Abstract
Dengue virus is a positive-strand RNA virus and a member of the genus Flavivirus, which includes West Nile, yellow fever, and tick-borne encephalitis viruses. Flavivirus genomes are translated as a single polyprotein that is subsequently cleaved into 10 proteins, the first of which is the viral capsid (C) protein. Dengue virus type 2 (DENV2) and other mosquito-borne flaviviruses initiate translation of C from a start codon in a suboptimal context and have multiple in-frame AUGs downstream. Here, we show that an RNA hairpin structure in the capsid coding region (cHP) directs translation start site selection in human and mosquito cells. The ability of the cHP to direct initiation from the first start codon is proportional to its thermodynamic stability, is position dependent, and is sequence independent, consistent with a mechanism in which the scanning initiation complex stalls momentarily over the first AUG as it begins to unwind the cHP. The cHP of tick-borne flaviviruses is not maintained in a position to influence start codon selection, which suggests that this coding region cis element may serve another function in the flavivirus life cycle. Here, we demonstrate that the DENV2 cHP and both the first and second AUGs of C are necessary for efficient viral replication in human and mosquito cells. While numerous regulatory elements have been identified in the untranslated regions of RNA viral genomes, we show that the cHP is a coding-region RNA element that directs start codon selection and is required for viral replication.
The four dengue virus serotypes (DENV1 to -4) are members of the genus Flavivirus in the family Flaviviridae, which includes such medically important human pathogens as West Nile virus (WNV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), and hepatitis C virus. The genus Flavivirus includes arboviruses transmitted by mosquitoes or ticks, as well as species that are not transmitted by a known arthropod vector (30). Dengue virus is transmitted to humans by Aedes aegypti and Aedes albopictus mosquitoes, resulting in tens of millions of cases of dengue fever annually, with 2.5 billion people at risk for infection worldwide (58). A subset of infections progress to the more severe disease, dengue hemorrhagic fever/dengue shock syndrome, leading to an estimated 500,000 hospitalizations each year, primarily among children (58). Despite the public health impact of dengue virus, a vaccine is not yet available commercially, and treatment of infection remains largely supportive. In addition, much of the basic virology of dengue virus infections remains to be elucidated.
The flaviviruses contain a capped, positive-sense RNA genome that is translated as a single polyprotein, which is cleaved co- and posttranslationally by host and viral proteases to yield three structural and seven nonstructural proteins (30). During cap-dependent translation, initiation occurs when the cap-binding complex eukaryotic initiation factor 4F (eIF4F) and cofactor eIF4B bind the 5′ cap structure. Recruitment of the 43S preinitiation complex by eIF4F leads to formation of the 48S complex, which scans the 5′ untranslated region (UTR), unwinding the secondary structure until it locates a start codon (45). Extensive secondary structure in the 5′ UTR can impede the scanning complex from reaching the start codon (35). A portion of the time, the initiation machinery recognizes the first start codon it reaches, usually AUG. However, the efficiency of a given start codon is dependent upon the sequences immediately upstream and downstream of the start codon, known as the Kozak consensus sequence in vertebrates: gccRccAUGG (uppercase letters indicate functionally important positions, and boldface letters indicate the start codon) (31). Functionally, the most important positions have been demonstrated to be the −3 and +4 positions relative to the A of AUG (33, 36). Ninety-seven percent of vertebrate mRNAs have a purine residue at the −3 position (32), and a purine at the −3 site and a guanine at the +4 site can dramatically increase recognition of a start codon in vitro and in cells (33, 36).
The start codon of the flavivirus polyprotein is located at the beginning of the capsid protein (C), the dimeric alpha-helical protein component of the viral nucleocapsid (30). The solution structure of DENV C suggests that the highly basic carboxy terminus both forms a dimerization domain and interacts with the viral RNA (40). The N terminus is disordered in solution, but given that it is also highly positively charged, it has been proposed that it may interact with the viral RNA and form a regular structure upon RNA binding (24). The N termini of the flavivirus capsid proteins are not well conserved, but the overall positive charge and protein length are maintained (4), suggesting that the amino-terminal residues are important in the viral life cycle. Despite this, we have noted that the start codons of DENV1 to -3 and many of the mosquito-borne flaviviruses are in a poor initiation context, predicting that N-terminally truncated capsid products would be produced by leaky scanning. Nevertheless, sequencing of the capsid protein supports the first AUG as the start codon that is utilized (4).
It has been reported that introducing RNA secondary structure downstream of a start codon in a poor initiation context can enhance recognition of the suboptimal codon (33, 34). The optimal distance from the start codon for a hairpin to direct initiation is 12 to 15 nucleotides (nt), which corresponds to the footprint of a ribosome paused over a start codon (37). It is believed that the scanning initiation machinery pauses at the structural element in order to unwind it, allowing the ribosome to remain in contact with an AUG in a poor initiation context (34, 37, 51).
Given the lack of a strong initiation context among many of the mosquito-borne flaviviruses, we hypothesized that secondary structure present downstream of the start codon could be responsible for the high efficiency of initiation from the first AUG. Here, we describe a mechanism for translation initiation codon selection by DENV2 involving a phylogenetically conserved RNA secondary-structure element in the capsid coding region that likely also plays a role in another step of the viral life cycle.
MATERIALS AND METHODS
Secondary-structure prediction.
RNA secondary structures were predicted using mfold 3.1 (41, 62). In cases where multiple structures were predicted, the most stable structure was chosen. Reported ΔG values reflect efn2 refinement (the mfold free-energy computation incorporating coaxial stacking and the Jacobson-Stockmeyer theory for multibranched loops) (41, 62). For phylogenetic consensus structure prediction, sequences were first aligned with ClustalX 1.8 (52) software and then processed by the RNAalifold module of the Vienna RNA Package (21).
Construction of DNA constructs.
The C-FLAG constructs were generated by splicing by overlap extension-PCR (SOE-PCR) by amplifying the T7 promoter, 5′UTR, and first 270 nt of the capsid gene from the infectious clone (IC) of DENV2 strain 16681 (pD2/IC-30P, hereafter referred to as pD2/IC; a gift of R. Kinney, Centers for Disease Control and Prevention, Fort Collins, CO) and fusing the product to a 3XFLAG epitope and the 3′ UTR of DENV2 16681. The 3XFLAG region was formed by overlapping primers using the sequence from p3xFLAG-CMV-10 (Sigma, St. Louis, MO) without the initiation codon and with a terminal stop codon (TAG). Mutations were introduced by SOE-PCR or by QuickChange site-directed mutagenesis (Stratagene, La Jolla, CA). SOE-PCR products were digested with SacI and XbaI and ligated into pUC19 (New England Biolabs, Beverly, MA). Infectious-clone variants containing mutations in the 5′ UTR or C coding region were generated by amplifying the T7 promoter and the first 1,391 nt of the transcribed region of pD2/IC and introducing mutations by SOE-PCR or QuickChange. The resulting products were then digested with SacI and SphI and ligated into SacI/SphI-digested pD2/IC. IC variants containing mutations in the 3′ UTR were generated by amplifying the NS5 coding region and 3′ UTR of pD2/IC and introducing mutations by SOE-PCR. The resulting PCR products were digested with AvrII and XbaI and ligated into AvrII/XbaI-digested pD2/IC. Primer sequences are available upon request.
In vitro transcription.
