Abstract
Cherry leaf roll virus (CLRV) belongs to the Nepovirus genus within the family Comoviridae. It has a host range which includes a number of wild tree and shrub species. The serological and molecular diversity of CLRV was assessed using a collection of isolates and samples recovered from woody and herbaceous host plants from different geographical origins. Molecular diversity was assessed by sequencing a short (375-bp) region of the 3′ noncoding region (NCR) of the genomic RNAs while serological diversity was assessed using a panel of seven monoclonal antibodies raised initially against a walnut isolate of CLRV. The genomic region analyzed was shown to exhibit a significant degree of molecular variability with an average pairwise divergence of 8.5% (nucleotide identity). Similarly, serological variability proved to be high, with no single monoclonal antibody being able to recognize all isolates analyzed. Serological and molecular phylogenetic reconstructions showed a strong correlation. Remarkably, the diversity of CLRV populations is to a large extent defined by the host plant from which the viral samples are originally obtained. There are relatively few reports of plant viruses for which the genetic diversity is structured by the host plant. In the case of CLRV, we hypothesize that this situation may reflect the exclusive mode of transmission in natural plant populations by pollen and by seeds. These modes of transmission are likely to impose barriers to host change by the virus, leading to rapid biological and genetic separation of CLRV variants coevolving with different plant host species.
Cherry leaf roll virus (CLRV) was first described in 1955 by Posnette and Cropley as causing a disease of sweet cherry (Prunus avium L.) in England (7). Since then it has been shown to exhibit a wide natural host range including a variety of herbaceous and woody plants. Some of the most common natural hosts of CLRV are common birch (Betula pendula Roth), black elderberry (Sambucus nigra L.), English walnut (Juglans regia L.), and sweet cherry. The virus is widely distributed and has been detected throughout Europe, the former U.S.S.R., North America, Chile (13), New Zealand, Australia, China (18), and Japan. CLRV is naturally transmitted through seeds and pollen (1, 18). It is a member of the genus Nepovirus (46) but, unlike the majority of other members of this genus, CLRV is not considered to be transmitted by soil-borne nematodes (45).
CLRV has a bipartite single-stranded positive-sense RNA genome estimated to be about 15 kb total, with RNA-1 and RNA-2 sizes estimated at about 8.2 and 6.8 kb, respectively (29). Both RNAs are separately encapsidated in isometric particles (18). The genomic RNAs have a genome-encoded protein (VPg) covalently linked at their 5′ terminus and are polyadenylated at their 3′ terminus (4, 12).
CLRV belongs to the subgroup C nepoviruses. They are characterized by a large, separately encapsidated RNA-2 with a long (1.2 to 1.6 kb) 3′ noncoding region which is identical or almost identical to that of RNA-1 (2). It has been speculated that this very high conservation of the 3′ NCR between the two genomic RNAs could be the result of an RNA recombination mechanism acting as part of the RNA-2 replication process of these viruses (37, 39, 23).
To date, very little information is available on the molecular or serological variability of CLRV isolates, but many isolates of CLRV are known and have been distinguished by virulence on experimental hosts, by differences in reactivity with polyclonal antisera in agarose gel immunodiffusion analyses (9, 16, 17, 19, 20, 41) or by nucleic acid hybridization analyses (26). The isolates or strains of CLRV that have been most studied include the type (cherry) strain, the elm mosaic strain, the rhubarb strain, the golden elderberry strain, the red elder ringspot strain, the dogwood ringspot strain, the birch strain, the walnut ringspot and walnut yellow vein strains, and the blackberry and red raspberry strains (18).
In this study, CLRV isolates and samples recovered from a range of woody plants from different geographical regions surveyed within Germany as well as isolates from other countries have been analyzed for their serological and molecular diversity using a set of monoclonal antibodies and the nucleotide sequence of a 375-bp PCR-amplified fragment of the 3′ NCR. The results obtained demonstrate a strong correlation between the serological and molecular properties of the isolates and indicate that host plant species may be a major factor in defining the structure of CLRV populations.
MATERIALS AND METHODS
Virus samples.
The list of CLRV-infected samples and isolates used in this study, together with their country of origin and their original host are provided in Table 1. All virus isolates were maintained in Chenopodium quinoa plants by mechanical inoculation of crude leaf homogenates prepared in 0.01 M sodium phosphate buffer (pH 7.0), using Celite as an abrasive. For serological analysis, virus isolates recovered after propagation in C. quinoa plants and reference isolates propagated in C. quinoa were used, whereas for phylogenetic analysis PCR products amplified either directly from the leaves of the original host plants (codes ending with s in Table 1) or from leaves of C. quinoa plants after virus propagation were included. Most virus isolates recovered in Germany in this study have been propagated once in C. quinoa; in a few cases, two to three successive passages were done. Infected leaves were stored at −20°C before use, while for long term storage of virus isolates, infected leaves of C. quinoa were dried over calcium chloride and stored at 4°C.
TABLE 1.
