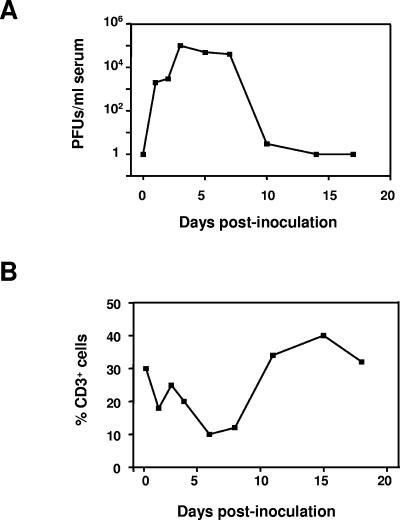
The analysis of total blood lymphocytes count showed a decrease in the percentage of lymphocytes starting at day 2 postinoculation and began to recover by day 10 (Fig. 1). Indeed, the drop in lymphocyte count coincided with the peak in viremia by day 2 or 3 postinoculation (Fig. 1). In order to determine which lymphocyte subsets were involved in the observed lymphopenia, PBLs from FMDV-infected and control animals were obtained and labeled with T- and B-cell-specific antibodies. Double labeling with anti-CD4 and anti-CD8 antibodies were used to differentiate T lymphocyte subpopulations.
FIG. 1.
Decrease in numbers of T cells at the peak of viral replication in pigs infected with FMDV C-S8c1. Large White × Landrace pigs were inoculated with 105 PFU of FMDV C-S8c1 in the coronary band and two pigs per day were sacrificed after inoculation. (A). Virus titers in serum were determined by plaque assay at the indicated time points postinoculation. Results represent the mean viral titers for two pigs per group per time point. (B) Percentage of CD3+ T cells in PBLs determined by flow cytometry analysis as described in Materials and Methods. Each point represents the mean for two animals per time point.
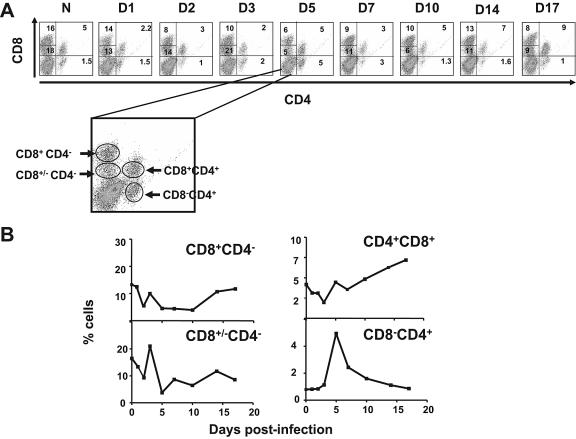
The lymphocyte populations CD3+ CD4− CD8+, containing cytotoxic T cells (CTL), and CD3+ CD4− CD4− /+ T cells were significantly depleted beginning as early as 2 days postinoculation (P < 0.05) (Fig. 2). The CTLs decreased by day 2 and at day 14 rebounded, although they never reached the level found in control animals. The CD3+ CD4+ CD8+/− T-cell subpopulation decreased by day 5 postinoculation, and, interestingly, by day 17 this cell subpopulation still represented 9% of the total lymphocytes, much below the value shown by naïve animals.
FIG. 2.
Decrease of the percentage of T-cell subpopulations in PBLs during acute infection of pigs with FMDV. PBLs were isolated (Materials and Methods) from FMDV C-S8c1-infected as well as uninfected (N) pigs. CD8+ and CD4+ cells obtained from PBLs were labeled with specific antibodies for these cell subsets (see Materials and Methods). (A). Dot plots showing flow cytometry analysis of double staining with anti-CD4 and anti-CD8 antibodies of PBLs. The magnified plot shows the different T-cell subpopulations determined by this technique: CD3+ CD8+ CD4− CTLs, CD3+ CD8+/− CD4− T cells, CD3+ CD4− CD4+ naïve T-helper cells, and CD3+ CD8+ CD4+ memory T cells. In each dot plot is indicated the percentage of each of these populations. (B) Based on the populations of cells determined by the dot plots, the curve plots show the percentage of each population over the 17-day observation period. Each point is the average for two pigs.