C-FLAG RNAs were generated via in vitro transcription by incubating XbaI-linearized DNA with 5 mM each GTP, CTP, and UTP; 0.36 mM ATP; 1.8 mM 7mG(5′)ppp(5′)A cap analog (New England Biolabs, Beverly, MA); 40 mM Tris-Cl, pH 7.9; 2.5 mM spermidine; 26 mM MgCl2; 0.01% Triton X-100; 10 mM dithiothreitol; 4 units/ml inorganic pyrophosphatase; 320 units/ml Superase•in (Ambion, Austin, TX); and 6.4 μl/ml T7 polymerase (a gift of K. Collins, University of California, Berkeley) for 2 h at 37°C. Eighty units/ml of DNase RQ-1 (Promega, Madison, WI) was added, and the reaction mixtures were incubated at 37°C for an additional 15 min. Free nucleotides were removed by gel filtration chromatography on a Micro Bio-Spin P-30 Tris column (Bio-Rad Laboratories, Hercules, CA). Infectious-clone RNAs were generated by in vitro transcription with the RiboMax Large Scale RNA Production System (T7) (Promega) with the following modifications to the manufacturer's protocol: 5 mM each GTP, CTP, and UTP; 1 mM ATP; and 5 mM 7mG(5′)ppp(5′)A cap analog incubated for 4 h at 30°C with the addition of 2 mM ATP after 30 min. All DNA templates were generated by digestion with XbaI and were gel purified using the QIAquick Gel Extraction kit (QIAGEN, Valencia, CA).
Cell culture.
Baby hamster kidney cells (BHK-21 clone 15) were grown in minimal essential medium-alpha (Gibco, Carlsbad, CA) with 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, pH 7.5, and 5% fetal bovine serum (FBS) (HyClone, Logan, UT) at 37°C in 5% CO2. Human hepatoma (Hep3B) cells were grown in Dulbecco's modified Eagle's medium (Gibco) with penicillin, streptomycin, HEPES, and 10% FBS at 37°C in 5% CO2. Aedes albopictus (C6/36) cells were grown in Leibovitz's L-15 medium (Gibco) with penicillin, streptomycin, HEPES, and 10% FBS (C-FLAG transfections) or 5% FBS (pD2/IC transfections) at 28°C.
RNA transfection.
Hep3B cells were seeded in 24-well plates, grown to 70% confluence, and transfected with 1 μg C-FLAG RNA using Lipofectamine 2000 (Invitrogen), followed by a 4-h incubation at 37°C in 5% CO2. C6/36 cells were transfected with 1 μg C-FLAG RNA with TransMessenger Transfection Reagent (QIAGEN) and incubated for 3 h at 28°C. Transfected C6/36 cells were washed in 1 ml L-15 medium and incubated for an additional 21 h at 28°C. All cells were lysed in 50 μl lysis buffer (125 mM NaCl, 50 mM Tris-Cl, pH 8.0, 10% glycerol, and 1% NP-40), and the lysate was clarified by centrifugation. For transfections with infectious-clone RNA, Hep3B and C6/36 cells were seeded in 24-well plates, grown to 50% confluence, and transfected as described above but were washed three times in 1 ml culture medium at 2 h posttransfection and then incubated for a total of 72 h. The supernatants were collected, and the viral titer was assessed by plaque assay. Cellular RNA was harvested from a duplicate well at 2 h posttransfection using the RNEasy Mini kit (QIAGEN), and the transfection efficiency was determined by quantitative reverse transcription (qRT)-PCR.
Quantitative RT-PCR.
To control for the transfection efficiency of viral RNAs, a duplicate well was transfected and cells were harvested after 2 h, at which time DENV2 RNAs had not undergone replication (11, 13). Intracellular RNA was extracted with the RNEasy Mini Kit (QIAGEN). qRT-PCR of viral RNA from transfected cells was performed on an Applied Biosystems 7300 using LUX Fluorogenic Primers and the Superscript III Platinum One-Step Quantitative RT-PCR system (Invitrogen, Carlsbad, CA). Anti-DENV2 NS1 primers were labeled with 6-carboxy-fluorescein, and anti-β-actin primers were labeled with 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein. Primer sequences are available upon request.
Immunoblotting.
Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 14% acrylamide gel and transferred to 0.2 μm Protran nitrocellulose (Schleicher & Schuell BioScience, Keene, NH). The immunoblots were incubated with anti-FLAG M2 monoclonal antibody (Sigma) at a dilution of 1/20,000, followed by incubation with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Jackson Immunoresearch, West Grove, PA) at a dilution of 1/40,000. The immunoblots were imaged on a Chemi-Doc EQ system (Bio-Rad), and band quantitation was conducted with QuantityOne software (Bio-Rad). The maximum linear range detectable in this system was determined to be 32-fold by serial dilution.
Plaque assay.
Plaque assays for virus titration were conducted as described previously (12). Briefly, BHK-21 monolayers were grown to 70% confluence in 12-well plates and incubated with serially diluted transfection supernatants for 2 h at 37°C in 5% CO2. The wells were subsequently overlaid with minimal essential medium-Eagle's medium (Sigma), 1% SeaPlaque low-melting-point agarose (Cambrex, Rockland, ME), and 5% FBS; incubated for 5 days; and fixed with 4% formaldehyde. The wells were stained with 0.1% crystal violet in 20% ethanol, and PFU per ml were calculated.
Viral cDNA sequencing.
A C6/36 cell monolayer was infected with plaque-positive supernatant from pD2/IC-AUG1mut-transfected C6/36 cells and incubated for 10 days, at which point the viral titer was detectable by plaque assay. Viral RNA was extracted from the supernatant using the QIAamp Viral RNA Mini kit (QIAGEN) and amplified by RT-PCR using the Superscript III Platinum One-Step RT-PCR system (Invitrogen). The RT-PCR product was extracted using the QIAquick PCR Cleanup kit (QIAGEN) and submitted for sequencing at the University of California, Berkeley, DNA Sequencing Facility (Berkeley, CA).
Statistical analysis.
Calculations of the mean and standard deviation (SD) were performed by Microsoft Excel 2004 for Macintosh (Microsoft Corporation, Redmond, WA). A statistically significant difference was defined as a P value of <0.05 by Student's t test, which was performed in Microsoft Excel.
RESULTS
Translation of DENV2 capsid initiates from multiple start codons.
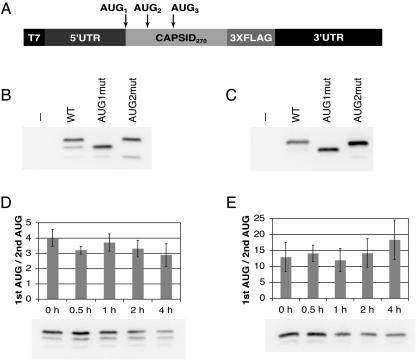
To determine whether translation of the DENV2 capsid protein initiates from multiple sites, constructs were designed that consisted of the 5′ UTR and the first 270 nt of the C gene of DENV2 fused to a 3XFLAG epitope and the DENV2 3′ UTR under the control of the T7 RNA polymerase promoter (C-FLAG) (Fig. 1A). In vitro-transcribed reporter RNAs were transfected into human hepatoma (Hep3B) or Aedes albopictus (C6/36) cells, chosen to represent the natural host and vector of DENV, respectively. Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and initiation from the first, second, or third AUG was assessed as a shift in molecular weight on an immunoblot. Initiation from the first start codon was favored in both Hep3B (Fig. 1B) and C6/36 (Fig. 1C) cells; however, leaky scanning did occur, and products that initiated from the second and third AUGs were observed. To ensure that the observed products corresponded to the assigned initiation codons, control RNAs were constructed in which the first or second start codons were mutated. Translation products from the AUG1mut and AUG2mut RNAs analyzed in parallel with the wild-type (wt) C-FLAG products confirmed the use of the first, second, and third start codons of C (Fig. 1B and C). Despite the poor initiation context of the DENV2 C protein, the full-length translation product represented the dominant band in both cell types.
FIG. 1.