Cherry leaf roll virus-infected samples and isolates used in this study
| Sample codea | Original hostb | Geographic origin | Yr | Accession no. | Origin of isolate or infected leaf samplec |
|---|---|---|---|---|---|
| E120 | Common birch | Berlin-Spandau, Germany | 2001 | AJ877118 | This study |
| E499s | Common birch | Berlin-Zehlendorf-Berkaer Str., Germany | 2002 | AJ877119 | This study |
| E896s | Common birch | Berlin-Zehlendorf-Thielallee, Germany | 2003 | AJ877120 | This study |
| E696s | Common birch | Klövensteen, Germany | 1995 | AJ877121 | Received from M. Bandte |
| E111 | Common birch | Klövensteen, Germany | 1996 | AJ877122 | Received from M. Bandte |
| E806 | Common birch | United Kingdom | Unknown | AJ877123 | Received from T. A. Jones |
| I2-RNA-1 | Common birch | United Kingdom | 1984 | S84124 | EMBL |
| I2-RNA-2 | Common birch | United Kingdom | 1984 | S84125 | EMBL |
| E1469 (I2 in 39) | Common birch | United Kingdom | 1984 | AJ877124 | Received from J. I. Cooper |
| E836s | River birch | Hannover-Herrenhausen, Germany | 2003 | AJ877125 | This study |
| E327 | Sweet cherry | Bonn, Germany | 1990 | AJ877127 | Received from J. Hamacher |
| E803 (C in 16, 17) | Sweet cherry | United Kingdom | Unknown | AJ877128 | Received from T. A. Jones |
| E1472 (CH125) | Sweet cherry | United Kingdom | 1955 | AJ877129 | Received from J. I. Cooper |
| E676s | Black elderberry | Helgoland, Germany | 2002 | AJ877130 | This study |
| E485 | Black elderberry | Fischland, Germany | 2002 | AJ877131 | This study |
| E603 | Black elderberry | Werder, Germany | 2002 | AJ877132 | This study |
| E583 | Black elderberry | Neuruppin, Germany | 2002 | AJ877133 | This study |
| E119s | Black elderberry | Berlin-Zehlendorf-Lentzeallee I, Germany | 2001 | AJ877134 | This study |
| E622s | Black elderberry | Berlin-Zehlendorf-Lentzeallee II, Germany | 2002 | AJ877135 | This study |
| E839s | Black elderberry | Berlin-Zehlendorf-Lentzeallee II, Germany | 2003 | AJ877136 | This study |
| E541s | Black elderberry | Berlin-Zehlendorf-Bitterstr., Germany | 2002 | AJ877137 | This study |
| E443 | Black elderberry | Berlin-Neuköllin, Germany | 2002 | AJ877138 | This study |
| E441 | Black elderberry | Aschersleben, Germany | 2002 | AJ877139 | This study |
| E950s | Black elderberry | Aschersleben, Germany | 2003 | AJ877140 | This study |
| E568 | Black elderberry | Balve, Germany | 2002 | AJ877141 | This study |
| E576 | Black elderberry | Fellinghausen, Germany | 2002 | AJ877142 | This study |
| E492 | Black elderberry | Sümeg, Hungary | 2002 | AJ877143 | This study |
| PV-0276 | Black elderberry | Bonn-Siebengebirge, Germany | Unknown | AJ877144 | Received from DSMZ |
| E804 (G in 16, 17) | Golden elderberry | U.S.A. | 1967 | AJ877145 | Received from T. A. Jones |
| E326 | English walnut | Bonn-Oberkassel, Germany | 1990 | AJ877146 | Received from J. Hamacher |
| E648 | English walnut | France | Unknown | AJ877147 | Received from P. Gentit |
| 4WJUG | English walnut | United Kingdom | Unknown | AJ877148 | Received from T. A. Jones |
| E800 | English walnut | United Kingdom | Unknown | AJ877149 | Received from T. A. Jones |
| GAY | English walnut | Gaydon, United Kingdom | Unknown | AJ877126 | Received from T. A. Jones |
| E156 (Hungary-3) | English walnut | Trans-Danubia, Hungary | 1984 | AJ877150 | Received from R. Zsovák-Hangyál |
| CTIFL | English walnut | France | Unknown | AJ877151 | Received from P. Gentit |
| Ludmila | English walnut | Slovakia | Unknown | AJ877152 | Received from L. Slovakova |
| W8-RNA-1 | English walnut | U.S.A. | 1980 | Z344265 | EMBL |
| W8-RNA-2 | English walnut | U.S.A. | 1980 | CL24694 | EMBL |
| E697s | Mountain ash | Hamburg-Osdorfer Born, Germany | 1993 | AJ877153 | This study |
| E695s | Mountain ash | Pinneberg, Germany | 1997 | AJ877154 | Received from M. Bandte |
| E693 | Mountain ash | Titisee-Neustadt, Germany | 2000 | AJ877155 | This study |
| E141s | Hombeam | Niedereimer, Germany | 2001 | AJ877156 | This study |
| E575s | Ground elder | Fellinghausen, Germany | 2002 | AJ877157 | This study |
| E325 | European ash | Schwäbische Alb, Germany | 1987 | AJ877158 | Received from J. Hamacher |
| E678s | European ash | Andechs, Germany | 2002 | AJ888533 | This study |
| E698s | European ash | Idar-Oberstein, Germany | 1992 | AJ888534 | This study |
| E113 (BEG in 19) | European beech | Bonn-Siebengebirge, Germany | 1992 | AJ877159 | Received from J. Hamacher |
| E801 (E in 16, 17) | American elm | U.S.A. | 1970 | AJ877160 | Received from T. A. Jones |
| E797 (D in 16, 17) | Flowering dogwood | U.S.A. | 1972 | AJ877161 | Received from T. A. Jones |
| E802 (RUB in 19) | Red raspberry | New Zealand | 1978 | AJ877162 | Received from T. A. Jones |
| E805 (BB in 17) | Blackberry | United Kingdom | 1973 | AJ877163 | Received from T. A. Jones |
| E1636 | Grapevine | Neustadt-W.-Königsbach, Germany | 2001 | AJ877164 | Received from U. Ipach |
| E395 | Rhubarb | Bornheim, Germany | 1987 | AJ877165 | Received from J. Hamacher |
| R25-RNA-1 | Rhubarb | United Kingdom | 1983 | S84126 | EMBL |
| Chinese chive | Chinese chive | Japan | 2004 | AB168098 | EMBL |
| Rumex AGBC | Sheep's sorrel | Japan | 2004 | AB168099 | EMBL |
| Rumex acetosella-21 | Sheep's sorrel | Japan | 2004 | AB168100 | EMBL |
Sample codes ending with s denote amplification of RT-PCR products directly from original leaf. Otherwise, RT-PCR products were amplified after recovery of virus isolates in the indicator plant species Chenopodium quinoa.
Common birch, European white birch (Betula pendula Roth), river birch (Betula nigra L.), sweet cherry (Prunus avium L.), black elderberry (Sambucus nigra L.), American golden elderberry (Sambucus canadensis L.), English walnut (Juglans regia L.), mountain ash (Sorbus aucuparia L.), hornbeam (Carpinus betulus L.), ground elder (Aegopodium podagraria L.), European ash (Fraxinus excelsior L.), European beech (Fagus sylvatica L.), American elm (Ulmus americana L.), flowering dogwood (Cornus florida L.), American red raspberry (Rubus idaeus L.), blackberry (Rubus procerus Muell), grapevine (Vitis vinifera L.), rhubarb (Rheum rhaponticum L.), Chinese chive (Allium tuberosum Rottl. ex Spreng.), and sheep's sorrel (Rumex acetosella L.).
EMBL indicates sequences that were obtained from the EMBL database; DSMZ indicates an isolate that was obtained from the German Collection of Microorganisms and Cell cultures.
Immunocapture reverse transcription-PCR amplification of CLRV cDNAs.
