Abstract
We investigated the replication and transmission of avian influenza A viruses in two species thought to be intermediate hosts in the spread of influenza A viruses in live poultry markets: Chinese ring-necked pheasants and chukar partridges. All 15 hemagglutinin subtypes replicated in pheasants, and most subtypes transmitted to naïve contact pheasants, primarily via the fecal-oral route. Many viruses were shed from the gastrointestinal tract of experimentally inoculated pheasants for 14 days or longer. Virus was isolated from the cloacal swabs of one contact pheasant for an unprecedented 45 days. Chukar partridges were less susceptible to infection with avian influenza viruses. The viruses that replicated in chukar partridges were isolated for 7 days after experimental inoculation, predominantly from the respiratory tract. We detected high neutralizing antibody titers with correspondingly low levels of serum hemagglutination inhibition antibody titers in pheasants and chukar partridges when chicken red blood cells were used in serological analyses. When horse erythrocytes were used, antibody titers were comparable to those obtained by using the neutralization assay. More importantly, the results suggested that pheasants can serve as a reservoir of influenza virus. Because of their continuous asymptomatic infection and longer stay in the markets, pheasants are ideal “carriers” of influenza A viruses. Their continued presence in live markets contributes to the perpetuation and genetic interaction of influenza viruses there. On the basis of our findings, it does not make good sense to ban quail but not pheasants from the live markets.
Influenza A viruses are a major disease problem in birds and humans as well as in some lower mammals. Since the first reported isolation of influenza A virus from wild birds in 1961, influenza A viruses have been isolated from 90 avian species representing 12 of the 50 orders of birds (2, 35). In waterfowl, almost every combination of hemagglutinin (HA) and neuraminidase subtypes have been isolated; the most common subtypes are H3, H4, and H6 (11, 17). Therefore, wild aquatic birds, such as ducks and geese, serve as the natural reservoir for influenza A viruses. A growing body of evidence shows that stable lineages of influenza viruses are being established in chickens. In domestic poultry, such as chickens, the most prominent influenza A viruses include the H3, H5, H6, H7, and H9 subtypes (18).
The emergence of influenza A viruses that threaten both human and veterinary public health continues to concern us. The H2, H5, H7, and H9 subtypes are considered to have high pandemic potential, but all 16 subtypes may possess this potential (41). The threat posed by the continuing evolution and interspecies transmission of H5N1 influenza viruses became apparent in 1997, when 6 of 18 infected humans died in Hong Kong (6). This incident marked the first time that H5N1 avian influenza A viruses had been transmitted directly to humans. The viruses came from poultry in the live animal markets (wet markets), which have more recently been identified as breeding grounds for both influenza and severe acute respiratory syndrome corona virus (3, 28, 30, 42). Live bird retail markets are widespread throughout Southeast Asia and also operate in some U.S. cities. These markets house both terrestrial and aquatic birds such as chickens, pigeons, ducks, geese, quail, pheasants, chukar partridges, and guinea fowl. Chickens and pigeons are the predominant species sold. This mix of birds provides the ideal conditions for interspecies transmission and propagation of influenza A viruses. After the 1997 H5N1 incident in Hong Kong, ducks and geese, the primary sources of influenza virus, were removed from the markets, imported separately, and sold killed and chilled. Practices in Hong Kong's live poultry markets were further changed after the H5N1 virus reemerged in 2001 and 2002 (32). During these later outbreaks, researchers determined that quail support the replication of at least 14 of the 15 HA subtypes of influenza viruses as well as swine influenza viruses of the H1 and H3 subtypes (19). However, “minor poultry” (pheasants, chukar partridges, and guinea fowl) are still sold in the live bird markets; because of their higher price, they tend to stay in these markets longer than other poultry, such as chickens, that remain in the markets only a day or two (8).
Limited reports indicate that influenza A viruses can replicate in these minor poultry species. An avian influenza virus of the H5N2 subtype was isolated from a dead pen-raised chukar partridge during a wildlife survey conducted after an outbreak of lethal avian influenza in 1983 in Pennsylvania (21). The H5N2 virus (A/Chicken/Pennsylvania/1370/83 [A/CK/PA/1370/83]) isolated from a chicken during this outbreak was later used to experimentally inoculate ducks, gulls, and pheasants (45). In this experiment, all birds were susceptible to infection with A/CK/PA/1370/83. Pheasants shed the virus in feces for up to 15 days, although most showed no clinical signs of disease, and those that did show signs recovered within 2 days (45). In another study, influenza A virus of the H9N2 subtype was isolated from adult ring-necked pheasants for the first 7 days after experimental inoculation, and it was transmitted to contact birds, which showed no apparent clinical signs of disease (12). Their longer stays in the markets may enable these birds to act as asymptomatic carriers of influenza viruses for days to weeks. These avian species' role in the introduction and spread of influenza to domestic poultry remains unclear. However, it is clear that we need to know more about the scope of the avian reservoir. Not only do we need to know which species are infected but we also need to know whether they are hosts to the viruses. Our study examined the replication and transmission of influenza A viruses representative of 15 HA subtypes in pheasants and chukar partridges to better understand the role of these species in the ecology and evolution of the viruses. Our results suggest that replication and transmission of influenza A viruses are species dependent and that pheasants shed virus for prolonged periods of time. Chukar partridges were less susceptible to infection with the viruses tested in this study than were pheasants.
MATERIALS AND METHODS
Viruses.
The viruses used in this study (Table 1) were sent to the repository of St. Jude Children's Research Hospital from World Health Organization laboratories, particularly the one at Hong Kong University. Because wild aquatic birds such as ducks are the natural reservoir for influenza viruses, we chose the most recent viruses isolated from wild ducks for use in this study. We further restricted the subtypes to Eurasian isolates, because Asia is considered to be the epicenter of influenza virus pandemics (31). Because H13 has not been isolated from ducks, an isolate from a gull was selected for this influenza virus subtype (34). Representatives of each virus subtype most frequently isolated from the American lineage were also selected for this study. Viruses of the American lineage were used by Makarova et al. (19) for studies of quail (Coturnix coturnix), which will allow for a direct comparison between quail and the birds used in this study. Representatives of each subtype of influenza viruses isolated from humans (H1N1 and H3N2) and swine (H1N1 and H3N2) were included because we wanted to determine whether mammalian viruses can establish respiratory infections in these birds. The human viruses used in this study (A/HK/1/68 [H3N2] and A/USSR/90/77 [H1N1]) are egg adapted with a minimum number of passages (three to five) in embryonated chicken eggs. All viruses were propagated in 10-day-old embryonated chicken eggs, and the 50% egg infectious dose (EID50) was determined by the Reed and Muench method (Table 1) (24). Hemagglutinin subtypes were confirmed using subtype-specific antisera in a hemagglutination inhibition (HI) assay (22).
TABLE 1.
Influenza A viruses used in this study
| HA subtype | Influenza A virus | Abbreviation | Virus titer (EID50 [log10/ml])
|
|
|---|---|---|---|---|
| In original virus stock | In pheasant drinking water (3 dpi) | |||
| Avian viruses | ||||
| H1 | A/Wild Duck/Shantou/520/01 (H1N9) | DK H1 | 10.00 | 6.25 |
| H2 | A/Duck/Nanchang/2-0492/00 (H2N9) | DK H2 | 8.75 | 5.50 |
| H3 | A/Duck/Korea/S10/03 (H3N2) | DK H3 | 9.25 | 2.50 |
| A/Mallard/Alberta/31/01 (H3N9) | Mal H3 | 8.25 | 4.50 | |
| H4 | A/Duck/Mongolia/218/01 (H4N6) | DK H4 | 9.25 | 5.00 |
| A/Mallard/Alberta/119/00 (H4N6) | Mal H4 | 8.25 | 3.75 | |
| H5 | A/Duck/Hokkaido/447/00 (H5N3) | DK H5 | 9.25 | <1.00 |
| H6 | A/Duck/Shantou/5540/01 (H6N2) | DK H6 | 8.75 | 4.00 |
| A/Mallard/Alberta/206/96 (H6N8) | Mal H6 | 9.30 | 5.50 | |
| H7 | A/Mallard/Netherlands/12/03 (H7N3) | Mal H7 | 9.25 | 6.50 |
| H8 | A/Mallard/Alberta/194/92 (H8N4) | Mal H8 | 9.75 | 5.75 |
| H9 | A/Duck/Hong Kong/Y280/97 (H9N2) | DK H9 | 9.50 | 4.50 |
| H10 | A/Duck/Hong Kong/562/79 (H10N9) | DK H10 | 8.50 | 5.00 |
| H11 | A/Duck/Stantou/1411/00 (H11N2) | DK H11 | 8.75 | 4.25 |
| A/Mallard/Alberta/122/99 (H11N9) | Mal H11 | 8.50 | 3.75 | |
| H12 | A/Duck/Kirgiz/956/87 (H12N2) | DK H12 | 9.50 | 3.00 |
| H13 | A/Gull/Astrachan/227/84 (H13N6) | Gull H13 | 9.50 | 2.50 |
| H14 | A/Mallard/Astrachan/263/82 (H14N5) | Mal H14 | 9.25 | <1.00 |
| H15 | A/Wedgetailed Shearwater/W. Australia/2576/79 (H15N9) | SW H15 | 9.25 | 3.25 |
| Mammalian viruses | ||||
| H1N1 | A/Swine/IA/3421/90 | 7.50 | <1.00 | |
| A/USSR/90/77 | 9.25 | <1.00 | ||
| H3N2 | A/Swine/TX/4199-2/98 | 8.75 | 2.50 | |
| A/Hong Kong/1/68 | A/HC/1/68 | 9.50 | 1.00 | |
Animals and experimental infections.