Translation of DENV2 capsid initiates from multiple AUGs. RNAs consisting of the DENV2 5′ UTR, the first 270 nt of the capsid gene, a 3XFLAG epitope, and the DENV2 3′ UTR (C-FLAG) were transfected into Hep3B and C6/36 cells. (A) Schematic diagram of C-FLAG RNA constructs. (B) Anti-FLAG immunoblot of C-FLAG-transfected Hep3B cells at 4 h posttransfection. (C) Anti-FLAG blot of transfected C6/36 cells at 24 h posttransfection. (D) Anti-FLAG immunoblot of Hep3B cells treated with 10 μg/ml cycloheximide (CHX) at 3 h posttransfection, incubated for 30 min, and lysed at the time indicated. The efficiency of codon usage is expressed as a ratio of the higher-molecular-weight C-FLAG isoform (1st AUG) to the lower-molecular-weight isoform (2nd AUG), and the mean ratio and SD were calculated from four experiments. (E) Anti-FLAG immunoblot of C6/36 cells treated as in panel D with the addition of CHX at 18 h posttransfection. The graph is as in panel D; the mean ratio and SD were calculated from five experiments. −, no RNA transfection (control).
One possible explanation for the greater amount of the full-length C-FLAG product is that the larger isoform is more stable in cells. To assess the relative stabilities of the C-FLAG isoforms produced from the first two start codons, Hep3B and C6/36 cells were transfected with C-FLAG RNAs, incubated for the times indicated, and treated with cycloheximide to prevent further translation. The ratios of the two products were compared over a period of 4 h. The ratio of the two largest C-FLAG isoforms did not change significantly over time in either Hep3B or C6/36 cells (Fig. 1D and E); therefore, instability of the truncated product cannot account for the apparent efficient usage of the first start codon despite its poor initiation context.
A conserved hairpin structure is predicted in the capsid gene coding region.
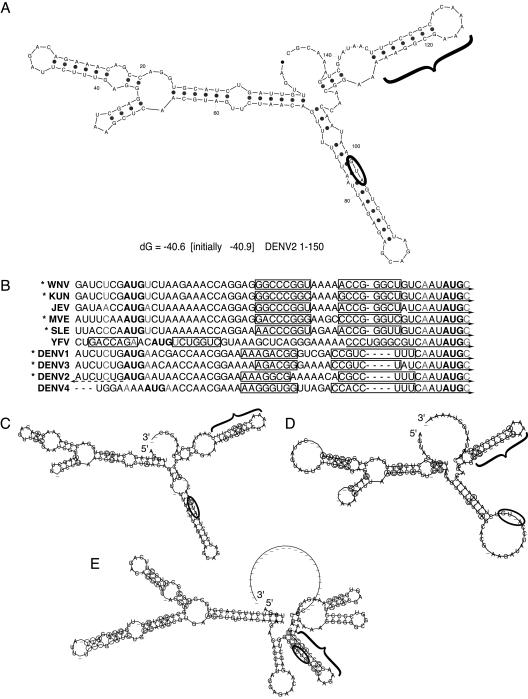
To determine whether downstream secondary structure might be responsible for the efficiency of first start codon usage in translation of C-FLAG, the structure of the first 150 nt of DENV2 was predicted using mfold (41, 62). A hairpin element (cHP) was predicted to form 14 nt downstream of the start codon in the C coding region of DENV2 (Fig. 2A). This cHP structure is maintained when small segments, as well as the full-length virus, are folded and is present in the majority of computed structures (data not shown).
FIG. 2.
A conserved hairpin element is predicted among mosquito- and tick-borne flaviviruses. (A) The RNA secondary structure of the first 150 nt of DENV2 was predicted by mfold; the start codon is circled, and the cHP is indicated by a bracket. (B) Alignment of start codons and the first in-frame AUG of mosquito-borne flaviruses, with predicted cHP stem regions outlined by rectangles. The −3 and +4 positions are indicated in gray letters. Viruses with a poor initiation context are indicated by asterisks. 5′ CS regions are indicated by solid arrows. The DENV2 5′ UAR region is indicated by a dashed arrow. (C) Phylogenetic consensus structure based on aligned sequences of DENV1, DENV2, DENV3, and DENV4 as computed by RNAalifold, with the start codon circled and the cHP indicated with a bracket. Covariant residues are circled. (D) Phylogenetic consensus structure of the mosquito-borne Japanese encephalitis serogroup viruses, WNV, Kunjin virus, JEV, St. Louis encephalitis virus, and Murray valley encephalitis virus, as in panel C. (E) Phylogenetic consensus structure of the tick-borne viruses: tick-borne encephalitis, Omsk hemorrhagic fever, Kyasanur Forest disease, and Powassan viruses, as in panel C.
Having established that DENV2 initiates efficiently from a weak start codon and that it may encode the cHP to compensate for the poor initiation context, we sought to determine whether other flavivirus genomes might include a similar structure. The start codon context and secondary structure were analyzed for the flaviviruses for which sequences of the 5′ UTR and C were available. Of the mosquito-borne viruses examined, DENV1, DENV2, DENV3, Murray valley encephalitis virus, WNV, Kunjin virus and St. Louis encephalitis virus, had unfavorable contexts at both positions (Fig. 2B). Only three of the mosquito-borne viruses analyzed had favorable contexts at either the −3 or +4 position: DENV4, YFV, and JEV (Fig. 2B). In the mfold-predicted secondary structures of the mosquito-borne flaviviruses, the cHP element was present 12 to 16 nt downstream of the start codon in all but one case (YFV; data not shown). Although the predicted YFV structure reveals a hairpin in approximately the same position relative to the 5′ end of the genome as the other mosquito-borne flaviviruses, the YFV start codon, which is in a favorable initiation context, is located farther downstream and therefore is not in a position to be potentially regulated by the cHP (Fig. 2B and data not shown).
The conservation of the cHP among flaviviruses was further confirmed by phylogenetic secondary-structure analysis using RNAalifold (21). Among the dengue viruses and among flaviviruses of the Japanese encephalitis virus serogroup, the cHP was maintained by covariation to preserve the overall secondary structure despite changes to the primary sequence (Fig. 2C and D). It is noteworthy that the reported predicted structures of the 5′ UTRs of the mosquito-borne viruses are also maintained by covariation. In addition, among the tick-borne flaviviruses analyzed, the cHP was also maintained at approximately the same position relative to the 5′ end (Fig. 2E). However, similar to YFV, the cHP of the tick-borne flaviviruses contains the start codon, which is in a strong initiation context (Fig. 2E and data not shown).
The DENV2 cHP directs start codon selection.
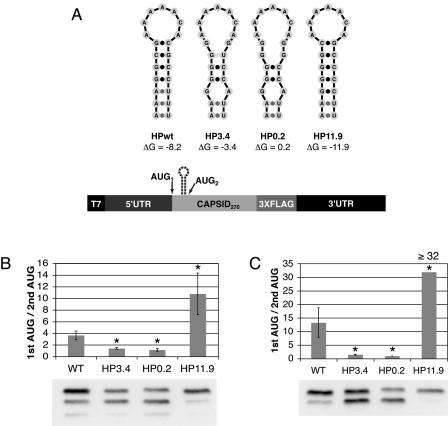
Given the position of the predicted cHP relative to the initiation site and the apparent conservation of the structure in the viruses examined, we sought to determine the role of the DENV2 cHP in translation initiation from the first start codon. If the cHP were directing initiation by causing the scanning translation machinery to stall, then disruption of the cHP would be predicted to increase initiation from the downstream AUG and stabilization of the cHP should increase initiation from the first start codon. To test the function of the cHP, mutations were introduced into C-FLAG constructs that were predicted to disrupt or further stabilize the cHP as computed by mfold. Two disrupted hairpins were constructed, with free energies of −3.4 kcal/mol (HP3.4) and +0.2 kcal/mol (HP0.2) when folded independently (Fig. 3A). RNAs were transfected into Hep3B and C6/36 cells, and translation products were analyzed by immunoblotting. Mutations that were predicted to disrupt the cHP decreased initiation from the first AUG in both Hep3B and C6/36 cells. In Hep3B cells, the ratio of the higher-molecular-weight to the lower-molecular-weight C-FLAG isoform decreased 61% in the HP3.4 and 68% in the HP0.2 constructs compared to the wt cHP (Fig. 3B). Similarly, in C6/36 cells, the ratio of the full-length C-FLAG to the truncated product decreased 89% in the HP3.4 and 93% in the HP0.2 constructs in comparison to the wt (Fig. 3C). Conversely, increasing the ΔG of the hairpin to −11.9 kcal/mol (HP11.9) enhanced initiation from the first start codon nearly threefold in Hep3B cells and at least 2.4-fold in C6/36 cells over the wt cHP (Fig. 3B and C, respectively). Thus, the cHP directs first-AUG selection proportional to its stability.