Immunocapture was done according to Werner et al. (47) using a concentration of 3 μg/ml of a polyclonal CLRV antiserum produced against an ash isolate of CLRV and kindly provided by J. Hamacher, University of Bonn, Germany. First-strand cDNA synthesis was done directly in the immunocapture tubes in a total reaction volume of 20 μl using 20 units/μl Moloney murine leukemia virus reverse transcriptase (Fermentas), 1 mM deoxynucleoside triphosphate mix, 5 μM antisense primer RW1 (5′-GTCGGAAAGATTACGTAAAAGG-3′, complementary to positions 1716 to 1737 of sequence S84124 used as a reference). PCR amplification was done in a total volume of 100 μl using 2 μl of reverse transcription product, 1.5 mM MgCl2, 0.025 units/μl Taq DNA polymerase (Fermentas), 0.2 μM antisense primer RW1, and 0.2 μM sense primer RW2 (5′-TGGCGACCGTGTAACGGCA-3′, complementary to positions 1322 to 1339 of S84124) in a Robocycler PCR machine (Stratagene). For both reverse transcription and PCR steps, the reaction buffers were those recommended by the supplier. The cycling scheme used was 2 min of denaturation at 95°C followed by 35 cycles at 51°C annealing for 30 seconds, 72°C extension for 30 seconds, and 95°C denaturation for 1 min, with a final extension for 2 min at 51°C and 5 min at 72°C.
Cloning and sequencing of CLRV cDNA fragments.
Sequence analysis was done either directly on uncloned PCR products purified using QIAEX II microcolumns (QIAGEN) or after cloning in the pGEM-T-Easy plasmid (Promega) transformed in Escherichia coli JM109 (Promega)-competent cells according to recommendations of the supplier. Recombinant plasmids were purified using Nucleospin columns (Macherey and Nagel) before sequencing.
Nucleotide sequence, phylogenetic and character analyses.
Multiple sequence alignments were done using CLUSTALX (40). Trees were constructed using three methods: neighbor joining with Kimura two-parameter distance using CLUSTALX and MEGA2 (22), maximum likelihood using Phylip (10), and Bayesian analysis with the general time reversible substitution model with gamma-distributed rate variation using MrBayes 2.0 (14). Branch support was assessed by bootstrapping (neighbor joining and maximum likelihood; 1,000 replicates) and Markov Chain Monte Carlo (Bayesian analysis) methods.
For Bayesian analysis, four Markov chains of 2,100,000 generations were run to estimate posterior probabilities. Trees were sampled every 1,000 generations and the first 600,000 generations were discarded as burn-in. Thus, the resulting consensus tree with posterior branch probabilities was based on 1,500 sampled trees. Other phylogenetic algorithms (minimum evolution, maximum parsimony) generally yielded similar topologies and bootstrap values (data not shown). Phylogenetic trees were visualized using the programs Njplot (34) and TREEVIEW (33). Recombination analyses were done using the programs Geneconcv (38) and RDP2 (27).
From sequence and serological data, trees were reconstructed for 24 CLRV isolates using the neighbor-joining algorithm in MEGA2. Eight serological characters were used representing the enzyme-linked immunosorbent assay (ELISA) reactivity of the CLRV isolates with the polyclonal antiserum and the seven monoclonal antibodies. The serological characters were encoded in three state parameters (no reactivity, partial reactivity and full reactivity) assuming that there can be free interconversion (mutation) between those three states. For sequence distance and serological matrices bootstrapping with 1,000 permutations was done and from reconstructed trees a consensus tree was calculated. Measures of similarity between the consensus trees obtained from sequence and serological data were calculated using partition, triplet, quartet and other metrics using the program COMPONENT 2.0 (32). The similarity values obtained for the sequence and serological tree pair were compared with similarity values obtained from tree pairs composed of the consensus sequence tree and of 1,000 to 10,000 random trees generated using COMPONENT 2.0.
Average diversities, Nei's Gst coefficient of differentiation (30), and genetic distances (p-distances) were calculated on nucleotide identity or using the Kimura two-parameter method using MEGA2. Correlations of genetic, geographic, and host species matrices were assessed with the Mantel test (24) based on Pearson correlation with a two-tailed P value, a level of significance of 0.05 and 10,000 random permutations. The genetic distances between the isolates were expressed in a matrix of pairwise nucleotide divergence percentages, geographical distances were expressed in a matrix of pairwise geographic distances in kilometers calculated from global positioning data, and pairwise host species association was expressed in a matrix coding 0 if virus sequences were recovered from the same host species and 1 if virus sequences were recovered from different host species. All sequences reported in this article have been deposited in the EMBL database.
Production of monoclonal antibodies and ELISA serological assays.
Following inoculations of BALB/c mice with a single injection of 60 μg of purified CLRV particles of the CTIFL walnut isolate (Table 1), monoclonal antibodies (MAbs) secreting hybridomas were obtained using standard procedures (8). Screening of the hybridomas was done using a triple antibody sandwich ELISA in which particles of the homologous CLRV isolate were first trapped using purified immunoglobulin Gs from a polyclonal antiserum raised against the same isolate. Following selection and cloning by serial dilution, seven MAbs were finally obtained in this way. Analysis of the reactivity of the various MAbs against a range of CLRV isolates was done using the same triple antibody sandwich ELISA procedure. As a control, a double antibody sandwich ELISA assay (5) was done using coating immunoglobulin G's and alkaline phosphatase-conjugated immunoglobulin G's purified and prepared from the polyclonal antiserum described above.
Nucleotide sequence accession numbers.
The 50 sequences reported in this paper have been deposited in the EMBL database under accession numbers AJ877118 to AJ877165, AJ888533, and AJ888534.
RESULTS
Analysis of nucleotide variability of CLRV.
CLRV infection of trees in forest stands, nurseries, public parks, and gardens was identified at 16 locations in Germany during a survey from 2001 to 2004 by immunocapture reverse transcription-PCR using symptomatic leaf material and/or by mechanical inoculation to indicator plants followed by immunocapture reverse transcription-PCR. The PCR detection assay was based on the CLRV-specific PCR primers described by Werner et al. (47). These primers amplify an approximately 416-bp fragment (approximately 375 bp, excluding the primers) corresponding to the extreme 3′ part of the CLRV 3′ noncoding region. There are only two bases between the end of the downstream primer used and the poly(A) tail of the genomic RNAs. In many cases, mechanical transmission to indicator plants was not successful but amplification and analysis of reverse transcription-PCR products directly from the original infected field material was possible, allowing the molecular analysis of the 3′ NCR.