By contrast, the CD3+ CD4+ CD4− naïve T-helper cells were not depleted and immediately, starting at day 3 postinoculation, their percentage increased significantly. Such an increase peaked at day 5 postinoculation, declining until day 14, when normal levels were reached. Similarly, the CD3+ CD4+ CD8+ T-cell subset, containing memory T-helper lymphocytes, showed a significant decline at days 1 to 3 postinoculation but started to recover by day 5 with a substantial expansion until day 17 postinoculation, the last day analyzed. These results indicate that CTLs and CD4+ CD8+/− T cells were depleted at the onset of the viremia but unable to expand after the virus was cleared from serum. Conversely, the T helper cells, memory and naïve, were slightly depleted at the peak of the viremia (days 2 to 3 postinoculation) but rapidly expanded by day 5 postinoculation.
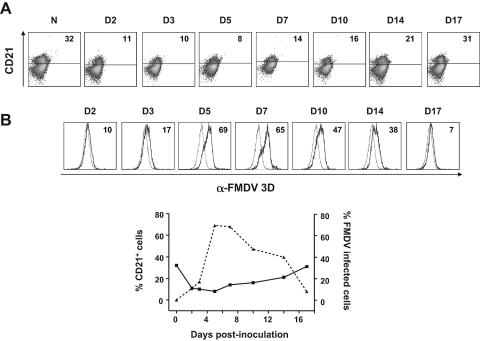
We next evaluated the B-cell composition in PBLs from FMDV-infected and control animals by CD21 staining and flow cytometry analyses. Similar to T cells, CD21+ cells were significantly depleted starting at day 1 postinoculation (Fig. 3A). However, at day 7 postinoculation CD21+ cells started to recover until day 17, when a normal value of B cells was obtained. Therefore, there was depletion of B cells at the onset of the viremia that went back to normal levels once the virus had been cleared.
FIG. 3.
Replication of FMDV-C-S8c1 in CD21+ B lymphocytes. PBLs isolated from FMDV-infected pigs were labeled with CD21 monoclonal antibody to determine the percentage of B cells. (A). Dot plots showing the percentage of CD21-positive cells determined by flow cytometry analyses. The number on each dot plot refers to the percentage of CD21+ cells. (B) Double staining with CD21 and 3H11-Alexa 647 to determine the percentage of FMDV-infected B cells. The histograms show the CD21+ cells positive for 3H11 in PBLs from FMDV-infected (dark line) and uninfected (light line) pigs. The number in each histogram shows the cells positive for 3H11 over the background of uninfected pigs. The curve plot shows the percentage of CD21+ cells and 3H11+ CD21+ cells over the 17-day period analyzed.
Lymphocyte depletion in secondary lymphoid organs.
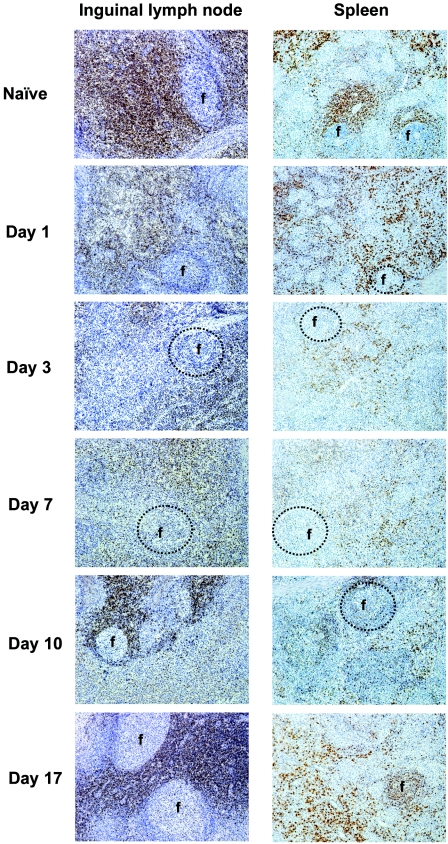
Early T-cell depletion in the periphery of FMDV-infected pigs may be explained either by altered trafficking or by cell death. In order to evaluate the possibility of altered T-cell traffic, we examined the T-cell distribution in different lymphoid compartments following FMDV infection, using immunohistochemistry of tissue sections with anti-CD3 antibody. We found that depletion of total T cells occurred in inguinal lymph nodes, spleen (Fig. 4), and prescapular lymph node (data not shown). By day 1 postinoculation, the number of T cells was significantly diminished and by day 3 postinoculation there were barely any T cells in all the organs observed.