FIG. 3.
The DENV2 cHP regulates translation initiation site selection. (A) Schematic diagram of constructs used to test different HP free energies. (B) Anti-FLAG immunoblot of C-FLAG-transfected Hep3B cells at 4 h posttransfection. The efficiency of codon usage is expressed as a ratio of the higher-molecular-weight C-FLAG isoform (1st AUG) to the lower-molecular-weight isoform (2nd AUG). The error bars indicate SDs; the data are derived from four experiments. (C) Anti-FLAG immunoblot of transfected C6/36 cells at 24 h posttransfection as in panel B. The graph is as in panel B; the data represent the averages of three experiments. The ratio of 32 reflects the maximum difference detectable by immunoblotting under the conditions described. *, P < 0.001 relative to wt.
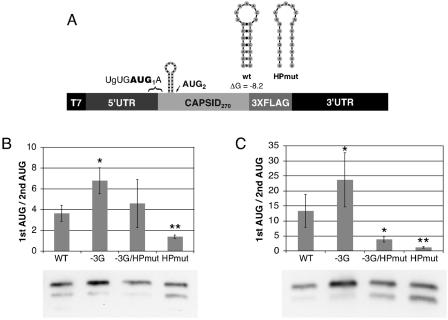
Having demonstrated that the DENV2 cHP functions in start site selection, we subsequently evaluated the efficiency of first-AUG selection by the cHP compared to start site selection directed by an improved start codon context. To assess start codon selection directed by context, a construct was designed in which the −3 position was changed to a guanine residue in conjunction with both a wt cHP and mutations predicted to disrupt formation of the stem of the cHP (−3G and −3G/HPmut, respectively) (Fig. 4A). In both cell types, pairing the −3G mutation with the wt cHP resulted in the increased recognition of the first AUG compared to either the −3G mutation alone or the wt cHP alone. In Hep3B cells, the wt cHP directed start codon selection similarly to the −3G/HPmut RNA (ratios of first- to second-AUG usage, 3.6 and 4.6 for wt and −3G/HPmut, respectively) (Fig. 4B). As expected, the wt cHP and −3G/HPmut RNAs exhibited greater initiation from the first AUG than the HPmut alone in Hep3B cells (Fig. 4B). In C6/36 cells, the wt cHP directed initiation 3.4 times more efficiently than did the −3G/HPmut RNA, while both the wt cHP and −3G/HPmut constructs favored the first AUG compared to the HPmut alone (Fig. 4C). Therefore, the cHP directs first-AUG selection as well as or better than an improved start codon context alone and can enhance the effect of a stronger initiation context in human and mosquito cells.
FIG. 4.
Comparison of first start codon selection by DENV2 cHP to selection by improved start codon context. (A) Schematic diagram of constructs used to test the efficiency of first start codon selection by the cHP or by initiation context. (B) Anti-FLAG immunoblot of C-FLAG-transfected Hep3B cells at 4 h posttransfection. The efficiency of codon usage is expressed as a ratio of the higher-molecular-weight C-FLAG isoform (1st AUG) to the lower-molecular-weight isoform (2nd AUG). The error bars indicate SDs; the data are derived from five experiments. (C) Anti-FLAG immunoblot of transfected C6/36 cells at 24 h posttransfection as in panel B. The graph is as in panel B; the data represent the averages of four experiments. *, P < 0.05 relative to wt; **, P < 0.01 relative to −3G/HPmut.
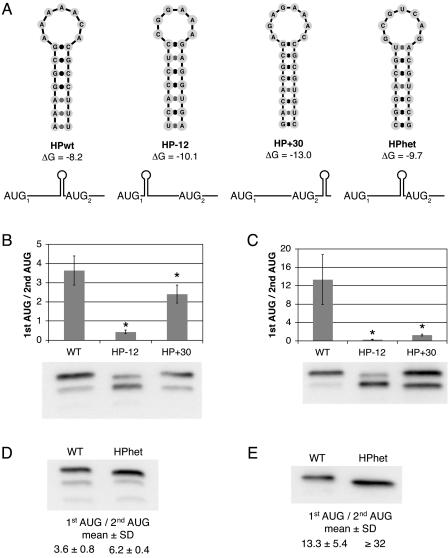
The DENV2 cHP functions in a position-dependent, sequence-independent manner.
The proposed mechanism by which coding-region secondary structure directs start codon selection predicts that the cHP would exert its effect in a position-dependent manner. To assess the position dependence of the cHP in directing first-AUG usage, two C-FLAG mutants were constructed in the HPmut background that created predicted hairpins either 12 nt upstream (HP−12) or 30 nt downstream (HP+30) from its natural position (Fig. 5A). Moving the cHP upstream decreased initiation from the first AUG 88% in Hep3B cells and 98% in C6/36 cells (Fig. 5B and C). In C6/36 cells, shifting the cHP downstream decreased first start codon selection by 91% (Fig. 5C). Repositioning the cHP downstream had a less dramatic, albeit significant, effect in Hep3B cells, decreasing initiation from the first AUG by 34% (Fig. 5B). To verify that the cHP itself and not a sequence motif within it is responsible for start codon selection, a C-FLAG construct was derived in which the cHP sequence was replaced with a heterologous sequence predicted to form a hairpin of similar ΔG (HPhet) (Fig. 5A). In both Hep3B and C6/36 cells, the change of cHP sequence did not decrease its ability to direct start site selection (Fig. 5D and E). Initiation from the first AUG in the HPhet construct increased 1.7-fold in Hep3B cells and at least 2.4-fold in C6/36 cells, consistent with its greater free energy (−9.7 kcal/mol versus −8.2 kcal/mol, respectively) (Fig. 5A). Overall, the position dependence and sequence independence of the cHP support the model of ribosome stalling in cHP-mediated start codon selection.
FIG. 5.
The DENV2 cHP directs start codon selection via a position-dependent, sequence-independent mechanism. (A) Schematic diagrams of constructs used to test the position and sequence dependence of the DENV2 cHP in regulating start site selection. (B) Anti-FLAG immunoblot of C-FLAG-transfected Hep3B cells at 4 h posttransfection. The efficiency of codon usage is expressed as a ratio of the higher-molecular-weight C-FLAG isoform (1st AUG) to the lower-molecular-weight isoform (2nd AUG). The error bars indicate SDs; the data are derived from three experiments. (C) Anti-FLAG immunoblot of transfected C6/36 cells at 24 h posttransfection as in panel B. The graph is as in panel B; the data represent the averages of three experiments. (D) Anti-FLAG immunoblot of transfected Hep3B cells at 4 h posttransfection as in panel B. The mean ratio and SD are calculated from four experiments. (E) Anti-FLAG immunoblot of transfected C6/36 cells at 24 h posttransfection as in panel B. The table is as in panel D; the data represent the averages of four experiments. The ratio of 32 reflects the maximum difference detectable by immunoblotting under the conditions described. *, P < 0.01 relative to wt.
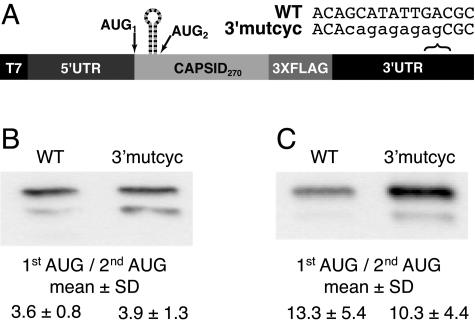
5′-3′ cyclization is not required for cHP-mediated start codon selection.