Six-week-old Chinese ring-necked pheasants (Phasianus colchicus) (Ideal Poultry, Cameron, TX) and adult chukar partridges (Alectoris chukar) (Ideal Poultry) were used in this study. Serum samples from each bird were collected and tested before inoculation to ensure that the birds were serologically negative for avian influenza virus. Three pheasants were inoculated with 107 EID50 in a total volume of 1.0 ml of phosphate buffered saline (PBS) intraocularly (0.2 ml), intranasally (0.4 ml), and intratracheally (0.4 ml). One hour after inoculation, two uninfected pheasants were placed into the cage with the inoculated pheasants. The birds were observed daily, and tracheal and cloacal swabs were obtained on days 3, 5, 7, and 10 postinoculation (p.i.) to detect virus. Swabs were also obtained every other day beginning on day 12 p.i. to determine the length of time the birds shed virus. Three 10-day-old embryonated chicken eggs were inoculated with 0.1 ml of the sample medium for each swab collected and incubated for 48 h at 35°C, after which time allantoic fluid from each egg was evaluated to detect influenza virus infection by the hemagglutination test using 0.5% chicken red blood cells (CRBCs). If at least one of the three eggs was positive by the hemagglutination test, the bird from which the sample was collected was considered to be positive for influenza virus infection. Serum samples were collected from all surviving birds 14 days postinoculation (dpi). All these studies were performed in an animal facility approved for use by the U.S. Department of Agriculture.
Drinking water (1.0 ml) was sampled on day 3 p.i. because drinking water containers were inside the cages with the pheasants, and both infected and contact birds shared the water source. Virus titers in the drinking water were determined in 10-day-old embryonated chicken eggs in accordance with the Reed and Muench method (24).
Serological analysis by HI and virus neutralization (VN) assays.
Serum samples collected from each bird before infection and 14 dpi were treated with receptor-destroying enzyme and tested for HI antibodies to the virus with which the birds were infected; we used a hemagglutination inhibition assay with 0.5% CRBCs, as previously described (22). Viruses were diluted to contain four agglutinating units in sterile PBS solution. The HI assay was also performed by using 1% horse red blood cells (HRBCs) in a 0.5% bovine serum albumin (BSA)-PBS solution, and titers were read after a 60-min incubation period (36).
VN assays were performed in Madin-Darby canine kidney (MDCK) cells plated in microtiter plates, as previously described (15). The 50% tissue culture infectious dose (TCID50) was determined for each virus. Briefly, 10-fold serial dilutions of the virus were made from 10−1 to 10−10 in 1× minimal essential medium (Invitrogen, CA) with 4% BSA and TPCK [l-(tosylamido-2-phenyl) ethyl chloromethyl ketone]-treated trypsin (Worthington Biochemical Corporation, Lakewood, NJ) (1 μg/ml). Dilutions of the virus were added to the MDCK cells (4 wells for each dilution; 200 μl/well), and the cells were incubated for 48 h at 35°C. The contents of each well were tested for hemagglutination by incubating 50 μl of tissue culture supernatant with 50 μl of 0.5% CRBCs for 30 min. The TCID50 was calculated by the Reed and Muench method. Serum samples collected 14 dpi were treated with receptor-destroying enzyme and heat inactivated as mentioned above. Twofold serial dilutions of serum samples, beginning at 1:20, were made in 1× minimal essential medium containing 4% BSA and TPCK-treated trypsin (1 μg/ml). The homologous virus was standardized to contain 200 TCID50, an equal volume of virus was added to each serum dilution, and the mixture was incubated for 60 min at 35°C. Confluent monolayers of MDCK cells in 96-well plates were washed three times with PBS, and 200 μl of virus-antiserum mixture was added to each well (4 wells per mixture). After incubation at 35°C for 72 h, 50 μl of supernatant from each well was tested for HA activity by using 0.5% CRBCs. Geometric mean titers were calculated for each serum sample.
RESULTS
Replication of avian influenza A viruses in pheasants.
To determine the susceptibility of pheasants to infection with avian influenza A viruses, groups of three pheasants were inoculated via the natural route (intranasally, intraocularly, and intratracheally) with one virus of each of the 15 HA subtypes (Table 1). None of the 69 pheasants showed clinical disease signs during the 14-day observation period; however, two died: one (A/Mallard/Alberta/122/99 [Mal H11] group) died on day 9 p.i. and the other (A/Mallard/Netherlands/12/03 [Mal H7] group) had a head injury and died on day 11 p.i. Virus was isolated from both birds on days 3 and 5 p.i. only. The late deaths of these pheasants were most likely due to fight-induced wounds unrelated to the influenza virus infection.
Avian influenza A viruses of the 15 HA subtypes tested replicated in the inoculated pheasants, and most subtypes were detected in both the tracheal and cloacal swabs (Table 2). The A/Duck/Nanchang/2-0492/00 (DK H2) virus was detected solely in the cloacal swabs; the A/Gull/Astrachan/227/84 (Gull H13) and A/Wedgetailed Shearwater/W. Australia/2576/99 (SW H15) isolates were detected only in the tracheal swabs. Viruses were considered short-term (≤10 days) or long-term (≥14 days) shedders (Table 2). Short-term shedders included avian influenza A viruses of seven subtypes. One virus, SW H15, was detectable for only 5 days postinfection, while four viruses were shed for 7 dpi. The remaining two viruses (A/Duck/Korea/S10/03 [DK H3] and A/Duck/Stantou/1411/00 [DK H11]) were detected for 10 days following experimental inoculation of the pheasants.
TABLE 2.
Replication studies in Chinese ring-necked pheasants
| Virus | Method of infection | No. of birds positive 5 dpi/total no. of birds
|
No. of days of virus shed | |
|---|---|---|---|---|
| Trachea | Cloaca | |||
| DK H1 | Inoculated | 2/3 | 2/3 | 14a |
| Contact | 1/2 | 2/2 | 10 | |
| DK H2 | Inoculated | 0/3 | 2/3 | 20b |
| Contact | 0/2 | 1/2 | 18 | |
| DK H3 | Inoculated | 2/3 | 2/3 | 10 |
| Contact | 1/2 | 1/2 | 14a | |
| Mal H3 | Inoculated | 2/3 | 3/3 | 14a |
| Contact | 1/2 | 2/2 | 10 | |
| DK H4 | Inoculated | 3/3 | 3/3 | 14a |
| Contact | 0/2 | 1/2 | 10 | |
| Mal H4 | Inoculated | 3/3 | 3/3 | 7 |
| Contact | 1/2 | 2/2 | 7 | |
| DK H5 | Inoculated | 1/3 | 1/3 | 14a |
| Contact | 0/2 | 1/2 | 20b | |
| DK H6 | Inoculated | 3/3 | 3/3 | 7 |
| Contact | 1/2 | 1/2 | 10 | |
| Mal H6 | Inoculated | 2/3 | 3/3 | 14a |
| Contact | 2/2 | 2/2 | 12 | |
| Mal H7 | Inoculated | 3/3 | 3/3 | 20b |
| Contact | 2/2 | 2/2 | 16 | |
| Mal H8 | Inoculated | 2/3 | 2/3 | 16 |
| Contact | 1/2 | 2/2 | 18 | |
| DK H9 | Inoculated | 3/3 | 2/3 | 7 |
| Contact | 2/2 | 1/2 | 7 | |
| DK H10 | Inoculated | 3/3 | 3/3 | 18 |
| Contact | 2/2 | 2/2 | 45 | |
| Mal H11 | Inoculated | 3/3 | 1/3 | 14 |
| Contact | 0/2 | 0/2 | 16 | |
| DK H11 | Inoculated | 1/3 | 3/3 | 10 |
| Contact | 1/2 | 1/2 | 10 | |
| DK H12 | Inoculated | 3/3 | 2/3 | 20 |
| Contact | 1/2 | 2/2 | 23 | |
| Gull H13 | Inoculated | 3/3 | 0/3 | 7 |
| Contact | 0/2 | 0/2 | 0 | |
| Mal H14 | Inoculated | 3/3 | 0/3 | 23 |
| Contact | 0/2 | 0/2 | 0 | |
| SW H15 | Inoculated | 2/3 | 0/3 | 5 |
| Contact | 1/2 | 0/2 | 7 | |
Birds were monitored for only 14 dpi.