FIG. 4.
Immunohistochemical staining of CD3+ cells of spleen and inguinal lymph node from uninfected and FMDV C-S8c1-infected pigs. Tissue sections (7 μm) were immunostained as described in the Materials and Methods section with anti-CD3 antibody. The chromogen used in the immunostaining procedure was diaminobenzidine, which gives a brown product where primary antibody binds; sections were counterstained with Mayer's hematoxylin. Five time points were chosen as representative of the observed 17-day period. Circles indicate follicular areas (f) or where they should have been in those tissue sections where follicular areas were missing (days 1, 3, and 7 postinoculation).
Starting at day 10 postinoculation, an increase in T-cell number was observed until day 17 postinoculation, when the number of T cells was very similar to that of naïve animals, mainly in the lymph nodes. In the spleen, however, the number of T cells at day 17 postinoculation was still below the normal levels. Moreover, a loss of lymphoid follicles (B-cell-dependent areas mainly), and reduced cellularity in parafollicular T-cell-dependent areas were observed by day 3 postinoculation (Fig. 4, circles show where follicles were expected to be).
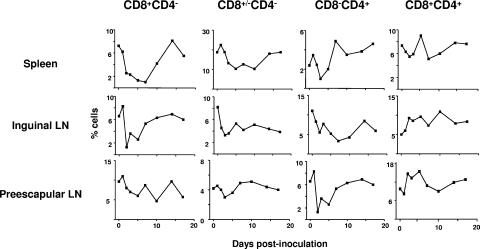
We next determined by flow cytometry the population of T cells that were depleted in these lymphoid organs. Double staining with anti-CD4 and anti-CD8 antibodies was used to quantify the percentage of each T-cell population. Thus, we observed a profound decline by day 3 postinoculation of CD3+ CD4− CD4+ T cells in the spleen and prescapular lymph node (Fig. 5), and values started to recover by day 5 postinoculation. However, in the superficial inguinal lymph node, the deepest decline was by day 7 postinoculation and recovered by day 14 postinoculation. The CD3+ CD8+ CD4− and CD3+ CD8+/− CD4− T cells declined by day 5 postinoculation in all tested organs, and reached normal levels by day 14 postinoculation.
FIG. 5.
T-cell populations in spleen and lymph nodes from FMDV-infected pigs. Cells were isolated from the spleen, inguinal lymph node, and prescapular lymph node and double stained with CD4 and CD8 monoclonal antibody (Material and Methods). The graphs show the percentage of each T-cell population obtained by flow cytometry analyses (see Materials and Methods and Fig. 2) at different times postinoculation. Each time point is the average for two animals.
In general, the reduction in the number of CD3+ CD8+ CD4− and CD3+ CD8+ /− CD4− T cells found in lymphoid organs was very similar to that found in blood. Double CD4/CD8-positive cells were increasing with the time in both lymph nodes (inguinal and prescapular) but not in the spleen, where a decline starting at day 1 postinoculation was observed. In this organ, the CD3+ CD8+ CD4+ cells started to recover by day 5 postinoculation. Interestingly, in the prescapular lymph node there was an expansion between days 2 and 7 postinoculation of double-positive cells and a decline started at day 10 postinoculation, although at day 17 normal levels were reached. Thus, the results indicate that depletion of T cells can be associated with a decrease of CD8-positive cells and, to a minor extent, of CD4-positive cells. Furthermore, although it is difficult to completely rule out the possibility that some cells may have been trafficking to unusual sites that we have not examined, the results indicate that the observed depletion of T cells is unlikely to be attributable to redistribution of these cells into organs.
Infection of T cells by FMDV.