Reverse transcription-PCR products amplified from CLRV isolates that had been propagated in indicator plants and maintained by different laboratories world-wide were also included in the analysis (Table 1). In order to determine whether passage of virus isolates through C. quinoa resulted in genetic change of isolates and whether CLRV populations in the natural host plants comprised a mixture of genotypes, comparisons were made between sequences obtained directly from natural host plants and from C. quinoa plants after virus propagation. The sequences of CLRV directly amplified from a birch tree in Klövensteen, Germany, in 1995 with those from a virus isolate recovered from the same tree in 1996 and subsequently propagated 14 times in C. quinoa were identical in the 375 bp of the 3′ NCR (samples E696s and E111in Table 1 and Fig. 1).
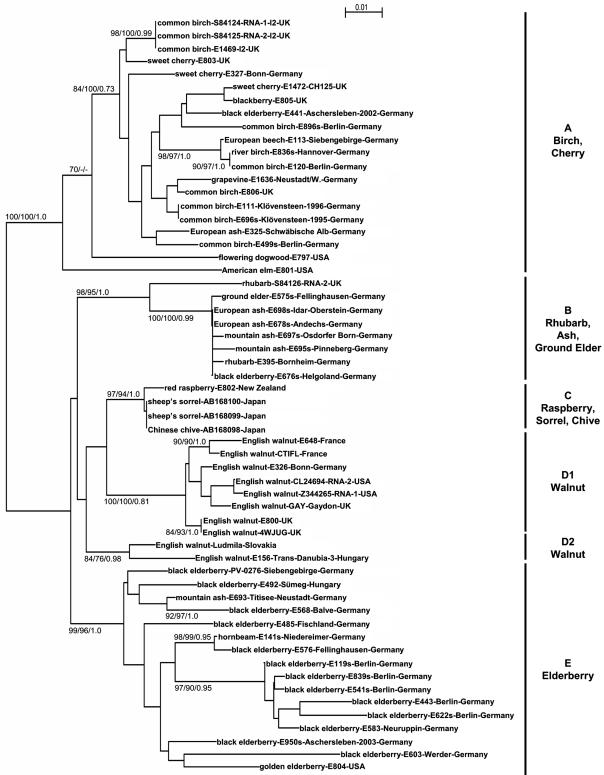
FIG. 1.
Phylogenetic tree reconstructed from the nucleotide sequence of a 3′-terminal genomic fragment (375 bp) of the cherry leaf roll virus genome amplified from original host plants or from isolates recovered from various hosts. Details about isolates are shown in Table 1. All data obtained from the EMBL nucleotide database are indicated by accession numbers. Data analysis and tree construction were done by neighbor-joining, maximum likelihood, and Bayesian analyses using the CLUSTALX, Phylip, and MrBayes programs. Bootstrap values (n = 1,000) or probability estimate values larger than 70% are indicated at branch nodes for neighbor-joining/maximum-likelihood/Bayesian analysis. Major phylogenetic groups are indicated by a bold line on the right.
There was no evidence in the sequence traces generated from infected leaf samples from the natural host plants or from virus isolates propagated in C. quinoa for the occurrence of mixed genotypes in any of the samples listed in Table 1. Two different samples of the cherry isolate CH125 described by Cropley (7) showed identical sequences in 375 bp of the 3′ NCR when amplified from dried samples from 1980 and 1993, separated by 13 years of experimental propagation in C. quinoa. Similarly, sequences of the 3′ NCR obtained from dried samples of the birch isolate I2 from 1991, 1992, and 1997 were confirmed to be identical to the sequence published by Scott et al. (39).
CLRV isolates CH125 and I2 have been propagated in C. quinoa at least four times a year, in total approximately 30 to 50 times since isolation. These results indicate that in natural hosts infection with mixed CLRV genotypes is not common and that propagation in C. quinoa is unlikely to have resulted in selection of a specific subpopulation, or in other genetic changes. The sequence composition of the 3′ NCR seems to be highly stable and not to change rapidly when the virus is propagated in C. quinoa, allowing the comparison of sequences obtained directly from the original host plant species and sequences obtained upon propagation in C. quinoa.
In order to evaluate the genetic variability of CLRV between different hosts, the sequence of reverse transcription-PCR fragments obtained from various samples and isolates was determined. Given that the PCR primers used can anneal to both genomic RNAs, it was initially necessary to evaluate the possibility that two different sequences, corresponding to the two genomic RNAs, might be detected. As a first step, the sequences of individual plasmid cDNA clones (four of birch E120 and six of elderberry E119s, Table 1) were determined. The various sequences obtained for each of these samples proved identical or almost identical (not more than one nucleotide difference) suggesting that the region targeted shows very little intra-isolate variability for RNA-1, RNA-2, and the total RNA population. This was confirmed by the fact that for 31 further infected leaf samples and isolates, analysis of the nucleotide sequence of two independent cDNA clones or of two independently obtained reverse transcription-PCR products revealed no differences in sequence for any of the samples. Thus, despite the fact that the two genomic RNAs contribute to the PCR product to be sequenced and despite the potential significant intra-isolate variability of plant RNA virus isolates (36), the intra-isolate variability of the 3′ NCR of CLRV appears to be consistently low. It was therefore decided to generate two single sequences (determined on both strands) for all further isolates and samples.
PCR amplification and sequencing was done for a total of 50 samples (34 isolates propagated in C. quinoa, 16 leaf samples from the original host plant) which were either collected in Germany or obtained from various colleagues (Table 1). Finally, the homologous region from CLRV sequences present in databases (8 sequences) were also included in the analysis, so that a data set of 58 sequences in total was analyzed.
Multiple sequence alignment of these sequences revealed both point mutations and significant indel variation. As a consequence, the size of the sequences obtained varied from 362 bp (E648) to 380 bp (E156). Pairwise comparisons revealed an average divergence between sequences of 8.5% ± 0.9% calculated using a nucleotide identity distance and discounting all indel positions (results not shown). This average value, however, covers very different situations since identical sequences were obtained for pairs, triplets, or quadruplets of samples in six cases (E800-4WJUG; Z34426S-CL24694, E1469-S84124, E120-E836s, AB168098-AB168099-AB168100, and E676s-E695s-E678s-E698s)while a maximal divergence of 17.0% was observed between samples E622s and E896s (results not shown). Remarkably, these two most diverse sequences originated from two geographically very close locations in Berlin (about 2 km apart) but were amplified from leaves of different hosts: elderberry and birch, respectively.