Birds were monitored for only 21 dpi.
Short-term shedders.
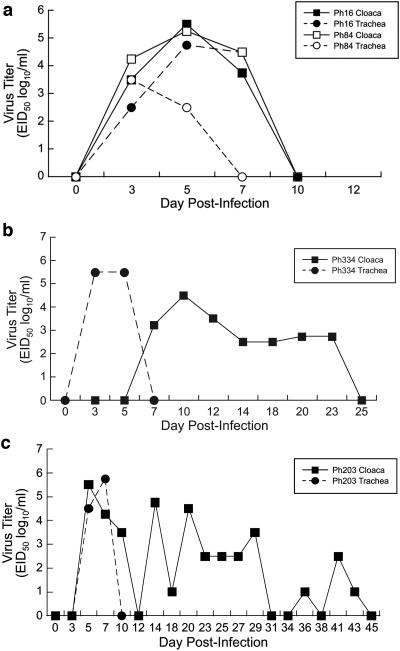
Replication of viruses that shed for ≤10 days followed the standard pattern associated with infection with an influenza A virus, as shown by the example in Fig. 1a. By day 3 p.i., a titer of 104.25 EID50/ml of virus was detectable from swabs obtained from the cloaca, and a titer of 103.5 EID50/ml of virus was detectable in samples from the trachea. Virus replication peaked on day 5 p.i. (titer of 105.5 EID50/ml). By day 7 p.i., virus was no longer detectable from tracheal swabs, and the titer in the cloacal swab fell to 104.5 EID50/ml. After day 7 p.i., virus titers declined until virus was no longer detectable at 10 dpi.
FIG. 1.
Length of virus shedding from pheasants infected with avian influenza A viruses. (a) Short-term shedders. These viruses were shed for ≤10 days from pheasants. Virus titers of an infected bird in the Mal H4 group (Ph16) and a contact bird in the Mal H3 group (Ph84) are shown. (b) Long-term shedders. These viruses were shed for ≥14 days. Virus titers of an infected bird in the Mal H14 group are shown. (c) Virus titers of a contact bird in the DK H10 group are shown.
Long-term shedders.
Long-term shedders included viruses that were shed for 14 days or longer from inoculated pheasants and included the 12 remaining avian viruses used in this study (Table 2). Six virus subtypes were shed for at least 14 days from inoculated pheasants, one was shed for 16 days, and one was shed for 18 days. Inoculated pheasants in three virus groups shed virus for 20 dpi; one virus (A/Mallard/Astrachan/263/82 [Mal H14]) was shed from the gastrointestinal tract for 23 days. Shedding from birds infected with the Mal H14 virus followed an atypical pattern. In the example shown in Fig. 1b, virus was undetectable from cloacal swabs on days 3 and 5 p.i.; however, high titers of virus (105.5 EID50/ml) were isolated from the tracheal swabs on these days. As virus titers from tracheal swabs decreased, they increased in cloacal swabs (peak titer, 104.5 EID50/ml at day 10 p.i.). Virus titers dropped to their lowest (102.5 EID50/ml) on day 14 p.i., and virus was no longer detectable by 25 dpi.
Transmission of avian influenza viruses among pheasants.
Transmission of influenza A viruses among avian species allows the viruses to be perpetuated in live bird markets. To determine whether they are transmitted in the pheasant population, two naïve pheasants were placed in the same cage with three pheasants that had been inoculated approximately 1 h earlier. Two of the 46 contact pheasants developed clinical signs of disease. On day 7 p.i., conjunctivitis developed in the left eye of a pheasant exposed to A/Duck/Hong Kong/Y280/97 (H9N2) (DK H9). The condition resolved itself within 2 days, and the bird survived to the end of the study. One pheasant exposed to A/Duck/Nanchang/2-0492/00 (H2N9) appeared lethargic and had ruffled feathers on days 6 and 7 p.i., and it died on day 8 p.i. At necropsy, virus was detected in the lungs (2.5 log10/ml) and intestine (2.25 log10/ml) but not in the brain or liver. Two other contact birds died, one in the A/Wild Duck/Shantou/520/01 (DK H1) group (11 dpi) and one in the A/Mallard/Alberta/194/92 (Mal H8) group (14 dpi). Virus was isolated from the gastrointestinal tract of both birds until their deaths (10 dpi and 12 dpi, respectively). At necropsy, no virus was isolated from the brains, lungs, liver, or intestine of the pheasant in the DK H1 group. The other pheasant did not undergo necropsy.
Most avian influenza A viruses of all HA subtypes were transmitted to naïve contact pheasants. Exceptions were the Gull H13 and Mal H14 subtypes (Table 2). Thirteen of the virus subtypes that were transmitted to contact birds replicated in both the respiratory and gastrointestinal tracts. However, the SW H15 virus was detected only in the tracheal swabs of the contact birds, similar to what was found among the infected pheasants. The DK H2, A/Duck/Kirgiz/956/87 (DK H12), and A/Mallard/Alberta/119/00 (Mal H4) viruses were detectable only in the cloacal swabs of contact birds.
We grouped viruses according to the number of days they were shed from the contact birds; however, some groupings were different from what they had been for the infected pheasants (Table 2). In contact birds, eight viruses were short-term shedders, and nine viruses were identified as long-term shedders. The DK H3 virus, which had been shed from both the trachea and cloaca of infected birds for 10 days, was shed for at least 14 days in contact birds. The DK H1, A/Mallard/Alberta/31/01 (Mal H3), and A/Duck/Mongolia/218/01 (DK H4) viruses were categorized as long-term shedders in inoculated pheasants and as short-term shedders in contact birds because they were no longer detectable in swab samples after 10 dpi. Contact birds shed three virus subtypes (Mal H4, DK H9, and SW H15) for 7 days; the remaining five subtypes were shed for 10 dpi. Replication of viruses that shed for ≤10 days followed the standard pattern associated with influenza virus infection (Fig. 1a). By 3 dpi, titers in the trachea and cloaca were 102.5 EID50/ml and 103.5 EID50/ml, respectively. Virus titers peaked by day 5 p.i. (104.75 EID50/ml for trachea and 105.5 EID50/ml for cloaca). On day 7, virus titers then dropped to 104.5 EID50/ml (trachea) and 103.75 EID50/ml (cloaca) until virus was no longer detectable on day 10 p.i. Of the nine viruses categorized as long-term shedders, contacts in the A/Mallard/Alberta/206/96 (Mal H6) group were positive on day 3 p.i. and shed virus until day 12 p.i., while one contact bird shed the DK H3 virus for at least 14 dpi. Contact birds shed two virus subtypes (Mal H7 and Mal H11) for 16 dpi, and birds in the DK H2 and Mal H8 groups shed virus for 18 dpi. One contact bird in the A/Duck/Hokkaido/447/00 (DK H5) group shed for 20 dpi, and a pheasant in the DK H12 group shed for 23 dpi. Most notably, one of the two contact birds from the A/Duck/Hong Kong/562/79 (DK H10) virus group continued to shed virus from the gastrointestinal tract for 45 dpi. Long-term virus shedding followed an atypical pattern of replication. Virus titers in the cloaca peaked (105.5 EID50/ml) for the example shown in Fig. 1c on day 5 p.i. and then dropped to undetectable levels on day 12 p.i. However, on day 14 p.i., a titer of 104.75 EID50/ml of virus was detected in the cloaca swab, after which virus titers in the cloaca fell to 102.5 EID50/ml. On days 31 and 34 p.i., virus was isolated; however, titers were <1 log. On day 41 p.i., the titer in the cloaca rose to 102.5 EID50/ml again and then dropped to an undetectable level by day 48 p.i. To confirm the finality of virus shedding in this bird, it was swabbed until day 55 p.i., when the samples were negative three consecutive times.