To combine phenotypic characterization of various immune cell subsets with the detection of viral antigen, we established a flow cytometry-based assay using the FMDV 3D-specific monoclonal antibody 3H11 and the FMDV 3A-specific monoclonal antibody 2C2. The 3D and 3A proteins were chosen as suitable target antigens because expression of both proteins requires viral replication.
PBLs from FMDV-infected pigs at different times postinoculation were stained for surface markers and intracellular FMDV 3D or 3A as described in Materials and Methods. Viral 3D and 3A were readily detectable in all major T lymphocyte populations although only data for 3D staining is shown for simplification (Fig. 6). The CD3+ CD8+/− CD4− T-cell subset positive for viral antigen increased over time so that by day 10 postinoculation 15% of CD8+/− CD4− T cells contained FMDV antigens, to decline to almost undetectable levels of viral antigen by day 17 postinoculation. In contrast, the percentage of viral antigen-positive cells in the subpopulation of CD3+ CD8+ CD4− αβ-CTLs increased to 10% by day 3 postinoculation and dropped to less than 5% by day 5 postinoculation. By day 7 postinoculation this percentage increased again to 10% to reach a maximum of positive cells at 10 days postinoculation.
FIG. 6.
Replication of FMDV in subpopulations of T cells. T cells from PBLs were stained with anti-CD4 and -CD8 antibodies and cytometric analysis was carried out. Intracellular staining with monoclonal antibody 3H11 specific for FMDV 3D protein determined the percentage of double-positive cells for 3H11-Alexa647 and CD8+ CD4− cells (A), CD8+ CD4+ cells (B), CD8+ /− CD4− cells (C), and CD4− CD4+ cells (D). The histograms show the percentage of 3H11-positive cells in each T-cell population of infected (dark line) and uninfected control (dotted plot) pigs. The number indicates the percentage of 3H11-positive cells over the control pigs. The curve plots for each T-cell population show the percentage of infected cells over the 17 days analyzed.
The infectivity curve of CD3+ CD8+ CD4+ memory T cells was very similar to that of CTLs, showing a sudden drop by day 5 postinoculation and a maximum of infection by day 10 postinoculation. The CD3+ CD4− CD4+ naïve Th lymphocytes showed the highest percentage of viral antigen-positive cells of all the T-cell subsets examined. By day 1 postinoculation more than 25% of cells were positive for viral antigen, and this dropped markedly by day 5 to almost undetectable levels. By day 10 postinoculation about 20% of cells were positive for viral antigen and this declined to undetectable levels by day 17. The label of PBLs with CD21 and FMDV 3D monoclonal antibody showed a higher percentage of positive cells for viral antigen on B cells (more than 60% by day 5 postinoculation) (Fig. 3B).
In view of the above data, we investigated whether the cells positive for viral antigen were productively infected with FMDV. Our flow cytometry-based assay allows rapid detection of viral antigen in defined immune cell subsets, yet it does not measure the level of infectious virus that may be present, and the uptake of viral antigens in the absence of actual infection cannot be excluded. In order to evaluate this, we cocultured PBLs from infected swine with monolayers of BHK cells, which are susceptible to FMDV infection. Previous to adding the PBLs to BHK cells, the PBLs were treated with a phosphate buffer of pH 6.0 to eliminate any virus bound to the cellular membrane that could account for the infectivity.
These cocultures yielded cytopathic effect at 24 h, similar to diluted serum from blood samples (Fig. 7A). In addition, an infectious center assay on BHK cells was done to quantify viral production by cells. More that 103 PFU per 104 cells were found by day 5 postinoculation, declining by day 14 postinoculation to almost undetectable levels (Fig. 7B). Thus, the pattern of infection found by flow cytometry really reflected a productive infection of lymphocytes by FMDV.
FIG. 7.
Viral production from PBLs isolated from FMDV C-S8c1-infected pigs. (A) PBLs were cultured over BHK cells susceptible to FMDV infection. BHK cells at 24 h after addition of PBLs from infected pigs. The PBLs were isolated from pigs infected at day 5, 10, and 17 postinoculation. Complete cytopathic effect can be observed from cocultured BHK cells and PBLs from pigs at day 5 postinoculation. (B) Infectious center assay of PBLs from infected pigs on BHK cells. (C) Replication curve of FMDV C-S8c1 on PBLs. PBLs from naïve pigs were infected in vitro with FMDV C-S8c1 and viral production was determined. Triangles correspond to infection wash with PBS after adsorption; squares correspond to infection wash with pH 6.0 phosphate buffer after adsorption.