The flavivirus genome includes complementary sequences (CS) in the 5′ and 3′ ends whose basepairing activity is required for RNA synthesis during viral replication (9, 26, 47, 60, 61). Moreover, basepairing between the 5′ and 3′ ends has been demonstrated in the absence of an intact viral replication complex (2, 60). The 5′ cyclization domain (5′ CS) is located in the capsid coding region immediately downstream of the cHP in DENV2 (Fig. 2B). To determine whether the potential of the RNAs to cyclize between the 5′ and 3′ ends contributes positively or negatively to the cHP's ability to direct start codon selection, mutations were made in the 3′ cyclization domain (3′ CS) as described previously (3′mutcyc) (Fig. 6A) (61). In both Hep3B and C6/36 cells, no significant difference in start codon usage was detectable between the wt and 3′mutcyc constructs (Fig. 6B and C), indicating that the potential for the RNA to cyclize via the CS domains is not required for cHP-directed first-AUG selection.
FIG. 6.
5′-3′ cyclization is not required for cHP-mediated start codon selection. (A) Schematic diagram of constructs used to test the impact of potential 5′-3′ cyclization on initiation site selection. (B) Anti-FLAG immunoblot of C-FLAG-transfected Hep3B cells at 4 h posttransfection. The efficiency of codon usage is expressed as a ratio of the higher-molecular-weight C-FLAG isoform (1st AUG) to the lower-molecular-weight isoform (2nd AUG). The data are derived from three experiments. (C) Anti-FLAG immunoblot of transfected C6/36 cells at 24 h posttransfection as in panel B. The table is as in panel B; the data represent the averages of three experiments.
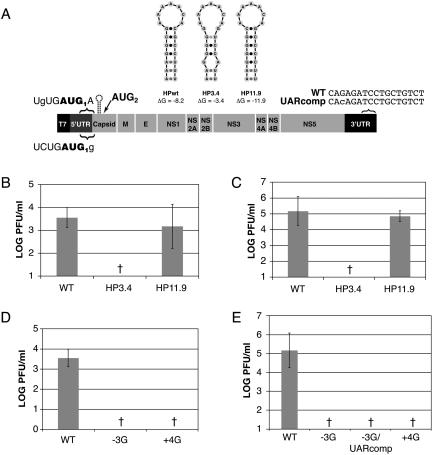
The DENV2 cHP and −3 position are required for efficient viral replication.
The conservation of the cHP element among mosquito- and tick-borne flaviviruses implies that this structure is critical for viral replication. To test the requirement for the DENV2 cHP in the viral life cycle, mutations were made in the infectious clone of DENV2 prototype Thai strain 16681 (pD2/IC) that were predicted to disrupt the cHP (Fig. 7A). Viral RNA was in vitro transcribed and transfected into Hep3B and C6/36 cells, and viral replication was assessed after 72 h by plaque assay. In Hep3B and C6/36 cells, disruption of the cHP (pD2/IC-HP3.4) reduced viral replication by at least 2.5 log units and 4 log units, respectively, to levels undetectable by plaque assay (Fig. 7B and C). Restoration of the hairpin structure (pD2/IC-HP11.9) rescued viral replication to levels similar to wt (Fig. 7B and C). The defect in viral replication cannot be attributed to amino acid changes that resulted from mutations to the cHP (K7R, A7G, and T11S), as the identical changes were present in the HP11.9 variant, which replicated to wt levels in both cell types. Thus, the cHP is required for efficient viral replication in human and mosquito cells.
FIG. 7.
The cHP is required for efficient DENV2 replication. In vitro-transcribed IC RNAs were transfected into Hep3B and C6/36 cell monolayers, and viral replication was assessed after 72 h by plaque assay. The titers were normalized to transfection efficiency as determined by qRT-PCR at 2 h posttransfection. (A) Schematics of IC variants utilized to study the role of the cHP and of the nucleotides that make up the DENV2 initiation context. (B) Viral titers are expressed as PFU per ml from IC-transfected Hep3B cells. One log unit reflects the limit of detection of a standard plaque assay. The error bars indicate SD; the data are derived from at least four experiments. (C) Viral titers from IC-transfected C6/36 cells. The graph is as in panel B; the data are derived from at least four experiments. (D) Viral titers from IC-transfected Hep3B cells. The graph is as in panel B; the data are derived from at least four experiments. (E) Viral titers from IC-transfected C6/36 cells. The graph is as in panel B; the data are derived from at least three experiments. †, not detectable.
Among DENV serotypes 1 to 3, a cytosine residue is conserved at the −3 position relative to the start codon (Fig. 2B), maintaining the first AUG in a poor initiation context. To determine whether this conserved position plays a role in the viral life cycle, the −3 position was mutated to a guanine to improve the initiation context (pD2/IC-3G). Mutation of this single nucleotide reduced viral replication by greater than 2.5 log units in Hep3B and 4 log units in C6/36 cells to levels below the limit of detection by plaque assay (Fig. 7D and E). The −3 position is contained within a region known as the 5′ upstream AUG region (UAR), which participates in basepairing with the 3′ end of the genome (3′ UAR) to circularize the viral RNA (2). To determine whether the −3G mutation exerted its effect by disrupting basepairing between the 5′ and 3′ UARs, a compensatory mutation in the 3′ UAR (pD2/IC-3G/3′UARcomp) (Fig. 7A) was made. The 3′UARcomp mutation in combination with the −3G mutation resulted in a decrease in viral replication to undetectable levels in C6/36 cells (Fig. 7E). Similar results were obtained in Hep3B cells (data not shown). Like the −3G infectious-clone variants, mutation of the +4 position to a guanine residue (pD2/IC-+4G) to improve the initiation context resulted in a level of viral replication undetectable by plaque assay in both Hep3B and C6/36 cells (Fig. 7D and E, respectively). Thus, the cHP and the conserved nucleotides that make up the DENV2 start codon context are required for efficient viral replication in cells.
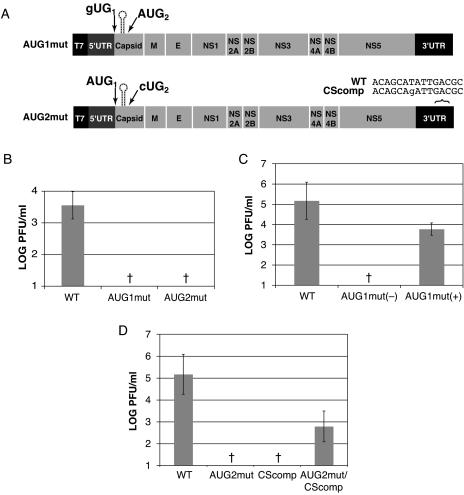
Both the first and second DENV2 capsid AUGs are required for viral replication.
The results of C-FLAG translation experiments indicate that the DENV2 capsid protein is translated from multiple start codons, producing full-length and shorter C isoforms. To determine whether both of the first two AUGs are required for the DENV2 viral life cycle, the first and second AUGs were mutated (pD2/IC-AUG1mut and pD2/IC-AUG2mut, respectively) (Fig. 8A). Hep3B and C6/36 cells were transfected with viral RNA, and replication was assessed by plaque assay. Mutation of the first AUG decreased viral replication in Hep3B cells by greater than 2.5 log units to levels below the limit of detection (Fig. 8B). In C6/36 cells, however, lower levels of viral replication from the AUG1mut variant were detected in three of five experiments. In the three experiments where a low level of viral replication was observed, the difference between wt and AUG1mut was approximately 1.4 log units (Fig. 8C). Surprisingly, sequencing of the progeny virus yielded the input GUG mutation of the first start codon. Mutation of the second AUG resulted in a decrease in viral titers of greater than 2.5 log units in Hep3B cells and 4 log units in C6/36 cells to undetectable levels (Fig. 8B and D, respectively). Similar to the −3G mutation, mutation of AUG2 resulted in a disruption of the cyclization of the genome that occurs through the 5′ and 3′ CS domains (9, 19, 26, 47, 61). To determine whether the defective replication of pD2/IC-AUG2mut was the result of disrupted cyclization, a compensatory mutation was made in the 3′ CS domain to restore basepairing (Fig. 8A). The single 3′CScomp modification alone (pD2/IC-3′CScomp) resulted in a lack of detectable viral replication in C6/36 cells (Fig. 8D). The variant combining both mutations (pD2/IC-AUG2mut/3′CScomp) did replicate, albeit at lower levels than the wt, in C6/36 cells (a decrease of 2.4 log units) (Fig. 8D). Similar results were obtained in Hep3B cells (data not shown). The decreased viral titers produced by the AUG1mut and AUG2mut pD2/IC variants, therefore, confirm the importance of the first and second AUGs in the viral life cycle.