Viruses are transmitted among wild aquatic birds mainly through the fecal-oral route, because virus replicates in the intestines of ducks that excrete high concentrations into lake water through feces (44). Drinking water was shared by the infected and contact birds, and because the water source was inside the cage with the birds, the water became contaminated with fecal material. High titers of virus were detected in drinking water sampled on day 3 p.i. (range, <1 to 6.25 log10/ml; mean, 3.97 log10/ml) for all subtypes in which transmission occurred. The exception was the DK H5 virus (Table 1). The Gull H13 virus, which was not transmitted to contact pheasants, was isolated from tracheal swabs of infected pheasants. Due to long-term shedding from the cloaca of pheasants, the fecal-oral route is most likely the route of transmission in these birds. However, the aerosol route may have been responsible for transmission of the SW H15 virus, because it was detected in only the trachea of both infected and contact birds.
Failure of swine and human influenza A viruses to replicate in pheasants.
Because it has been shown that mammalian viruses can replicate to a limited extent in Japanese quail, researchers have concluded that avian species can serve as intermediate hosts in the ecology of influenza A viruses (19). To determine whether swine and human influenza A viruses can replicate in pheasants, we tested two recent North American swine isolates (Table 1) representing the classical swine H1N1 viruses and the H3N2 subtype. Neither was detected in the respiratory or intestinal tract of the pheasants on day 3, 5, or 7 p.i. (data not shown).
We used human isolates from past pandemics (1968 and 1977) to test the ability of human influenza A viruses to replicate in pheasants. Theses isolates are considered to be more avian-like than those currently circulating in humans because some of their genes were inherited from avian lineages (14, 27). Neither human virus (H1N1 and H3N2) used in this study was detected in the pheasants. In addition, neither virus was detectable in the drinking water sampled on day 3 p.i. (data not shown).
Replication of avian influenza A viruses in chukar partridges.
We wanted to know whether the avian influenza viruses tested in pheasants behave in the same way in chukar partridges because this species may be kept in live markets for weeks. To explore this question, we inoculated chukar partridges with viruses representative of groups assigned based on results in the pheasants (Table 3). For example, a virus that had been excreted only from the cloaca of pheasants (DK H2) and another that had been detected exclusively in tracheal swabs of pheasants (Gull H13) were chosen.
TABLE 3.
Replication studies in chukar partridges
| Virus | Water sample (log10/ml) | Method of infection | No. of birds shedding/total no. of birds on dpi (titer[s] [log10/ml])a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3
|
5
|
7
|
10
|
|||||||
| Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | |||
| A/Sw/Iowa/3421/90 (H1N1) | 3 | Inoculated | 2/3 (1) | 0/3 | 2/3 (4.25, 3) | 0/3 | 0/2b | 0/2 | ND | ND |
| Contact | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
| A/Sw/TX/4199-2/98 (H3N2) | 3.5 | Inoculated | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
| A/Mallard/Alberta/31/01 (H3N9) | 2 | Inoculated | 1/3 (3.25) | 0/3 | 1/3 (1) | 0/3 | 0/2b | 0/2 | 0/2 | 0/2 |
| Contact | 2/2 (3.75, 1) | 0/2 | 2/2 (3.25, 3.5) | 0/2 | 2/2 (1, 3.25) | 0/2 | 0/2 | 0/2 | ||
| A/Mal/Alb/119/00 (H4N6) | 4.5 | Inoculated | 0/3 | 0/3 | 0/2c | 0/2 | 0/2 | 0/2 | ND | ND |
| Contact | 0/2 | 0/2 | 1/2 (3.5) | 0/2 | 1/2 (6.25) | 0/2 | ND | 0/2 | ||
| A/Mal/Alb/206/96 (H6N8) | Not tested | Inoculated | 2/3 (2.67) | 1/3 (1) | 1/3 (4.25) | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
| A/Mal/Alb/122/99 (H11N9) | 2.5 | Inoculated | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
| A/DK/Nan/2-0492/00 (H2N9) | 3.75 | Inoculated | 2/3 | 2/3 | 2/3 | 2/3 | 0/3 | 0/3 | ND | ND |
| Contact | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
| A/DK/Hok/447/00 (H5N3) | 2 | Inoculated | 2/3 | 1/3 | 1/3 | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 1/2 | 1/2 | 1/2 | 0/2 | 1/2 | 0/2 | 1/2 | 0/2 | ||
| A/Mal/Neth/12/03 (H7N3) | 3.75 | Inoculated | 2/3 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 1/2 | 0/2 | 2/2 | 0/2 | 1/2 | 1/2 | 0/2 | 0/2 | ||
| A/DK/HK/Y280/97 (H9N2) | 4.25 | Inoculated | 3/3 | 0/3 | 3/3 (4.5, 4.75, 5.75) | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 |
| Contact | 2/2 | 0/2 | 2/2 | 2/2 | 2/2 | 1/2 | 0/2 | 0/2 | ||
| A/DK/HK/562/79 (H10N9) | 5.25 | Inoculated | 3/3 | 2/3 | 3/3 (5.25, 5.25, 5.5) | 2/3 (3.5) | 0/3 | 1/3 | 0/3 | 0/3 |
| Contact | 2/2 | 0/2 | 2/2 | 0/2 | 1/2 | 1/2 | 0/2 | 0/2 | ||
| A/Gull/Ast/227/84 (H13N6) | 4.5 | Inoculated | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | ND | ND |
| Contact | 0/2 | 2/2 | 0/2 | 0/2 | 0/2 | 0/2 | ND | ND | ||
ND, not done.
One bird died 6 dpi.
One bird died 4 dpi.
No clinical signs of influenza virus infection were noted during the 14-day observation period in any of the chukar partridges used in this study. However, 3 of the 30 inoculated chukar partridges died during the study, 1 in the Mal H3 group (day 6 p.i.) and 2 in the Mal H4 group (days 4 and 10 p.i.). Virus was not isolated from these birds on either day they were swabbed, nor was it later isolated from the brain, lung, liver, or intestine of any chukar partridge, indicating that their deaths were unrelated to influenza virus infection.
Of the 10 avian influenza viruses tested, 8 were detectable in experimentally inoculated chukar partridges (Table 3), and all were short-term shedders in this species. One of these eight viruses (Gull H13) was detected only on day 3 p.i., five were detectable for 5 days p.i. (Mal H3, Mal H6, DK H2, DK H5, and Mal H7), and only two (DK H9 and DK H10) were detected for 7 days p.i. Four viruses were detectable only in the trachea of the inoculated birds, and four others were isolated from both the trachea and cloaca. The remaining two avian viruses (Mal H4 and Mal H11) were not detectable in either the trachea or cloaca of the inoculated chukar partridge. Only one of three chukar partridges inoculated with each of the North American isolates (Mal H3 and Mal H6) tested positive for virus replication, whereas the older (identified before 2000) Eurasian isolates replicated in all three inoculated chukar partridges, and the newer (identified after 2000) Eurasian isolates were detectable in two of the three experimentally inoculated birds.
Transmission of avian influenza viruses among chukar partridges.
To determine whether influenza A viruses are transmitted among chukar partridges, two naïve birds were placed in the same cage with the three experimentally inoculated birds approximately 1 h after they had been inoculated. Three of the 10 avian influenza viruses (DK H2, Mal H6, and Mal H11) tested did not transmit to naïve contact birds, even though appreciable amounts of virus were detected in the shared drinking water. The remaining seven viruses were transmissible. Of these viruses, six were detectable for at least 7 dpi. The other virus (DK H5) was isolated from the trachea of one of the two contact birds on day 10 p.i. The Gull H13 virus was isolated from the cloacal swabs of both contact birds only on day 3 p.i. In most cases, the contact birds shed virus for a longer time than did the infected birds. Although no virus was isolated from chukar partridges inoculated with the Mal H4 virus, one of two contact birds shed virus on days 3 and 7 p.i. (EID50 of 103.5 and 106.25, respectively). Presumably, transmission occurred via the shared drinking water because a substantial amount of virus was isolated on day 3 p.i. (104.5 EID50/ml). Virus isolated from the water in this group may have been a contaminant from the original inoculum. Transmissible viruses were detected mainly in the trachea of contact birds; however, by day 7 p.i., viruses such as DK H10 and Mal H7 were being excreted via the cloaca.