In order to reinforce the concept that FMDV infects PBLs productively, we infected PBLs isolated from a naïve pig in vitro with FMDV C-S8c1 at a multiplicity of infection of 5 PFU/cell. Cells were washed with pH 6.0 phosphate buffer or PBS after the adsorption time, and supernatants were harvested for titration on BHK cells. The results (Fig. 7C) show a productive infection of PBLs by FMDV C-S8c1. These data clearly demonstrate that FMDV C-S8c1 is able to infect PBLs in vitro as well as in vivo.
Apoptosis in secondary lymphoid tissues.
We considered the possibility that in situ cell death was responsible for the lymphoid depletion. In order to determine rates of apoptosis in several peripheral lymphoid organs, we visualized DNA fragmentation directly by the in situ TUNEL assay on tissue sections. In spleen sections, by day 2 postinoculation, a much reduced number of positive cells were found in the white pulp area, and the number of positive cells did not increase in the following days (Fig. 8). However, although at 2 days postinoculation several apoptotic cells could be visualized in the follicular areas in the inguinal lymph node, these apoptotic cells were also observed by day 14 postinoculation (Fig. 8), suggesting that a percentage of cells in the lymph node but not in the spleen of FMDV-infected pigs undergo apoptosis.
FIG. 8.
Apoptosis in spleen and inguinal lymph node from FMDV C-S8c1-infected pigs determined by TUNEL staining. Inguinal lymph node and spleen sections were staining using the TUNEL technique (see Materials and Methods). Five time points were chosen as representative of the observed 17-day period. Circles indicate where follicular areas are or should be (f).
Inhibition of T-cell proliferation.
We next determined whether the remaining T cells in the circulation were functional in inducing cellular immunity. We tested for the ability of whole PBLs to respond to the mitogen concanavalin A. In uninfected animals, there was an easily detected response to concanavalin A (Fig. 9). In contrast, starting at 1 day postinoculation, the capacity of the T cells to respond to concanavalin A was greatly reduced (stimulation index, ≅50 versus ≅150 in naïve animals). This weak T-cell response was found until day 7 postinoculation. By day 10 postinoculation, the T-cell response started to recover to reach a stimulation index higher than that in naïve animals by day 17 postinoculation. Stimulation of cells with higher concentrations of concanavalin A did not result in an increase in the response of T cells from those animals that are unable to respond to concanavalin A (data not shown). Thus, the virus is more likely involved in inhibiting T-cell function in the residual T cells.
FIG. 9.
T-cell proliferation in response to mitogen stimulation during acute infection of swine with FMDV. The T-cell proliferation response of PBLs isolated from pigs infected with FMDV C-S8c1 or control animals (naïve) to the mitogen concanavalin A was determined. Data are reported as the stimulation index. Standard deviations are indicated.
DISCUSSION
In this study we have provided a comprehensive analysis of the effects on lymphocytes of acute infection of swine with FMDV. Overall, we observe a profound lymphopenia and lymphoid depletion in all the lymphoid organs analyzed. This lymphoid depletion affects all T-cell subsets as well as B lymphocytes. More importantly, we describe, for the first time, that FMDV is able to replicate in lymphocytes in vivo. This viral replication may result in lymphoid depletion, since the levels of cellular death by apoptosis are not high enough to explain the significant decrease in lymphocyte levels. Moreover, those lymphocytes that survive do not respond to mitogen activation, indicative of an immunosuppressive state in the infected animals. The most important questions arising from these observations relate to the survival, phenotype, and function of virus-infected immune cells, as well as integration of these findings into the altered capacity of the host to mount an effective immune response.