FIG. 8.
Both the first and second AUGs of capsid play a role in the viral life cycle. In vitro-transcribed IC RNAs were transfected into Hep3B and C6/36 cell monolayers, and viral replication was assessed at 72 h by plaque assay. The titers were normalized to transfection efficiency as determined by qRT-PCR at 2 h posttransfection. (A) Schematic of IC variants utilized to study the roles of the first two AUGs in the capsid coding region. (B) Viral titers expressed as PFU/ml from IC-transfected Hep3B cells. One log unit reflects the limit of detection of a standard plaque assay. The error bars indicate SDs; the data are derived from at least three experiments. (C) Viral titers from IC-transfected C6/36 cells. The graph is as in panel B; the data are derived from at least five experiments. AUG1mut (−), not detectable in two of five experiments; AUG1mut (+), titerable virus detected in three of five experiments. (D) Viral titers from IC-transfected C6/36 cells. The graph is as in panel B; the data are derived from at least three experiments. †, not detectable.
DISCUSSION
In this report, we have investigated start codon selection and the role of a coding-region structural element in the life cycle of dengue virus type 2. We have shown phylogenetic evidence that a hairpin structure (cHP) is maintained among mosquito- and tick-borne flaviviruses and functions in start site selection in DENV2. The DENV2 cHP directs first-AUG usage proportional to its stability and performs as well as or better than a single nucleotide change to improve the initiation context in both human (Hep3B) and A. albopictus (C6/36) cells. The apparent position dependence and sequence independence of cHP-mediated translation initiation from the first AUG is consistent with the mechanism proposed for the role of coding-region secondary structure in start codon selection. Congruent with the maintenance of the cHP among flaviviruses, this cis element is necessary for the replication of DENV2 in Hep3B and C6/36 cells. Similarly, conserved residues immediately upstream and downstream of the start codon and the first and second AUGs of the capsid are required for efficient viral replication in both cell types.
cis-acting elements are frequently found in the UTRs of cellular and viral messages. Less common is the identification of regulatory elements located in the coding regions. However, it is likely that the coding region influences the secondary structure of the UTRs and that coding regions may also contain regulatory cis elements, as has been demonstrated in several viruses (15, 16, 18, 38). While structures in the untranslated regions of flaviviruses have been shown to regulate translation and viral RNA synthesis (1, 22, 44, 53, 60), no specific coding-region structures have been identified that regulate translation, replication, or encapsidation, with the exception of the 5′ CS, which forms a long-range interaction with the 3′ UTR (9, 19, 26, 47, 61). It is clear from studies in which subgenomic Kunjin virus replicons were complemented in trans with viral proteins that certain regions of flavivirus genomes are required to be present in cis for viral replication and assembly (27, 28, 39). Whether this reflects a requirement for regulatory RNA elements contained within the viral protein coding regions remains to be elucidated.
From studies involving C-FLAG fusion constructs, we have shown that the cHP modulates start codon selection in two cell types that represent the natural life cycle of the dengue viruses. Consistent with this model, the cHP enhances initiation site selection proportional to its free energy and exhibits sequence independence and position dependence in its modulation of translation initiation. Interestingly, usage of the first AUG when the hairpin was relocated upstream was less than that observed for the construct in which the cHP was simply disrupted. This may be the result of the first AUG being obscured by the new structure created 2 nt downstream of the start codon. Shifting the hairpin downstream from the wt position also decreased recognition of the first AUG. In both Hep3B and C6/36 cells, usage of the first start codon was greater than that observed for the construct with a disrupted cHP (HPmut) (Fig. 4). Although the maximum effect of enhancement has been reported to occur when a hairpin structure is located 14 nucleotides from the first AUG (34), the increased stability of the hairpin shifted downstream compared to the wt (−13.0 kcal/mol versus −8.2 kcal/mol) may contribute to its ability to influence start site selection. In both cases, the creation of a hairpin at another location might have altered the overall structure of the 5′ end in a way that influenced start site recognition, though in each case, changes to the mfold-predicted structure were minimal.
It is notable that the degree to which the cHP and mutant hairpins direct start codon selection is greater in C6/36 than in Hep3B cells. There are two possible explanations for this observation. First, the temperature at which C6/36 cells are grown is substantially lower than that for Hep3B cells (28°C versus 37°C). Free-energy calculations by mfold predict that this decrease in temperature will correspond to an increase in stability of the cHP by 1.5 kcal/mol. Alternatively, this may be the result of real differences between species. The mechanism by which ribosomes recognize start codons has been mapped not only to basepairing with the initiator tRNA-Met anticodon, but also to initiation factors, such as eIF1 and eIF1a, which dissociate improperly formed initiation complexes (48, 49); to eIF2, which may participate in proper anticodon presentation (10, 20, 51); and to general RNA binding proteins (42). In addition, start codon selection in cell-free translation systems has been shown to be dependent on the concentration of the RNA template (10), which has also been observed for the C-FLAG constructs (K. Clyde and E. Harris, unpublished observations). These findings support the assertion that the levels of individual translation factors influence start codon selection independently of the initiation context. It is therefore possible that the difference in efficiency seen between human and mosquito cells results from the differential expression or regulation of translation factors that modulate start codon recognition.
Attempts to compensate for the poor initiation context of the DENV2 start codon by altering the start codon context (−3G and +4G) in the infectious clone yielded viral variants that did not produce detectable titers. The −3 site in DENV2 lies within a 17-nt region that is conserved in all four DENV serotypes. It is not surprising that a mutation at this site caused a defect in viral replication. Moreover, in DENV4, a deletion in this motif was lethal to virus replication (6). It has been shown recently that this region, in addition to the CS, participates in circularization of the DENV genome (2). Mutation of the −3 site, in conjunction with two other substitutions by Alvarez et al. (2), decreased viral titers below the level of detection, and compensatory mutations to restore basepairing only partially rescued viral replication. It is not surprising, then, that the −3G variant of pD2/IC exhibits a defect in viral replication even in the context of a compensatory mutation in the 3′ UAR, confirming its role in viral replication beyond cyclization. In addition to the −3 site, the +4 site is also required for efficient viral replication. As this position is part of the coding region, this may reflect a preference at the amino acid level. These observations form a compelling argument for the evolution of the cHP as a mechanism for start site selection to overcome constraints on the nucleotides immediately upstream and downstream of the start codon.