Replication and transmission of swine influenza A viruses in chukar partridges.
To determine whether mammalian viruses can replicate in chukar partridges, we inoculated partridges with one isolate from each of the two predominant lineages currently circulating in swine in North America. No clinical disease signs were observed in the inoculated birds; however, one bird in the swine H1 group died on day 6 p.i. Because no virus was isolated from this bird on either day it was swabbed, its death was most likely unrelated to the virus inoculation. The swine H3 virus was not detected, whereas the swine H1 virus was detected on days 3 and 5 p.i. in the trachea of two of the three infected chukar partridges. Neither swine virus was transmitted to naïve contact chukar partridges, although virus was isolated (103 EID50/ml and 103.5 EID50/ml, respectively) from shared drinking water sampled 3 dpi.
Comparison of the replication of influenza A viruses in pheasants and chukar partridges.
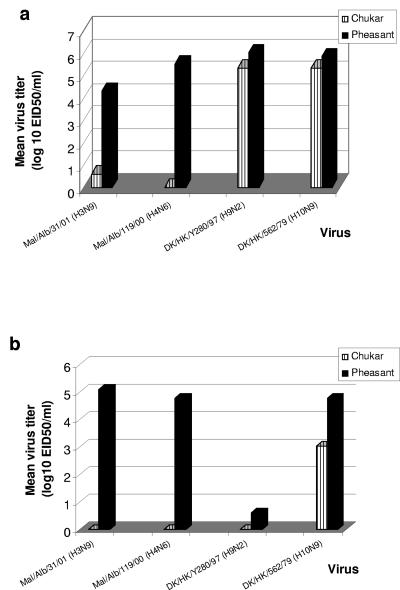
To further compare the replication efficiency of influenza A viruses in these bird species, we chose four viruses and determined their titers from swabs obtained on day 5 p.i. from both bird species, because influenza virus replication in most species peaks at day 5 p.i. (Fig. 2). Two of the selected viruses were classified as short-term (≤10 days) shedders (Mal H4 and DK H9) and two were classified as long-term (≥14 days) shedders (Mal H3 and DK H10) in pheasants. The Mal H4 virus was not detected in the three infected chukar partridges; however, it was isolated from tracheal (mean titer, 105.52 EID50/ml) and cloacal (mean titer, 104.78 EID50/ml) swabs of all three infected pheasants on days 3 and 5 p.i. The DK H9 virus was categorized as a short-term shedder in pheasants, in which it was detected in swabs from both the trachea and cloaca; however, replication in chukar partridges was restricted to the respiratory tract (range, 104.5 EID50/ml to 105.75 EID50/ml). The DK H9 virus was isolated from all three inoculated pheasants; titers of DK H9 virus detected from tracheal swabs (106.5 EID50/ml) were higher than those of the other three virus subtypes. The Mal H3 virus was isolated from tracheal swabs of one of the three infected chukar partridges on days 3 and 5 p.i. (virus titer, 101 EID50/ml on day 5 p.i.). This virus was isolated from tracheal and cloacal samples of all three infected pheasants up to 7 dpi. At least one bird shed virus from the gastrointestinal tract for a minimum of 14 dpi. Virus titers detected from tracheal swabs of pheasants infected with Mal H3 (102.5 EID50/ml) were lower than those of the other three virus subtypes. The DK H10 virus replicated in the trachea and gastrointestinal tract of three experimentally inoculated pheasants for 7 dpi. In two of the three birds, the virus was shed from the gastrointestinal tract for 18 days; however, it was detectable in experimentally inoculated chukar partridges (tracheal and cloacal samples) for only 7 dpi.
FIG. 2.
Comparison of virus titers in pheasants and chukar partridges (5 dpi). (a) Tracheal titers. (b) Cloacal titers. Titers were determined for all positive swabs collected 5 dpi. We categorized the Mal H4 (Mal/Alb/119/00) and DK H9 (DK/HK/Y280/97) viruses as short-term shedders and the Mal H3 (Mal/Alb/31/01) and DK H10 (DK/HK/562/79) viruses as long-term shedders on the basis of results obtained from the inoculation of pheasants.
Although the main site of virus replication in the chukar partridges was the respiratory tract, average titers of all four viruses detected from the tracheal swabs of pheasants were higher than those from chukar partridges. However, no significant difference was observed between the average virus titers from tracheal swabs of chukar partridges and those of pheasants for the DK H9 and DK H10 groups. Virus was detectable from the cloacal swabs obtained from chukar partridges only in the DK H10 group; one bird had a titer of 103.5 EID50/ml. Again, virus titers of cloacal swab samples from pheasants were higher than those from chukar partridges for all four virus groups, leading us to conclude that these four influenza A viruses replicate more efficiently in pheasants than in chukar partridges.
Serologic response of pheasants and chukar partridges to avian influenza viruses.
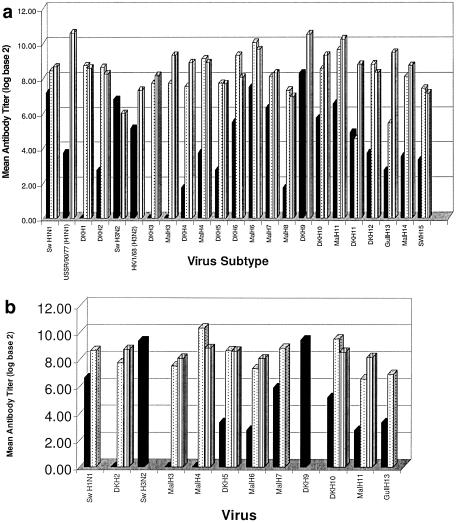
To determine the immune status of the pheasants and chukar partridges, we collected blood of all the birds used in this study twice to test for seroconversion to influenza A virus before challenge and at 14 dpi. Preinfection sera were tested by HI assay against the homologous virus with which the pheasants or chukar partridges were inoculated, and all of the birds were seronegative (<10) for influenza A virus (data not shown). We used VN and HI assays to test the serum from 14 dpi. The VN assay, which primarily detects HA antibodies, is the more sensitive test. It reflects the in vivo neutralization of the influenza virus more closely than does the HI assay, and it can be used to confirm the results of the HI test. Postinfection sera from all inoculated pheasants were tested by VN assay, and sera from seven groups of infected chukar partridges were tested to detect neutralizing antibodies against the homologous virus (Fig. 3a and b). Neutralizing antibodies were detectable in all sera tested by this assay, with the exception of sera from an infected pheasant in the A/Duck/Shantou/5540/01 (DK H6) group, in which a neutralization titer of <40 was detected (Table 4). In chukar partridges, the titers ranged from 160 to 640; the highest average neutralizing titer of 426 was detected in a bird from the DK H2 group. Although the titers for the birds in the Mal H4 group appear to be higher than those of birds in the DK H2 group, serum was available from only one infected chukar partridge in this group. In pheasants, neutralizing titers ranged from <40 to 1,810, and the highest average neutralization titers were detected from pheasants in the human H1N1 and DK H9 groups (1,565 and 1,493, respectively). We detected neutralization titers of <100 in one infected pheasant in each of three groups (DK H5, Mal H8, and SW H15). Interestingly, the lowest neutralization titers, 47 and 80, were detected in pheasants infected with the swine H3 virus.
FIG. 3.
Antibody titers in inoculated birds 14 days postinfection. (a) Antibody titers in inoculated pheasants. (b) Antibody titers in inoculated chukar partridges. Bars are the log (base 2) of the average titers from the three inoculated birds. Black bars are HI antibody titers determined using chicken red blood cells. Dotted bars are HI antibody titers determined using horse red blood cells, and hashed bars are neutralization titers. Serum HI antibody titers were not determined postinfection for the DK H9, swine (Sw) H3N2, and HK/1/68 (H3N2) virus groups. Neutralizing titers were not determined for the swine H1N1, swine H3N2, Mal H7, Mal H9, and Gull H13 groups of chukar partridge postinfection serum.
TABLE 4.