During the observed time of the infection (days 1 to 17 postinoculation), lymphopenia started when virus could be detected in blood, corresponding to the onset of the viremia, and lymphocyte levels recovered slightly by day 14, although they never reached the levels found in naïve animals (Fig. 1). This lymphopenia affects mainly CD8+ CD4− T cells (CD8+ CD4− and CD8+/− CD4−). Unlike some other cytopathic viruses, control of which has been shown to be independent of CD8+ T cells, it has been established that CD8+ T cells participate in the immune response to FMDV in cattle (5), although nothing is known for pigs.
FMDV infection results in a rapid reduction of major histocompatibility complex (MHC) class I expression on susceptible cells (22), which could impair presentation of viral peptides by FMDV-infected cells to CTLs, facilitating virus escape from this particular antiviral response of the host. On the other hand, monoclonal antibodies against CD8+ or anti-MHC class I molecules can inhibit proliferation of immune swine lymphocytes when stimulated with viral particles (5, 18). However, the precise contribution of CD8+ lymphocytes during the onset of the immune response against FMDV is still unclear.
In contrast to CD8+ T cells, CD4+ cells seem to expand at the peak of the viremia as might be expected, since they are essential for the production of neutralizing antibodies by B cells. This expansion is observed in blood but not in lymphoid organs, where a profound depletion of CD4+ T cells is found. This would have consequences in terms of the immunocompromising of the animals, not only in the face of FMDV, but also with respect to concomitant or secondary infections.
Our data regarding viral infection of lymphocytes suggest that the lymphoid depletion and T-cell dysfunction could be due to viral replication in lymphocytes. We need first to address whether the cells positive for viral antigen are productively infected with FMDV. Uptake of viral antigens by T cells in the absence of actual infection seems unlikely for several reasons: our own data show that coculture of PBLs with susceptible BHK cells yields cytopathic effect according to the percentage of cells positive to viral antigen by flow cytometry. We have shown productive infection of PBLs in vitro by FMDV C-S8c1. The expression of nonstructural proteins necessitates of viral replication, and this is the case for the detection of the 3D protein by monoclonal antibody. In fact, display of an FMDV-3D epitope relies on 3D neosynthesis rather than a long-lived pool of cellular 3D or encapsidated proteins. Together, these considerations indicate that the presence of viral 3D in T cells is the consequence of continuous replication, translation, and posttranslational processing of viral proteins, rather than the acquisition of isolated 3D in the absence of infection.
The infection of lymphocytes by FMDV described in this report seems to be unique for serotype C, since others have reported the lack of infection of lymphocytes in vivo by FMDV serotype O (1). FMDV, like other RNA viruses, exists and replicates as complex and dynamic mixtures of related genomes termed viral quasi-species (9, 10). This spectrum of related mutants is reflected in a continuous genetic variation with several biological consequences, affecting, among others, viral pathogenesis. Initial infection of different cell types by the two groups of viruses (serotype C and serotype O) may provide these viruses with access to different sites, and with different disease manifestations. Indeed, the infection of lymphocytes by a virus may alter the immune response elicited by the pathogen in a way still unexplored for FMDV. To our knowledge, no differences have been reported between the immune responses against serotypes C and O or other serotypes, although this may need additional investigation. Therefore, the case reported here is clear evidence of the implications of genetic variation of FMDV in vivo.
Taking into account other viral infections (16, 21, 23, 28), it appears that when apoptosis is implicated in the pathogenesis of lymphocyte depletion lesions, its incidence is usually increased and can be demonstrated by in situ detection in lymphoid tissues. Moreover, it appears that higher rates of apoptosis are found during the acute phase of infection, when active virus replication occurs. For example, this is the case in classical swine fever virus (28), African swine fever virus (15, 19), and chicken anemia virus (13) infections. Nevertheless, in our study, no increased apoptotic rates were found in any lymphocyte-depleted lesional stage. Thus, the emerging picture suggests that FMDV is not causing apoptosis of virus-infected lymphocytes in vivo. More likely the virus is destroying the cells and this is reflected in depletion and immunosuppression during the acute stage of the disease. Nevertheless, this is currently a topic that we are investigating in our laboratory.