Evaluation of this CLRV data set using either the Geneconv or the nine programs included in the RDP program package failed to provide significant evidence for the presence of recombination events in the data set which, however, represents only a small proportion (5%) of the CLRV genome.
Phylogenetic reconstructions using the neighbor-joining, maximum likelihood and Bayesian analyses yielded essentially similar clusterings so that only the neighbor-joining tree is shown in Fig. 1, with the bootstrap values and probability estimate values for all three methods indicated at the nodes. The tree shows the existence of several clusters of CLRV isolates, which are supported by very high bootstrap values, generally above 95%. Based on these analyses six major phylogenetic clusters of isolates are observed, some of them being composed of a large majority of isolates sharing the same original host. This was particularly evident for the two walnut groups (D1 and D2), the elderberry group (E) and, to a lesser extent, the birch-cherry group (A). On the other hand, two of the groups, C (raspberry, sorrel, chive) and B (rhubarb, ash, ground elder), contain isolates originating from a wider range of hosts. Two American isolates from American elm (E801) and dogwood (E797), cluster within the birch-cherry group but, in most analyses, are nevertheless significantly removed from the other isolates of this group.
Analysis of the serological variability of selected CLRV isolates.
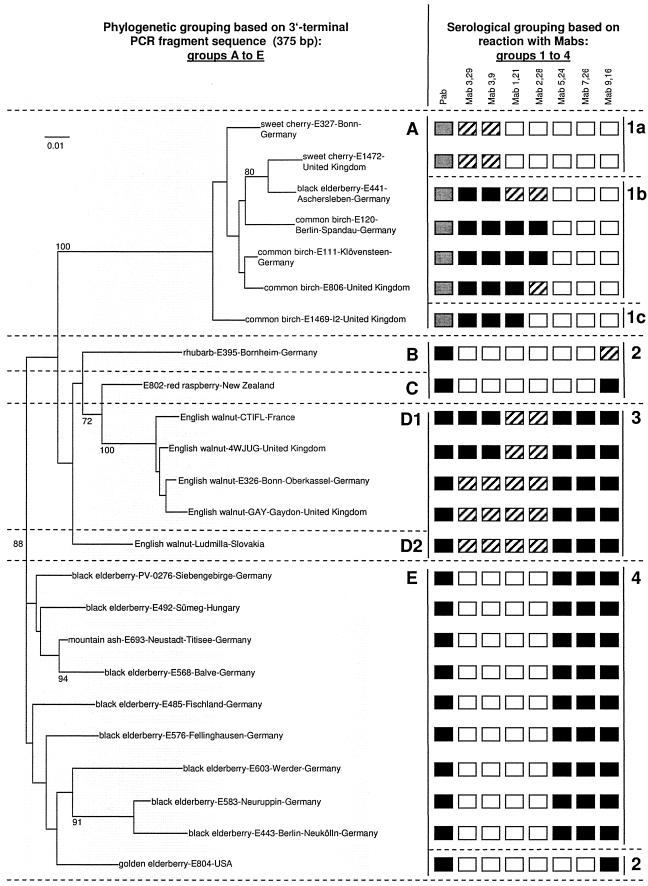
Using a panel of seven monoclonal antibodies produced against a French walnut isolate (CTIFL, Table 1), the serological variability of CLRV was assessed on a subset of 24 CLRV isolates which included at least one member of all six phylogenetic groups described above and were propagated in C. quinoa. As a control, all 24 isolates were assayed in parallel in a double antibody sandwich ELISA format (5) using a polyclonal antiserum raised against the CTIFL walnut isolate. Wide differences in the reactivity of the isolates toward both the polyclonal reagent and the various MAbs tested were observed (Fig. 2). By using the polyclonal antiserum, two groups of isolates could be identified on the basis of the optical densities observed in ELISA assays: one group of isolates consistently gave low ELISA readings (optical density < 0.3) while the second group generally produced much higher optical densities (optical density > 0.6 to 0.8) under similar assay conditions. The isolates giving low ELISA readings corresponded to the birch-cherry phylogenetic group (A in Fig. 2). None of the seven MAb was able to detect all isolates tested. Allowing for some minor variability, the 24 isolates can be classified into four main MAbs reactivity groups (Fig. 2).
FIG. 2.
Comparison of cherry leaf roll virus isolates obtained from different hosts by phylogenetic analysis of a 3′-terminal genomic fragment (375 bp) and by reactivity with a panel of monoclonal antibodies produced against a CLRV isolate from walnut. Phylogenetic groups A to E similar to the groups described in Fig. 1 are indicated. Nucleotide sequence analysis and phylogenetic tree construction was done as described for Fig. 1. Reactivities with the polyclonal antiserum and with the various monoclonal antibodies are indicated for each isolates by the boxes on the right of the phylogenetic tree. For the polyclonal reagents a gray box indicates low (<0.3) ELISA readings while a black bow indicates ELISA readings in excess of 0.6 to 0.8. For the monoclonal antibodies, a black box indicates ELISA readings in excess of 50% of the value obtained with the polyclonal reagents while a hatched box indicates values below this 50% value. Empty boxes indicate an absence of reactivity. MAb reactivity groups 1 to 4 are indicated at the extreme right of the figure.
All the walnut isolates tested, representing phylogenetic groups D1 and D2 originating from Germany, United Kingdom, Slovakia, and France (including the homologous CTIFL isolate used for the immunization), reacted with all of the seven MAbs and correspond to MAb reactivity group 3. In contrast, all the other isolates tested failed to react with one or more of the MAbs. Reactivity group 4 is characterized by positive reactions only with MAbs 5.24, 7.26, and 9.16 and constitutes a large group of elderberry isolates (PV-0276, E492, E568, E485, E576, E603, E583, and E443) together with a mountain ash isolate (E693). Reactivity group 1 consisted of a group of 7 isolates originating from various hosts, which typically showed low reactivity towards the polyclonal reagents (Fig. 2). This somewhat heterogeneous group, characterized by reactivity towards MAbs 3.29 and 3.9, but not towards MAbs 5.24, 7.26 and 9.16, can be divided further into at least three subgroups, based on the reactivity of individual isolates towards MAbs 1.21 and 2.28. Finally, three isolates (E395, E802, and E804) were characterized by the fact that they were recognized by MAb 9.16 only, and on this basis they were classified as reactivity group 2.
Comparison of phylogenetic and serological variabilities of CLRV.