Antibody titers in inoculated pheasants 14 dpi
| Virus | Antibody titer at 14 dpib for bird indicated
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HI
|
VN
|
||||||||
| CRBCs
|
HRBCs
|
||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Swine H1N1 | 80 | 320 | 40 | 80 | 640-1,280 | <10 | 320 | 640 | 269 |
| USSR/90/77 (H1N1) | <10 | <10 | 40 | <10 | <10 | <10 | 1,810 | 1,810 | 1,076 |
| DK H1 | <10 | <10 | <10 | 640-1,280 | 160 | 160 | 381 | 453 | 320 |
| DK H2 | <10 | <10 | 20 | 160 | 80 | 640-1,280 | 381 | 381 | 160 |
| Swine H3N2 | 20 | 160 | 160 | NT | NT | NT | 48 | 80 | NT |
| HK/1/68 (H3N2) | 15 | 30 | 60 | NT | NT | NT | 160 | 160 | 160 |
| DK H3 | <10 | <10 | <10 | 320 | 320 | <10a | 320 | 381 | 160 |
| Mal H3 | <10 | <10 | <10 | 320 | 160 | 160 | 640 | 640 | NT |
| DK H4 | <10 | <10 | 10 | 320 | 160 | 80 | 320 | 640 | NT |
| Mal H4 | <10 | 20 | 20 | 320 | >1,280 | 80 | 640 | 320 | NT |
| DK H5 | 20 | <10 | <10 | <10a | 160 | 320-640 | 320 | 95 | NT |
| DK H6 | 10 | 80 | NT | >1,280 | >1,280 | NA | <40 | 538 | NT |
| Mal H6 | 40 | 320 | NT | 640 | >1,280 | >1,280 | 320 | 1,280 | NT |
| Mal H7 | 80 | 80 | NT | 80 | 160-320 | NA | 320 | NT | NT |
| Mal H8 | <10 | <10 | 10 | 80 | 320 | 80 | 135 | 80 | 160 |
| DK H9 | 160 | 320 | 480 | NT | NT | NT | 1,280 | 640 | 2,560 |
| DK H10 | 40 | 80 | 40 | 160 | 320 | 640 | 320 | 320 | 1,280 |
| Mal H11 | 160 | 30 | NT | >1,280 | 320 | NA | 640 | 1,810 | NT |
| DK H11 | 40 | 10 | 40 | 20 | 10 | 40 | 640 | 320 | 381 |
| DK H12 | 40 | <10 | <10 | 640-1,280 | 320 | 80 | 320 | 320 | NT |
| Gull H13 | 20 | <10 | <10 | 40 | 80 | 10 | 761 | 761 | 640 |
| Mal H14 | 20 | 15 | <10 | 320 | 320-640 | 40 | 320 | 320 | 640 |
| SW H15 | <10 | 10 | 20 | 40 | 160 | 320 | 80 | 190 | 160 |
No virus was isolated from these birds.
NT, not tested.
Postinfection sera from all infected pheasants and chukar partridges were also tested against the homologous virus in the HI assay. In general, when the HI assay was performed by using CRBCs, most postinfection sera had low or undetectable antibody titers (<40). Six of the 23 avian viruses used in this study induced a detectable HI antibody response (>40) in at least one of the three inoculated pheasants, whereas low titers (range, 10 to 40) were detected in at least one of the three inoculated pheasants for eight avian viruses (Table 4). HI antibody titers were undetectable (<10) in sera from any of the three inoculated pheasants for the five remaining avian viruses used in this study. In most sera from pheasants inoculated with the mammalian viruses, HI activity was detected. The exception was the human H1N1 postinfection sera. Results were similar to those observed when CRBCs were used to test chukar partridge sera. HI antibodies against eight of the avian influenza A viruses were detected at levels below what is considered to be protective against infection (titers of ≤40), although the DK H10 virus produced a moderate HI antibody response in one of the three inoculated birds (80). The swine viruses induced high levels of HI antibodies in at least two of the three inoculated birds (mean titer, 100 for swine H1 and 667 for swine H3). The DK H9 and Mal H7 viruses also induced a measurable HI antibody response in all three infected chukar partridges (mean titers, 693 and 60, respectively). No correlation was made between serum HI antibody titers and length of virus shedding. Long-term shedders were equally as likely as short-term shedders to induce a measurable HI antibody response. Because some groups shed virus for more than 14 days, we suspected that these birds had not yet mounted a measurable immune response by 14 dpi. In sera collected from six groups of pheasants at 21 dpi and 28 dpi, HI antibody titers had not demonstrably increased from day 14 p.i. (data not shown).
Because the VN and HI assays measure the same serum antibody pool, the lack of detectable serum HI antibodies after infection of the birds may be due to the use of CRBCs in the HI assay. The ability of influenza viruses to agglutinate erythrocytes from different animal species is related to their receptor specificity. For this reason, we used horse red blood cells (HRBCs) in the HI assay of postinfection sera from pheasants and chukar partridges. After this modification to the test, HI serum antibodies were detectable with all avian viruses tested. The exceptions were the DK H9, swine H3, and human H3 viruses. These viruses could not be used to test postinfection sera because they did not agglutinate HRBCs. Interestingly, the swine H1 and human H1 viruses were able to agglutinate the HRBCs. However, when HRBCs were used, the test did not detect HI activity in the postinfection pheasant sera from the human H1 group. In most cases, titers measured in the HI assay by using HRBCs were >80, even when they were undetectable by using CRBCs. In a few instances, serum HI antibodies were undetectable (<10), even when the assay used HRBCs; however, in these birds, virus was not isolated from any sample postinfection, suggesting that they were not infected.
To compare the assay used to obtain antibody titers, we measured the average of the HI and VN antibody titers in the inoculated birds at 14 dpi and used the log (base 2) of this mean value as a measure of the antibody response to each virus (Fig. 3). The HI antibody titers measured by using CRBCs were not directly correlated with the VN antibody titers, which were higher than HI antibody titers for all avian influenza viruses tested, with the single exception mentioned above. Mean HI antibody titers measured by using HRBCs were comparable to titers obtained from the VN assay, with two exceptions. Mean antibody titers of sera from birds inoculated with DK H11 and Gull H13 viruses were similar when measured by HI assay using either CRBCs or HRBCs.
DISCUSSION
To elucidate the role of pheasants and chukar partridges in the ecology and evolution of influenza A viruses in live poultry markets, we tested avian influenza viruses of 15 HA subtypes, as well as several mammalian influenza viruses, to determine whether they can replicate in and be transmitted among these minor poultry species. Few or no clinical disease signs were observed in any of the birds monitored throughout this study. We did observe marked differences in the response of pheasants and chukar partridges to the viruses tested in this study, leading us to conclude that the site and length of replication and transmission of avian influenza viruses are host dependent. In our study, pheasants were susceptible to infection with avian influenza A viruses of all 15 HA subtypes tested, whereas chukar partridges were susceptible to infection with only 80% of the avian viruses tested for replication in this species. However, chukar partridges were able to support the limited replication of a swine virus, in contrast to pheasants, in which no mammalian viruses tested in this study were detectable after experimental inoculation. In addition, influenza A viruses were transmitted from infected pheasants and chukar partridges to naïve contact birds of the same species via the fecal-oral route. Long-term shedding of virus (>14 days) from the gastrointestinal tract of pheasants was observed; one contact pheasant shed virus for an unprecedented 45 days. In contrast, chukar partridges stopped shedding virus 7 dpi.