The function of the residual T cells, measured by the capacity to proliferate in response to mitogen activation, is clearly impaired. Considering the ability of the virus to infect lymphocytes, one of the mechanisms to prevent antiviral response is not only to destroy T cells but to interfere with T-cell function. In this way, the virus may create a favorable window time in which it can expand and be shed to the environment, favoring transmission. On the other side, the simple interaction between FMDV and lymphocytes could be insufficient to impair T-cell function. Further studies on the interaction of FMDV with other lymphoid cells such as dendritic cells are required to understand the network.
In conclusion, this study provides the first evidence that FMDV can infect lymphocytes in vivo, creating an immunosuppressive stage in which the virus is able to spread. This starts very shortly after infection, suggesting that the early innate immune system is affected. The cooperation between the innate and adaptive immune response shifts the balance between induction of an immune response and virus spread in favor of the immune response. The understanding of this cooperation in FMD would provide a basis for the design of better vaccines against this harmful animal disease.
Acknowledgments
We thank Esteban Domingo for invaluable discussions, support, and critical reading of the manuscript and Javier Domínguez for discussion and suggestions. We thank Marta Sanz-Ramos for great help with the PBL isolation and FACS staining.
This work was supported by grants AGL2004-00499 and BMC2001-1823-C02-01 from the MEC (Spain) and PAI AGR-0137 from the Andalucia Research Plan (Spain).
REFERENCES
- 1.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T-cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 92:61-73. [DOI] [PubMed] [Google Scholar]
- 2.Becker, Y. 1994. Need for cellular and humoral immune responses in bovines to ensure protection from foot-and-mouth disease virus (FMDV)-a point of view. Virus Genes 8:199-214. [DOI] [PubMed] [Google Scholar]
- 3.Brown, F. 1999. Control of foot-and-mouth disease by vaccination. Dev. Biol. Stand. 100:131-135. [PubMed] [Google Scholar]
- 4.Brown, F. 1992. New approaches to vaccination against foot-and-mouth disease. Vaccine 10:1022-1026. [DOI] [PubMed] [Google Scholar]
- 5.Childerstone, A. J., L. Cedillo-Baron, M. Foster-Cuevas, and R. M. Parkhouse. 1999. Demonstration of bovine CD8+ T-cell responses to foot-and-mouth disease virus. J. Gen. Virol. 80:663-669. [DOI] [PubMed] [Google Scholar]
- 6.Collen, T., J. Baron, A. Childerstone, A. Corteyn, T. R. Doel, M. Flint, M. Garcia-Valcarcel, R. M. Parkhouse, and M. D. Ryan. 1998. Heterotypic recognition of recombinant FMDV proteins by bovine T cells: the polymerase (P3Dpol) as an immunodominant T-cell immunogen. Virus Res. 56:125-133. [DOI] [PubMed] [Google Scholar]
- 7.Collen, T., L. Pullen, and T. R. Doel. 1989. T-cell-dependent induction of antibody against foot-and-mouth disease virus in a mouse model. J. Gen. Virol. 70:395-403. [DOI] [PubMed] [Google Scholar]
- 8.Cotran, R. S., V. Kumar, and T. Collins. 1999. Diseases of immunity. W.B. Saunders Company, Philadelphia, Pa.
- 9.Domingo, E., C. Biebricher, M. Eigen, and J. J. Holland. 2001. Quasispecies and RNA virus evolution: principles and consequences. Landes Bioscience, Austin, Tex.
- 10.Domingo, E., C. M. Ruiz-Jarabo, A. Arias, and C. Escarmis. 2004. Quasispecies dynamics and evolution of FOot-and-mouth disease virus, p. 261-304. In E. Domingo (ed.), Foot-and-mouth disease: current perspectives. Horizon Bioscience, Norfolk, England.