As can be seen in Fig. 2, a consistent correlation is observed between the MAbs reactivity groups and the phylogenetic groups defined by the analysis of the 375-bp sequence from the 3′ NCR. Phylogenetic group E (elderberry) and A (birch-cherry) correspond precisely to MAb reactivity groups 4 and 1, respectively. The two walnut phylogenetic groups (D1 and D2) show similar MAb reactivity and correspond to MAb reactivity group 3 while the two minor phylogenetic groups C (raspberry-sorrel-chive) and B (rhubarb-ash-ground elder) fall together in MAb reactivity group 2.
In order to validate the apparent correlation observed between the molecular variability and serological reactivity of CLRV isolates, the COMPONENT 2.0 program was used to compare the consensus phylogenetic tree reconstructed from the sequence data with the consensus tree generated from the serological data. The similarity values obtained by comparing the phylogenetic and serological trees were contrasted with the similarity values obtained by comparing the phylogenetic tree with 1,000 to 10,000 random trees generated using COMPONENT 2.0. In all cases (partition, triplet, quartet, other metrics), the values obtained when comparing the actual trees were more than 12 standard deviations above the average value obtained when comparing the molecular tree with random trees. In no trial did the best value obtained for the comparison with random trees get close to the value obtained when comparing the experimental trees, clearly indicating that the correlation observed between the serological and molecular clusterings is far better than would be expected by chance.
There was a single exception to this parallel clustering of isolates using the serological and molecular data: the golden elderberry isolate E804 was classified in the elderberry phylogenetic group E but had the same serological reactivity as isolates E802 (phylogenetic group C) and E395 (phylogenetic group B).
Genetic structure of CLRV populations.
The tendency of isolates of CLRV obtained from the same host to show similar serological and molecular properties was evaluated more precisely by calculating intra- and intersubpopulation diversities, together with Nei's Gst coefficient of differentiation between subpopulations (30). The effect of the country of origin of the samples was evaluated in a similar fashion. As a control, similar computations were also done using the clusters of samples defined by the phylogenetic analysis as subpopulations. The results of these calculations are presented in Table 2. Only subpopulations containing more than three samples were considered for the analysis presented in Table 2 to avoid possible confounding effects due to the limited representation of samples from some geographic regions or from some host plants in the data set. Results similar to those obtained using subpopulations containing a minimum of three samples were obtained when only subpopulations containing more than 5 or more than 10 samples per country of origin and more than 5 samples per host were considered.
TABLE 2.
Analysis of the contribution of various parameters to the genetic diversity of cherry leaf roll virus
| Parametera | Subpopulations defined by host speciesb | Subpopulations defined by country of originb | Subpopulations defined by phylogenetic groupb |
|---|---|---|---|
| Mean entire diversity | 0.089 ± 0.010 | 0.087 ± 0.009 | 0.085 ± 0.009 |
| Mean intrasubpopulation diversity | 0.044 ± 0.004 | 0.087 ± 0.009 | 0.022 ± 0.002 |
| Mean intersubpopulation diversity | 0.045 ± 0.006 | 0.003 ± 0.003 | 0.064 ± 0.008 |
| Nei's Gst coefficient of differentiation | 0.507 ± 0.029 | 0.037 ± 0.032 | 0.746 ± 0.021 |
Genetic distance and standard error. Standard error computation was done by bootstrap analysis with 1,000 replications.
For calculation of mean entire diversity, mean intrasubpopulation diversity, intersubpopulation diversity, and Nei's coefficient of differentiation, only sequences from groups with more than three members were included in the analysis (for subpopulations defined by host, country of origin, and phylogenetic group 35, 51, and 56 sequences, respectively).
Differentiation by country of origin was always found to be minimal (Gst values always below 11%), whereas strong differentiation by host of origin (Gst values between 40 and 55%) was observed. Thus, the country of origin does not contribute significantly to the structure of the CLRV populations. The intrasubpopulation diversity was very close to the total diversity observed for CLRV, indicating that CLRV populations in each country show a large degree of variability. A completely different situation arises when using the host origin to define the subpopulations analyzed. In this case, the inter- and intrasubpopulation diversities are roughly equal and a Gst value of 50% was obtained. These results show that 50% of the total CLRV diversity is distributed among subpopulations defined by the original host plant, indicating that there are considerable genetic differences between them. As a comparison, the use of the clusters of sequences defined by the phylogenetic analysis as subpopulations only further increases the Gst parameter to about 74%.
Although the analysis described above strongly supports the idea that CLRV samples sharing the same isolation host are more likely to be related than samples selected randomly, there are exceptions concerning some phylogenetic groups composed of samples from different hosts. This situation is particularly clear in the rhubarb-ash-ground elder group (B), which shows a particularly low diversity (0.018 ± 0.004) despite being composed of samples from 4 different isolation hosts. A somewhat similar situation applies to the birch-cherry group (A) which has a 0.038 ± 0.005 diversity and is composed of samples originating from 10 different hosts (common birch, sweet cherry, river birch, American elm, flowering dogwood, grapevine, European beech, blackberry, black elderberry, and European ash).
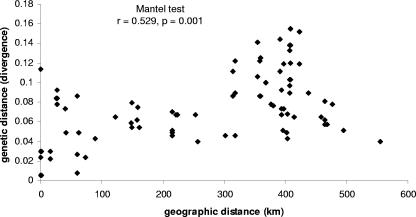
The possibility that the virus populations and subpopulations might be structured by the geographical distance between samples was evaluated using all 31 samples from Germany and all 14 elderberry samples from Germany. The correlation between geographic and genetic distance matrices was calculated using a Mantel test. Figure 3 shows a plot of the pairwise divergence between the 14 elderberry samples as a function of the distance between the places where these samples were initially collected. Although no obvious broad correlation can be observed on this plot, a Mantel test revealed a significant correlation (r = 0.529, P = 0.001) between the geographic and genetic distances for elderberry samples indicating that within Germany geographically close elderberry hosts are more likely to be infected by more closely related CLRV variants than geographically distant ones and that a substantial fraction (about 28%) of the total variance (r2) could be explained by geographic distance. An extreme counter-example to this relationship is, however, illustrated by samples E441 and E950s, which were recovered from infected elderberry trees from the same site in Aschersleben in Germany in 2002 and 2003 and belong to two different phylogenetic clusters (birch-cherry and elderberry, respectively; Fig. 1). No significant correlation between geographic distance and genetic distance was found when analyzing all 31 German CLRV isolates from 12 different hosts (r = 0.030, P = 0.510). A highly significant correlation was observed between the host species and the genetic distance (r = 0.336, P = 0.0001).