Viral nucleocapsid proteins are linked to diverse functions in the viral life cycle in addition to assembly (3, 7, 8, 25, 43, 46, 54, 59). A number of groups have reported that the flavivirus C protein localizes to the nuclei of infected cells (5, 46, 55, 56), and DENV C specifically interacts with hnRNP K and can reverse its repression of transcription (8). In JEV, nuclear translocation of C has been implicated in viral replication in cell culture and in neurotropism in mice (46). However, we know of no studies that have reported the requirement for expression of C from the first and second initiation codons. Here, we have shown that the full-length C isoform is required for efficient viral replication in human cells. Viral variants that are unable to express C from the second in-frame start codon were also replication deficient, and a compensatory mutation to restore basepairing with the 3′ CS only partially rescued replication. This may reflect the requirement of the virus to produce the N-terminally truncated C product, perhaps for a purpose other than assembly. If the requirement for the second AUG were entirely based on RNA sequence and a requirement for cyclization, then the second AUG would not necessarily be maintained in frame with respect to the first start codon, and we would expect to see sequence covariation at this site. However, this downstream, in-frame AUG is maintained in all the mosquito-borne flaviviruses analyzed (Fig. 2). We cannot rule out the possibility that the replication defect reflects a requirement for a Met residue at that position in C, although no functional motifs in DENV2 C were predicted to require this Met residue (Clyde and Harris, unpublished), with the exception of a bipartite nuclear targeting sequence that was found not to be involved in nuclear localization of DENV C (55). Somewhat surprising was the production of a low level of virus that lacks the first start codon in C6/36 cells (Fig. 8C). This may reflect a small amount of translation of C protein from a non-AUG codon that was not detectable in the C-FLAG constructs. It is also possible that a low level of revertant genome acted as a helper virus in packaging the AUG1mut variant; perhaps the lack of a first start codon confers upon the viral genome a selective advantage at another step in the viral life cycle prior to packaging. Alternatively, the viral life cycle may not absolutely require the full-length C protein. Overall, given the conservation of the first AUG and a second in-frame AUG among the mosquito-borne flaviviruses, it is not surprising that mutation of either would be unfavorable to viral replication.
The conservation of the cHP among all flaviviruses, regardless of the location of the start codon and of its context, suggests that it may play a role in the viral life cycle in addition to directing translation initiation from the first AUG. A dual role for such a structural element has also been described for hepatitis B virus (23). Using the infectious clone of DENV2 strain 16681, we showed that the cHP is required for viral replication in human and mosquito cells. A possible explanation for the decreased replication seen in the pD2/IC variant containing a disrupted cHP is the reduced level of full-length C protein that is produced. Alternatively, the truncated C isomer may inhibit nucleocapsid formation by acting as a dominant negative. However, given that the difference in level between full-length and truncated C was approximately 2.5-fold in Hep3B cells and slightly greater than 9-fold in C6/36 cells, either scenario would be unlikely to account entirely for the decrease in titer of 2.5 and 4 log units, respectively. This implicates the DENV2 cHP as a structural element involved at other stages of the viral life cycle, such as the regulation of translation, RNA synthesis, or viral assembly. A similar coding-region element has been shown to confer enhanced translation in Sindbis virus (15, 16), although we observed no pronounced differences in translation efficiency among the DENV2 C-FLAG constructs. RNA structures are also responsible for packaging of the viral genome in a number of RNA viruses (14, 17, 38, 50, 57). Therefore, it is possible that the cHP forms part of a flavivirus encapsidation signal. Finally, it has been shown that the beginning of the C coding region is required for flavivirus RNA synthesis in subgenomic RNA and reporter replicon systems (29, 60, 61). In addition, both the 5′ end of the WNV genome and the 3′ UTR are required for negative-strand synthesis, whereas only the 5′ end is necessary for positive-strand synthesis in vitro (47). Thus, it is possible that RNA sequences or structures act as replication signals or stabilize the replication machinery at the 3′ end of the negative strand. The cHP, therefore, may contribute to positive-strand (or both positive- and negative-strand) synthesis signals. We are in the process of determining which steps of the viral life cycle are regulated by the cHP.
RNA sequence and structural elements, primarily in the untranslated regions of the genome, have been linked to diverse processes in the flavivirus life cycle. Here, we have identified a conserved structure in the coding region of DENV2 that directs start site selection and is required for efficient viral replication in cell culture. Whereas the cHP is positioned to play a role in translation start site selection in most of the mosquito-borne flaviviruses, it is not situated to do so in the tick-borne viruses, although the structure is maintained by sequence covariation. This supports a role for the cHP at a step in the viral life cycle beyond enhanced first-AUG usage. Understanding of the specific cis elements that are utilized during viral replication will serve to broaden our knowledge of the basic biology of flaviviruses and will better inform design of vaccines and antiviral therapies.
Acknowledgments
We thank Jean-Yves Sgro and Ann Palmenberg for mfold prediction of the entire DENV2 strain 16681 genome, K. Jordan Walker and Anna-Marija Helt for assistance with cloning, and Richard Kinney for providing pD2/IC. We are grateful to Sondra and Milt Schlesinger, Suman M. Paranjape, Anna-Marija Helt, Dianna Edgil, and Katherine L. Holden for critical reading of the manuscript and/or helpful discussions.
Funding for this research was provided by NIH grant AI052324 and the Pew Charitable Trusts grant 26175C (E.H.), as well as by the Berkeley Fellowship for Graduate Study and the Zimmer Family Foundation scholarship program (K.C.).
REFERENCES
- 1.Alvarez, D. E., A. L. De Lella Ezcurra, S. Fucito, and A. V. Gamarnik. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200-212. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, D. E., M. F. Lodeiro, S. J. Luduena, L. I. Pietrasanta, and A. V. Gamarnik. 2005. Long-range RNA-RNA interactions circularize the dengue virus genome. J. Virol. 79:6631-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bampi, C., S. Jacquenet, D. Lener, D. Decimo, and J. L. Darlix. 2004. The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance. Int. J. Biochem. Cell. Biol. 36:1668-1686. [DOI] [PubMed] [Google Scholar]
- 4.Bell, J. R., R. M. Kinney, D. W. Trent, E. M. Lenches, L. Dalgarno, and J. H. Strauss. 1985. Amino-terminal amino acid sequences of structural proteins of three flaviviruses. Virology 143:224-229. [DOI] [PubMed] [Google Scholar]
- 5.Bulich, R., and J. G. Aaskov. 1992. Nuclear localization of dengue 2 virus core protein detected with monoclonal antibodies. J. Gen. Virol. 73:2999-3003. [DOI] [PubMed] [Google Scholar]
- 6.Cahour, A., A. Pletnev, M. Vazielle-Falcoz, L. Rosen, and C. J. Lai. 1995. Growth-restricted dengue virus mutants containing deletions in the 5′ noncoding region of the RNA genome. Virology 207:68-76. [DOI] [PubMed] [Google Scholar]
- 7.Callaway, A., D. Giesman-Cookmeyer, E. T. Gillock, T. L. Sit, and S. A. Lommel. 2001. The multifunctional capsid proteins of plant RNA viruses. Annu. Rev. Phytopathol. 39:419-460. [DOI] [PubMed] [Google Scholar]
- 8.Chang, C. J., H. W. Luh, S. H. Wang, H. J. Lin, S. C. Lee, and S. T. Hu. 2001. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 20:569-577. [DOI] [PubMed] [Google Scholar]
- 9.Corver, J., E. Lenches, K. Smith, R. A. Robison, T. Sando, E. G. Strauss, and J. H. Strauss. 2003. Fine mapping of a cis-acting sequence element in yellow fever virus RNA that is required for RNA replication and cyclization. J. Virol. 77:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasso, M. C., S. C. Milburn, J. W. Hershey, and R. J. Jackson. 1990. Selection of the 5′-proximal translation initiation site is influenced by mRNA and eIF-2 concentrations. Eur. J. Biochem. 187:361-371. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgil, D., M. S. Diamond, K. L. Holden, S. M. Paranjape, and E. Harris. 2003. Translation efficiency determines differences in cellular infection among dengue virus type 2 strains. Virology 317:275-290. [DOI] [PubMed] [Google Scholar]