The hemagglutination inhibition assay, routinely used to detect serum antibodies to influenza A viruses, relies on the inhibition of the binding between the hemagglutinin and the sialic acid of the red blood cell used. This assay is less sensitive than is the neutralization assay to the presence of serum antibodies induced by avian influenza viruses in mammalian species, including humans (1, 25). Less is known about the detection of antibodies by the HI assay in different avian species, although ducks have been reported to also lack detectable HI antibody responses to natural or experimental avian influenza infections (9, 16). Others have postulated that this inability of ducks to produce hemagglutinating antibody could be related to the structure of the main type of duck serum antibody, immunoglobulin Y (40). A direct comparison of the results obtained by using an HI assay with results obtained by using a neutralization assay demonstrated that the latter was better able to detect antibodies to avian H5N1 virus in adult human sera (25). Neutralizing antibodies were detected in all sera tested, even when antibodies were scarcely detectable by using the standard HI assay. Because the neutralization and HI assays measure the same serum antibody pool, we suspected that the HI assay failed to detect serum HI antibodies in the infected birds because chicken red blood cells were used. The ability of influenza viruses to agglutinate erythrocytes from different animal species is related to their receptor specificity. It has been shown that avian and equine influenza viruses bind preferentially to the N-acetylneuraminic acid α2,3-galactose (NeuAcα2,3-Gal) linkage, in contrast to human viruses, which prefer the NeuAcα2,6-Gal linkage (38). Chicken red blood cells contain a mixture of NeuAcaα2,3-Gal and NeuAcaα2,6-Gal linkages, whereas horse red blood cells contain almost exclusively NeuAcaα2,3-Gal linkages. In both pheasants and chukar partridges, postinfection serum antibody titers against avian influenza A viruses measured with HRBCs were comparable to titers measured by the neutralization assay. The mammalian H3 viruses as well as the DK H9 virus could not be tested by the HI assay using HRBCs because neither virus was able to agglutinate these erythrocytes. It has been shown that this H9 virus has receptor specificity similar to that of human H3N2 viruses (20). It is not surprising that the swine H1 virus was able to agglutinate the HRBCs, because swine viruses can bind both 2-3 and 2-6 sialic acids (13). On the basis of these results, it may be more appropriate to use HRBCs in the HI assay to detect serum antibodies to avian influenza A viruses.
Hong Kong health officials no longer permit quail to be sold in the live poultry markets because quail are suspected of transmitting influenza viruses to other birds. As reported previously by Makarova et al. (19), 14 of the 15 avian influenza A virus HA subtypes tested replicated in quail. The exception was the H15 subtype. These viruses, detectable for 3 to 6 dpi, were shed predominantly from the respiratory tract. The viruses tested in both chukar partridges and quail replicated in the respiratory tract and for approximately the same length of time. Overall, our findings suggest that among chukar partridges, more efficient replication and transmission of influenza viruses require adaptation. Pheasants, on the other hand, differed markedly from both quail and chukar partridges in their susceptibility to avian influenza viruses; the main site of replication was the gastrointestinal tract. In addition, virus titers in the cloacal and tracheal swabs of pheasants were higher than those of chukar partridges. However, pheasants and quail shed similar amounts of virus. Because of their continuous asymptomatic infection and longer stay in the markets, pheasants are ideal carriers of influenza A viruses. On the basis of these findings, it does not make good sense to ban quail but not pheasants from live bird markets.
Ducks are considered to be the natural reservoir of influenza A viruses because all known HA and neuraminidase subtypes have been found in these birds and because they are asymptomatic carriers (43). Furthermore, the replication of influenza virus occurs mainly in the intestinal tract of ducks (44). The avirulent nature of avian influenza infection in ducks may have resulted from adaptation to this host over centuries, creating a reservoir and thus ensuring the perpetuation of the viruses. Species other than the duck might be able to serve as a reservoir because not all subtypes of avian influenza viruses are present in ducks at any given time. In surveillance studies conducted in North America, H3, H4, and H6 subtypes predominated in wild ducks, whereas H1, H2, and H4 predominated in European ducks. The H13 and H14 subtypes have not been isolated from this bird population (29, 37). In addition, avian influenza A viruses of the H5 and H7 subtypes are usually pathogenic to chickens but do not typically cause lethality in ducks. For example, an H5 isolate from a 1983 outbreak in chickens (A/CK/PA/1370/83 [H5N2]) was 100% lethal to chickens but caused no disease in ducks (21). This same isolate was shown to be avirulent in experimentally inoculated pheasants, from which it was shed for 15 days in the feces. In our study, only low-pathogenic avian influenza viruses were used, and these viruses remained apathogenic for pheasants. Over time, influenza A viruses may have adapted to pheasants, in which they are now nonpathogenic. Pheasants, whose response to infection with all low-pathogenic HA subtypes tested was similar to that of ducks, may serve as another reservoir of avian influenza A viruses.
Influenza A viruses are shed for 2 to 4 weeks by ducks (43). It has been reported that ducks also lack HI antibody responses to natural or experimental avian influenza infections. In one study, a nonpathogenic H7 duck isolate was shed by orally inoculated ducks for 21 days, although serum HI antibodies were scarcely detectable. By contrast, in another study, a duck H3N2 influenza virus was shed for 18 days following experimental inoculation, and HI activity was not detected in the postinfection serum (9, 16). The genome maintained in the avian reservoir is said to be in evolutionary stasis, meaning that these viruses are optimally adapted to this host. The low rate of nucleotide substitutions and lack of strong positive selection pressure may explain the continued survival of the virus in the host and its stability. Selection of avian viruses in ducks is negative, and long-term survival favors viruses that have not changed or have changed very little. On the other hand, pheasants produced a good neutralizing antibody response to the viruses used for experimental inoculation, and yet they still shed virus for long periods of time. Thus the long-term shedding could be a by-product of positive selection pressure, and there could be antigenic changes associated with the extended shedding of virus. To determine whether antigenic drift variants are being selected in these birds, future investigations will include sequence analysis of the HA gene and serological analysis to further characterize viruses isolated from the pheasants at different time points. The development of antigenic drift variants could have serious consequences, because birds are the primary host of all influenza strains that have been introduced into mammals.
It is possible that influenza viruses are able to persist in pheasants because they are replicating in an immunologically privileged site. When the replicative ability and tissue tropism of five nonpathogenic duck viruses were evaluated in chickens, there was a general predilection of the viruses for kidney tissues and digestive tract tissues (5). In another study, viral nucleoprotein was found in the renal tubular epithelial cells of kidney tissues of chickens intravenously inoculated with an influenza virus of waterfowl origin. The nucleoprotein's presence there signified that the kidney is an important site for the replication of avian influenza virus of low pathogenicity (33). It has also been suggested that the bursa could serve as the primary site of influenza virus replication. Virus was isolated at a high rate (90% and 70%, respectively) from the bursa of both turkeys and ducks intravenously inoculated with influenza A viruses (5). High titers of a human-duck recombinant influenza virus were recovered from the bursa of ducks inoculated with the virus. The presence of high titers in the bursa suggests that this lymphoid organ could be the primary site of virus replication because the contents of the bursa empty into the cloaca and because lower levels of virus were detected in the feces (10). Influenza viruses detected from cloacal swabs could signify virus replication in either the lower intestine, the kidneys, or the bursa of Fabricius.
Until recently, it was thought that humans were infected with novel influenza viruses through swine because pigs can support the replication of both avian and human influenza A viruses (26). However, direct transmission of avian influenza viruses to humans has increased in recent years, beginning with the 1997 outbreak in which human infection by avian H5N1 was confirmed for the first time (4, 6). Two years later, two cases of human infection with avian H9N2 influenza were identified; and in The Netherlands in 2003, highly pathogenic avian H7N7 caused 89 confirmed cases of illness with one fatality during a fowl plague outbreak (7, 23). In late 2003, avian H5N1 was again directly transmitted to humans. By May 2005, 97 laboratory-confirmed cases of human infection had been identified in several Asian countries (World Health Organization, http://www.who.int/csr/disease/avian_influenza/country/cases_table-2005_05_09/en/index.html). No cases of human-to-human transmission have been confirmed, and only one incidence of probable transmission of avian H5N1 to humans has been reported. The continued introduction of avian viruses directly to humans favors the virus's chances of acquiring the gene or genes necessary for human-to-human transmission, because the genes of avian viruses have more opportunity to reassort with human influenza viruses (39). Live bird markets play a pivotal role in the distribution and genetic interaction of influenza viruses and also increase contact between poultry and humans. Closure of live poultry markets would markedly reduce the risk for chance reassortment to occur.
Acknowledgments
This study was supported by U.S. Public Health Service grant AI95357 and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank Margaret Carbaugh for editorial assistance.