FIG. 3.
Plot of pairwise genetic distance of the 3′ NCR (375 bp) (nucleotide identity) versus geographic distance (in km) for 14 sequences of cherry leaf roll virus obtained from elderberry hosts in Germany.
DISCUSSION
A significant degree of genetic variability was found within a short stretch (375 bp) of the 3′-terminal region of the viral genome of CLRV isolates and samples recovered from different geographical regions and host species (up to 17% divergence between sequences). Similarly, a high degree of serological variability was revealed using a panel of seven different monoclonal antibodies produced against a walnut isolate of CLRV. These results are consistent with previous analyses using polyclonal antisera and a smaller number of isolates that demonstrated significant serological variation between CLRV isolates (20, 16, 17, 9, 19, 41). Some of the phylogenetic and MAb reactivity groups appear to be composed mainly of isolates obtained from the same host. For the 3′-terminal untranslated regions of Satsuma dwarf-related nepovirus interserogroup, identities ranging from 67 to 92% and intraserogroup identities ranging from 92 to 96% have been reported (15). These values are similar to the interserogroup and intraserogroup identities observed in this study for CLRV (87 to 93% and 92 to 97%, respectively).
With two major exceptions, the groupings obtained based on the serological and phylogenetic relationships of the 3′ NCR are very similar. These findings suggest that the viral coat protein composition and dependent serological reactivity and the 3′ NCR sequence are representative of particular CLRV isolates and that recombination between the coat protein gene and the end of the 3′ NCR of distinct isolates may not be common for this virus.
The genomic nucleotide sequences have been determined for a number of different isolates of plant virus species and their phylogenetic grouping calculated previously. In some of these studies, isolates or strains have been found to show clustering affinities related to their geographical origin and natural spread, but grouping according to the original host plant species was almost always found to be much less pronounced (3, 11, 21, 31, 35, 42). In this study, no strong evidence for a grouping of CLRV 3′ NCR sequences according to their geographical origin could be found considering the countries of origin, but a statistically significant effect of distance on a smaller scale could be demonstrated using the set of 14 German elderberry isolates. In contrast, a strong relationship between CLRV genetic diversity and the original host plant species was observed.
Many host plants are represented by a single sample in this study so it is not possible to determine whether the same trend applies to all CLRV hosts. For those hosts for which several samples were analyzed there is, in general, a strong tendency for isolates from the same host to cluster together in the phylogenetic or serological analyses. Such a situation could be explained by two different but interdependent mechanisms, the inability of some viral isolates to infect some hosts (host specialization) or the inability of isolates infecting a given host to be transmitted to a different host species through the existence of ecological transmission barriers.
Comparison of host plant species and phylogenetic data reveal that the genetic isolation of host-specific CLRV variants is partial and not complete. Some CLRV sequences clustered in different phylogenetic groups to the majority of CLRV sequences from the same hosts. Thus, at least some members of the “nonelderberry” phylogenetic clusters (isolates E676s and E441) are, in fact, able to infect this host. A parallel situation clearly applies also to the mountain ash isolate E693. Also, three of the six phylogenetic clusters appear to correspond to samples originating from a variety of hosts. Even discounting the small raspberry-sorrel-chive cluster, the rhubarb-ash-ground-elder cluster totals four hosts for six CLRV sequences, while the larger birch-cherry cluster (20 sequences) totals 10 different hosts. Such observations strongly support the idea that CLRV isolates belonging to those clusters should have the ability to infect a broad range of potential hosts.
The most likely explanation for the strong influence of the host plant on the structure of CLRV populations compared to the strong influence of other factors on the structure of other nepovirus populations (44) seems therefore to be the existence of ecological barriers preventing efficient transmission of the virus between different host species. Such inter-specific transmission barriers would result in rapid genetic isolation of viral variants within given host populations and, over time, result in evolutionary divergence of these separate virus populations. The recovery of viral isolates belonging to “host-specific” phylogenetic clusters outside of these hosts (e.g., elderberry cluster isolate E693 in mountain ash) provides a first indication that these barriers to transmission are not absolute and that mechanisms exist for (probably) low frequency inter-specific transmission. Similarly, the observation that some phylogenetic clusters are composed of isolates from different hosts could be taken as an indication that the transmission barrier(s) may not have the same strength when considering transmission between different pairs or groups of host plant species.
Unlike most other plant viruses studied so far from a population genetics point of view, CLRV does not appear to have biological vectors. The natural transmission of CLRV to healthy plants occurs through pollination and by seeds. Although nematode transmission has been suspected for CLRV due to its taxonomic status in the Nepovirus genus, it has not been possible to confirm this (45). Transmission by other biological vectors, e.g., insects, has not been reported. However, CLRV has been detected by reverse transcription-PCR in the seed-feeding bug Kleidocerys resedae (47). Seed transmission is clearly a mechanism that does not allow inter-specific transmission. Previous studies have shown that CLRV particles are found both on the surface and inside the pollen grains produced by infected birch and walnut plants and have also provided evidence that pollen germination is required for virus transmission (25).
Virus transmission via pollen should result in a high level of species specificity, in particular if actual pollen germination and fertilization are required for infection of the receptor plant, as is the case for CLRV. Thus, pollen transmission of CLRV is very likely to present the kind of species specificity and barrier to cross-species infection that would be postulated from the genetic structure of CLRV populations reported here. Such a role of pollen transmission in the structure of CLRV populations was previously postulated by Cooper and Atkinson (6) on the basis of serological differences observed between CLRV isolates obtained from different host species.
Infection of plants by some nepoviruses via contaminated pollen (e.g., with the help of insects) through wounds might take place occasionally, although the epidemiological significance is not clear (28). Such a mechanism could be responsible for the species-to-species transmission of CLRV suggested by some of the multihost phylogenetic groups identified in this study. Many natural hosts of CLRV are perennial cultivated forest and garden plants which have been vegetatively propagated for many years, such as walnut and rhubarb (9, 43). These practices could also have contributed to the genetic isolation and adaptation of CLRV variants to some of its host species, especially where natural transmission by pollen and seed is limited.
Although further experimental work is clearly needed to fully validate these hypotheses, the results reported here provide, for the first time, strong evidence for a host-based selection of viral populations for a seed- and pollen-borne virus. The results also demonstrate that both the serological and molecular tools developed for this study allow the useful analysis of CLRV isolates in cultivated crops and also in wild host plants.