- 14.Fosmire, J. A., K. Hwang, and S. Makino. 1992. Identification and characterization of a coronavirus packaging signal. J. Virol. 66:3522-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frolov, I., and S. Schlesinger. 1996. Translation of Sindbis virus mRNA: analysis of sequences downstream of the initiating AUG codon that enhance translation. J. Virol. 70:1182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frolov, I., and S. Schlesinger. 1994. Translation of Sindbis virus mRNA: effects of sequences downstream of the initiating codon. J. Virol. 68:8111-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frolova, E., I. Frolov, and S. Schlesinger. 1997. Packaging signals in alphaviruses. J. Virol. 71:248-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garlapati, S., J. Chou, and C. C. Wang. 2001. Specific secondary structures in the capsid-coding region of giardiavirus transcript are required for its translation in Giardia lamblia. J. Mol. Biol. 308:623-638. [DOI] [PubMed] [Google Scholar]
- 19.Hahn, C. S., Y. S. Hahn, C. M. Rice, E. Lee, L. Dalgarno, E. G. Strauss, and J. H. Strauss. 1987. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 198:33-41. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto, N. N., L. S. Carnevalli, and B. A. Castilho. 2002. Translation initiation at non-AUG codons mediated by weakened association of eukaryotic initiation factor (eIF) 2 subunits. Biochem. J. 367:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofacker, I. L., M. Fekete, and P. F. Stadler. 2002. Secondary structure prediction for aligned RNA sequences. J. Mol. Biol. 319:1059-1066. [DOI] [PubMed] [Google Scholar]
- 22.Holden, K. L., and E. Harris. 2004. Enhancement of dengue virus translation: role of the 3′ untranslated region and the terminal 3′ stem-loop domain. Virology 329:119-133. [DOI] [PubMed] [Google Scholar]
- 23.Hwang, W. L., and T. S. Su. 1999. The encapsidation signal of hepatitis B virus facilitates preC AUG recognition resulting in inefficient translation of the downstream genes. J. Gen. Virol. 80:1769-1776. [DOI] [PubMed] [Google Scholar]
- 24.Jones, C. T., L. Ma, J. W. Burgner, T. D. Groesch, C. B. Post, and R. J. Kuhn. 2003. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 77:7143-7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaukinen, P., A. Vaheri, and A. Plyusnin. 2005. Hantavirus nucleocapsid protein: a multifunctional molecule with both housekeeping and ambassadorial duties. Arch. Virol. 150:1693-1713. [DOI] [PubMed] [Google Scholar]
- 26.Khromykh, A. A., H. Meka, K. J. Guyatt, and E. G. Westaway. 2001. Essential role of cyclization sequences in flavivirus RNA replication. J. Virol. 75:6719-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khromykh, A. A., P. L. Sedlak, and E. G. Westaway. 2000. cis- and trans-acting elements in flavivirus RNA replication. J. Virol. 74:3253-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khromykh, A. A., A. N. Varnavski, and E. G. Westaway. 1998. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 72:5967-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khromykh, A. A., and E. G. Westaway. 1997. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J. Virol. 71:1497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knipe, D. M., P. M. Howley, D. E. Griffin, and B. N. Fields. 2001. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 31.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozak, M. 1991. An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol. 115:887-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak, M. 1989. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol. Cell. Biol. 9:5073-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozak, M. 1990. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA 87:8301-8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak, M. 1986. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA 83:2850-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozak, M. 1986. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44:283-292. [DOI] [PubMed] [Google Scholar]
- 37.Kozak, M. 1991. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 266:19867-19870. [PubMed] [Google Scholar]
- 38.Kramvis, A., and M. C. Kew. 1998. Structure and function of the encapsidation signal of hepadnaviridae. J. Viral Hepat. 5:357-367. [DOI] [PubMed] [Google Scholar]
- 39.Liu, W. J., P. L. Sedlak, N. Kondratieva, and A. A. Khromykh. 2002. Complementation analysis of the flavivirus Kunjin NS3 and NS5 proteins defines the minimal regions essential for formation of a replication complex and shows a requirement of NS3 in cis for virus assembly. J. Virol. 76:10766-10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma, L., C. T. Jones, T. D. Groesch, R. J. Kuhn, and C. B. Post. 2004. Solution structure of dengue virus capsid protein reveals another fold. Proc. Natl. Acad. Sci. USA 101:3414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 42.McBratney, S., and P. Sarnow. 1996. Evidence for involvement of trans-acting factors in selection of the AUG start codon during eukaryotic translational initiation. Mol. Cell. Biol. 16:3523-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLauchlan, J. 2000. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J. Viral Hepat. 7:2-14. [DOI] [PubMed] [Google Scholar]
- 44.Men, R., M. Bray, D. Clark, R. M. Chanock, and C. J. Lai. 1996. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J. Virol. 70:3930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrick, W. C. 2004. Cap-dependent and cap-independent translation in eukaryotic systems. Gene 332:1-11. [DOI] [PubMed] [Google Scholar]
- 46.Mori, Y., T. Okabayashi, T. Yamashita, Z. Zhao, T. Wakita, K. Yasui, F. Hasebe, M. Tadano, E. Konishi, K. Moriishi, and Y. Matsuura. 2005. Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J. Virol. 79:3448-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nomaguchi, M., T. Teramoto, L. Yu, L. Markoff, and R. Padmanabhan. 2004. Requirements for West Nile virus (−)- and (+)-strand subgenomic RNA synthesis in vitro by the viral RNA-dependent RNA polymerase expressed in Escherichia coli. J. Biol. Chem. 279:12141-12151. [DOI] [PubMed] [Google Scholar]
- 48.Pestova, T. V., S. I. Borukhov, and C. U. Hellen. 1998. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 394:854-859. [DOI] [PubMed] [Google Scholar]
- 49.Pestova, T. V., and V. G. Kolupaeva. 2002. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 16:2906-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rein, A. 1994. Retroviral RNA packaging: a review. Arch. Virol. Suppl. 9:513-522. [DOI] [PubMed] [Google Scholar]
- 51.Sonenberg, N., J. W. B. Hershey, and M. Mathews. 2000. Translational control of gene expression, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilgner, M., T. S. Deas, and P. Y. Shi. 2005. The flavivirus-conserved penta-nucleotide in the 3′ stem-loop of the West Nile virus genome requires a specific sequence and structure for RNA synthesis, but not for viral translation. Virology 331:375-386. [DOI] [PubMed] [Google Scholar]
- 54.Tzeng, W. P., and T. K. Frey. 2005. Rubella virus capsid protein modulation of viral genomic and subgenomic RNA synthesis. Virology 337:327-334. [DOI] [PubMed] [Google Scholar]
- 55.Wang, S. H., W. J. Syu, K. J. Huang, H. Y. Lei, C. W. Yao, C. C. King, and S. T. Hu. 2002. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J. Gen. Virol. 83:3093-3102. [DOI] [PubMed] [Google Scholar]
- 56.Westaway, E. G., A. A. Khromykh, M. T. Kenney, J. M. Mackenzie, and M. K. Jones. 1997. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology 234:31-41. [DOI] [PubMed] [Google Scholar]
- 57.Whelan, S. P., and G. W. Wertz. 1999. The 5′ terminal trailer region of vesicular stomatitis virus contains a position-dependent cis-acting signal for assembly of RNA into infectious particles. J. Virol. 73:307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. 2002. Dengue and dengue haemorrhagic fever. Fact sheet no. 117. World Health Organization, Geneva, Switzerland.
- 59.Yang, J. S., M. P. Ramanathan, K. Muthumani, A. Y. Choo, S. H. Jin, Q. C. Yu, D. S. Hwang, D. K. Choo, M. D. Lee, K. Dang, W. Tang, J. J. Kim, and D. B. Weiner. 2002. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg. Infect. Dis. 8:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.You, S., B. Falgout, L. Markoff, and R. Padmanabhan. 2001. In vitro RNA synthesis from exogenous dengue viral RNA templates requires long range interactions between 5′- and 3′-terminal regions that influence RNA structure. J. Biol. Chem. 276:15581-15591. [DOI] [PubMed] [Google Scholar]
- 61.You, S., and R. Padmanabhan. 1999. A novel in vitro replication system for Dengue virus. Initiation of RNA synthesis at the 3′-end of exogenous viral RNA templates requires 5′- and 3′-terminal complementary sequence motifs of the viral RNA. J. Biol. Chem. 274:33714-33722. [DOI] [PubMed] [Google Scholar]
- 62.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]