REFERENCES
- 1.Beare, A. S., and R. G. Webster. 1991. Replication of avian influenza viruses in humans. Arch. Virol. 119:37-42. [DOI] [PubMed] [Google Scholar]
- 2.Becker, W. B. 1966. The isolation and classification of Tern virus: influenza A-Tern South Africa-1961. J. Hyg. (London) 64:309-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges, C. B., W. Lim, J. Hu-Primmer, L. Sims, K. Fukuda, K. H. Mak, T. Rowe, W. W. Thompson, L. Conn, X. Lu, N. J. Cox, and J. M. Katz. 2002. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997-1998. J. Infect. Dis. 185:1005-1010. [DOI] [PubMed] [Google Scholar]
- 4.Claas, E. C., J. C. de Jong, R. Van Beek, G. F. Rimmelzwaan, and A. D. Osterhaus. 1998. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine 16:977-978. [DOI] [PubMed] [Google Scholar]
- 5.Condobery, P. K., and R. D. Slemons. 1992. Biological properties of waterfowl-origin type A influenza viruses in chickens. Avian Dis. 36:17-23. [PubMed] [Google Scholar]
- 6.de Jong, J. C., E. C. Claas, A. D. Osterhaus, R. G. Webster, and W. L. Lim. 1997. A pandemic warning? Nature 389:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan, Y., K. F. Shortridge, S. Krauss, P. S. Chin, K. C. Dyrting, T. M. Ellis, R. G. Webster, and M. Peiris. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins, D. A., K. F. Shortridge, and P. L. Ng. 1987. Bile immunoglobulin of the duck (Anas platyrhynchos). II. Antibody response in influenza A virus infections. Immunology 62:499-504. [PMC free article] [PubMed] [Google Scholar]
- 10.Hinshaw, V. S., R. G. Webster, C. W. Naeve, and B. R. Murphy. 1983. Altered tissue tropism of human-avian reassortant influenza viruses. Virology 128:260-263. [DOI] [PubMed] [Google Scholar]
- 11.Hinshaw, V. S., R. G. Webster, and R. J. Rodriguez. 1979. Influenza A viruses: combinations of hemagglutinin and neuraminidase subtypes isolated from animals and other sources. Brief review. Arch. Virol. 62:281-290. [DOI] [PubMed] [Google Scholar]
- 12.Homme, P. J., and B. C. Easterday. 1970. Avian influenza virus infections. IV. Response of pheasants, ducks, and geese to influenza A-turkey-Wisconsin-1966 virus. Avian Dis. 14:285-290. [PubMed] [Google Scholar]
- 13.Ito, T. 2000. Interspecies transmission and receptor recognition of influenza A viruses. Microbiol. Immunol. 44:423-430. [DOI] [PubMed] [Google Scholar]
- 14.Kawaoka, Y., S. Krauss, and R. G. Webster. 1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63:4603-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kida, H., L. E. Brown, and R. G. Webster. 1982. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 122:38-47. [DOI] [PubMed] [Google Scholar]
- 16.Kida, H., R. Yanagawa, and Y. Matsuoka. 1980. Duck influenza lacking evidence of disease signs and immune response. Infect. Immun. 30:547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauss, S., D. Walker, S. P. Pryor, L. Niles, L. Chenghong, V. S. Hinshaw, and R. G. Webster. 2004. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 4:177-189. [DOI] [PubMed] [Google Scholar]
- 18.Liu, M., S. He, D. Walker, N. Zhou, D. R. Perez, B. Mo, F. Li, X. Huang, R. G. Webster, and R. J. Webby. 2003. The influenza virus gene pool in a poultry market in South central china. Virology 305:267-275. [DOI] [PubMed] [Google Scholar]
- 19.Makarova, N. V., H. Ozaki, H. Kida, R. G. Webster, and D. R. Perez. 2003. Replication and transmission of influenza viruses in Japanese quail. Virology 310:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matrosovich, M. N., S. Krauss, and R. G. Webster. 2001. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156-162. [DOI] [PubMed] [Google Scholar]
- 21.Nettles, V. F., J. M. Wood, and R. G. Webster. 1985. Wildlife surveillance associated with an outbreak of lethal H5N2 avian influenza in domestic poultry. Avian Dis. 29:733-741. [PubMed] [Google Scholar]
- 22.Palmer, D. F., M. T. Coleman, W. R. Dowdle, and G. C. Schild. 1975. Advanced laboratory techniques for influenza diagnosis. Immunology series no. 6, p. 51-52. U.S. Department of Health, Education, and Welfare, Washington, D.C.
- 23.Peiris, M., K. Y. Yuen, C. W. Leung, K. H. Chan, P. L. Ip, R. W. Lai, W. K. Orr, and K. F. Shortridge. 1999. Human infection with influenza H9N2. Lancet 354:916-917. [DOI] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 25.Rowe, T., R. A. Abernathy, J. Hu-Primmer, W. W. Thompson, X. Lu, W. Lim, K. Fukuda, N. J. Cox, and J. M. Katz. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholtissek, C. 1990. Pigs as ‘mixing vessels’ for the creation of new pandemic influenza A viruses. Med. Principles Pract. 2:65-71. [Google Scholar]
- 27.Scholtissek, C., W. Rohde, V. von Hoyningen, and R. Rott. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13-20.664248 [Google Scholar]
- 28.Senne, D. A., D. L. Suarez, J. C. Pedersen, and B. Panigrahy. 2003. Molecular and biological characteristics of H5 and H7 avian influenza viruses in live-bird markets of the northeastern United States, 1994-2001. Avian Dis. 47:898-904. [DOI] [PubMed] [Google Scholar]
- 29.Sharp, G. B., Y. Kawaoka, S. M. Wright, B. Turner, V. Hinshaw, and R. G. Webster. 1993. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol. Infect. 110:161-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shortridge, K. F., P. Gao, Y. Guan, T. Ito, Y. Kawaoka, D. Markwell, A. Takada, and R. G. Webster. 2000. Interspecies transmission of influenza viruses: H5N1 virus and a Hong Kong SAR perspective. Vet. Microbiol. 74:141-147. [DOI] [PubMed] [Google Scholar]
- 31.Shortridge, K. F., and C. H. Stuart-Harris. 1982. An influenza epicentre? Lancet ii:812-813. [DOI] [PubMed] [Google Scholar]
- 32.Sims, L. D., T. M. Ellis, K. K. Liu, K. Dyrting, H. Wong, M. Peiris, Y. Guan, and K. F. Shortridge. 2003. Avian influenza in Hong Kong 1997-2002. Avian Dis. 47:832-838. [DOI] [PubMed] [Google Scholar]
- 33.Slemons, R. D., and D. E. Swayne. 1990. Replication of a waterfowl-origin influenza virus in the kidney and intestine of chickens. Avian Dis. 34:277-284. [PubMed] [Google Scholar]
- 34.Stallknecht, D. E. 1998. Ecology and epidemiology of avian influenza viruses in wild bird populations: waterfowl, shorebirds, pelicans, cormorants, etc., p. 61-69. In D. E. Swayne and R. D. Slemons (ed.), Proceedings of the 4th International Symposium on Avian Influenza. U.S. Animal Health Association, Richmond, Va.
- 35.Stallknecht, D. E., and S. M. Shane. 1988. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 12:125-141. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson, I., J. M. Wood, K. G. Nicholson, and M. C. Zambon. 2003. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J. Med. Virol. 70:391-398. [DOI] [PubMed] [Google Scholar]
- 37.Suss, J., J. Schafer, H. Sinnecker, and R. G. Webster. 1994. Influenza virus subtypes in aquatic birds of eastern Germany. Arch. Virol. 135:101-114. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, Y., T. Ito, T. Suzuki, R. E. Holland, Jr., T. M. Chambers, M. Kiso, H. Ishida, and Y. Kawaoka. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825-11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungchusak, K., P. Auewarakul, S. F. Dowell, R. Kitphati, W. Auwanit, P. Puthavathana, M. Uiprasertkul, K. Boonnak, C. Pittayawonganon, N. J. Cox, S. R. Zaki, P. Thawatsupha, M. Chittaganpitch, R. Khontong, J. M. Simmerman, and S. Chunsutthiwat. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352:333-340. [DOI] [PubMed] [Google Scholar]
- 40.Warr, G. W., K. E. Magor, and D. A. Higgins. 1995. IgY: clues to the origins of modern antibodies. Immunol. Today 16:392-398. [DOI] [PubMed] [Google Scholar]
- 41.Webby, R. J., and R. G. Webster. 2003. Are we ready for pandemic influenza? Science 302:1519-1522. [DOI] [PubMed] [Google Scholar]
- 42.Webster, R. G. 2004. Wet markets—a continuing source of severe acute respiratory syndrome and influenza? Lancet 363:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webster, R. G., M. Yakhno, V. S. Hinshaw, W. J. Bean, and K. G. Murti. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood, J. M., R. G. Webster, and V. F. Nettles. 1985. Host range of A/Chicken/Pennsylvania/83 (H5N2) influenza virus. Avian Dis. 29:198-207. [PubMed] [Google Scholar]