- 11.English, R. V., C. M. Johnson, D. H. Gebhard, and M. B. Tompkins. 1993. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 67:5175-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass, E. J., and P. Millar. 1994. Induction of effective cross-reactive immunity by FMDV peptides is critically dependent upon specific MHC-peptide-T-cell interactions. Immunology 82:1-8. [PMC free article] [PubMed] [Google Scholar]
- 13.Jeurissen, S. H., F. Wagenaar, J. M. Pol, A. J. van der Eb, and M. H. Noteborn. 1992. Chicken anemia virus causes apoptosis of thymocytes after in vivo infection and of cell lines after in vitro infection. J. Virol. 66:7383-7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough, K. C., F. De Simone, E. Brocchi, L. Capucci, J. R. Crowther, and U. Kihm. 1992. Protective immune response against foot-and-mouth disease. J. Virol. 66:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oura, C. A., P. P. Powell, and R. M. Parkhouse. 1998. African swine fever: a disease characterized by apoptosis. J. Gen. Virol. 79:1427-1438. [DOI] [PubMed] [Google Scholar]
- 16.Resendes, A. R., N. Majo, J. Segales, E. Mateu, M. Calsamiglia, and M. Domingo. 2004. Apoptosis in lymphoid organs of pigs naturally infected by porcine circovirus type 2. J. Gen. Virol. 85:2837-2844. [DOI] [PubMed] [Google Scholar]
- 17.Roth, J. A. 1999. The immune system. Iowa State University Press, Ames, Iowa.
- 18.Saiz, J. C., A. Rodriguez, M. Gonzalez, F. Alonso, and F. Sobrino. 1992. Heterotypic lymphoproliferative response in pigs vaccinated with foot-and-mouth disease virus. Involvement of isolated capsid proteins. J. Gen. Virol. 73:2601-2607. [DOI] [PubMed] [Google Scholar]
- 19.Salguero, F. J., P. J. Sanchez-Cordon, M. A. Sierra, A. Jover, A. Nunez, and J. C. Gomez-Villamandos. 2004. Apoptosis of thymocytes in experimental African Swine Fever virus infection. Histol. Histopathol. 19:77-84. [DOI] [PubMed] [Google Scholar]
- 20.Samuel, A. R., and N. J. Knowles. 2001. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes). J. Gen. Virol. 82:609-621. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Cordon, P. J., S. Romanini, F. J. Salguero, A. Nunez, M. J. Bautista, A. Jover, and J. C. Gomez-Villamos. 2002. Apoptosis of thymocytes related to cytokine expression in experimental classical swine fever. J. Comp. Pathol. 127:239-248. [DOI] [PubMed] [Google Scholar]
- 22.Sanz-Parra, A., F. Sobrino, and V. Ley. 1998. Infection with foot-and-mouth disease virus results in a rapid reduction of MHC class I surface expression. J. Gen. Virol. 79:433-436. [DOI] [PubMed] [Google Scholar]
- 23.Sato, M., O. Mikami, M. Kobayashi, and Y. Nakajima. 2000. Apoptosis in the lymphatic organs of piglets inoculated with classical swine fever virus. Vet. Microbiol. 75:1-9. [DOI] [PubMed] [Google Scholar]
- 24.Sellers, R. F., A. I. Donaldson, and K. A. Herniman. 1971. Foot-and-mouth virus. Lancet i:1238. [DOI] [PubMed] [Google Scholar]
- 25.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de La Torre, and M. B. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobrino, F., M. Davila, J. Ortin, and E. Domingo. 1983. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology 128:310-318. [DOI] [PubMed] [Google Scholar]
- 27.Strohmaier, K., R. Franze, and K. H. Adam. 1982. Location and characterization of the antigenic portion of the FMDV immunizing protein. J. Gen. Virol. 59:295-306. [DOI] [PubMed] [Google Scholar]
- 28.Summerfield, A., S. M. Knotig, and K. C. McCullough. 1998. Lymphocyte apoptosis during classical swine fever: implication of activation-induced cell death. J. Virol. 72:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trautwein, G., and M. Hewicker-Trautwein. 1994. Immunopathogenesis of virus diseases of cats and dogs. Tierarztl Prax. 22:63-72. (In German.) [PubMed] [Google Scholar]
- 30.van Lierop, M. J., K. van Maanen, R. H. Meloen, V. P. Rutten, M. A. de Jong, and E. J. Hensen. 1992. Proliferative lymphocyte responses to foot-and-mouth disease virus and three FMDV peptides after vaccination or immunization with these peptides in cattle. Immunology 75:406-413. [PMC free article] [PubMed] [Google Scholar]