Acknowledgments
This work was supported by Konsul Karl und Gabriele Sandmann Stiftung (KKGS-Stiftung) and by Deutsche Forschungsgemeinschaft grant Bu 890/8-1.
We thank J. Hamacher, Rheinische Friedrich-Wilhelms-Universität Bonn, Germany; M. Bandte, Humboldt- Universität zu Berlin, Germany; the DSMZ, Braunschweig, Germany; U. Ipach, Dienstleistungszentrum Ländlicher Raum-Rheinpfalz, Neustadt/Weinstrasse, Germany; T. A. Jones, Scottish Research Crop Institute, United Kingdom; J. I. Cooper, CEH, Oxford, United Kingdom; R. Zsovák-Hangyál, Plant Health and Soil Conservation Service of County Fejér, Hungary; and Pascal Gentit, CTIFL Bergerac, France, for kindly providing CLRV isolates. U. Commandeur, Rheinisch-Westfälische Technische Hochschule Aachen, Germany, is gratefully acknowledged for technical help with sequencing. We also thank J. Hamacher, University of Bonn, Germany, for kindly providing the polyclonal antiserum produced against an ash isolate of CLRV used in this study and J. A. Walsh and C. E. Jenner for critical review of the manuscript.
REFERENCES
- 1.Bandte, M., and C. Büttner. 2001. Occurrence, transmission and diagnosis of cherry leaf roll nepovirus—a literature review. Pflanzenschutzberichte 59:1-19. [Google Scholar]
- 2.Borja, M. J., F. Sánchez, A. Rowhani, G. Bruening, and F. Ponz. 1995. Long, nearly identical untranslated sequences at the 3′ terminal regions of the genomic RNAs of cherry leafroll virus (walnut strain). Virus Genes 10:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Bousalem, M., E. Douzery, and D. Fargette. 2000. High genetic diversity, distant phylogenetic relationships and intraspecies recombination events among natural populations of Yam mosaic virus: a contribution to understanding potyvirus evolution. J. Gen. Virol. 81:243-255. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, M., and G. Bruening. 1995. A subgenomic RNA associated with cherry leafroll virus infections. Virology 211:22-41. [DOI] [PubMed] [Google Scholar]
- 5.Clark, N. F., and A. N. Adams. 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 34:475-483. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, J. I., and M. A. Atkinson. 1975. Cherry leaf roll virus causing a disease of Betula spp. in the United Kingdom. Forestry 48:193-203. [Google Scholar]
- 7.Cropley, R. 1961. Cherry leaf-roll virus. Ann. Appl. Biol. 49:524-529. [Google Scholar]
- 8.Desbiez, C., C. Scheibel, F. Granier, C. Robaglia, T. Delaunay, and H. Lecoq. 1996. Biological and molecular variability of zucchini yellow mosaic virus on the island of Martinique. Plant Dis. 80:203-207. [Google Scholar]
- 9.De Zoeten, G. A., J. A. Lauritis, and S. M. Mircetich. 1982. Cytopathology and properties of cherry leaf roll virus associated with walnut blackline disease. Etiology 72:1262-1265. [Google Scholar]
- 10.Felsenstein, J. 1989. PHYLIP—Phylogeny Inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 11.García-Arenal, F., A. Fraile, and J. Malpica. 2001. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 39:157-186. [DOI] [PubMed] [Google Scholar]
- 12.Hellen, C. U., and J. I. Cooper. 1987. The genome-linked protein of Cherry leaf roll virus. J. Gen. Virol 68:2913-2917. [Google Scholar]
- 13.Herrera, G., and M. Madariaga. 2001. Presence and incidence of grapevine viruses in the central zone of Chile. Agric. Téc. 61:393-400. [Google Scholar]
- 14.Huelsenbeck, J. P., and F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 15.Iwanami, T., Y. Kondo, M. Kobayashi, S. S. Han, and A. V. Karasev. 2001. Sequence diversity and interrelationship among isolates of satsuma dwarf-related viruses. Arch. Virol. 146:807-813. [DOI] [PubMed] [Google Scholar]
- 16.Jones, A. T. 1973. A comparison of some properties of four strains of cherry leaf roll virus. Ann. Appl. Biol. 74:211-217. [Google Scholar]
- 17.Jones, A. T. 1976. Serological specificity of isolates of cherry leaf roll virus from different natural hosts. Poljopr. Znan. Smotra. 39:527-532. [Google Scholar]
- 18.Jones, A. T. 1986. Cherry leaf roll virus. CM/AAB description of plant viruses no. 306. Commonwealth Mycological Institute, Kew, Surrey, England, and Association of Applied Biologists, Wellsbourne, Warwick, England.
- 19.Jones, A. T., R. Koenig, D.-E. Lesemann, J. Hamacher, F. Nienhaus, and S. Winter. 1990. Serological comparison of isolates of Cherry leaf roll virus from diseased beech and birch trees in a forest decline area in Germany with other isolates of the virus. J. Phytopathol. 129:339-344. [Google Scholar]
- 20.Jones, A. T., and A. F. Murant. 1971. Serological relationship between cherry leaf roll, elm mosaic and golden elderberry viruses. Ann. Appl. Biol. 69:11-15. [Google Scholar]
- 21.Krause-Sakate, R., O. Le Gall, H. Fakkfakh, M. Peypelut, M. Marrakchi, C. Varveri, M. A. Pavan, S. Souche, H. Lot, F. M. Zerbini, and T. Candresse. 2002. Molecular and biological characterization of Lettuce mosaic virus (LMV) isolates reveals a distinct and widespread type of resistance-breaking isolate: LMV-Most. Phytopathology 92:563-572. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular evolutionary genetics analysis software. Bioinformatics. 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall, O., T. Candresse, and J. Dunez. 1995. Transfer of the 3′ non-translated region of grapevine chrome mosaic virus RNA-1 by recombination to tomato black ring virus RNA-2 in pseudorecombinant isolates. J. Gen. Virol. 76:1285-1289. [DOI] [PubMed] [Google Scholar]
- 24.Mantel, N. 1967. The detection of disease clustering and generalized regression approach. Cancer Res. 27:209-220. [PubMed] [Google Scholar]
- 25.Massalski, P. R., and J. I. Cooper. 1984. The location of virus-like particles in the male gametophyte of birch, walnut, cherry naturally infected with cherry leaf roll virus and its relevance to vertical transmission of the virus. Plant Pathol. 33:255-262. [Google Scholar]
- 26.Massalski, P. R., and J. I. Cooper. 1986. Comparison of the genome RNA sequence homologies between isolates of cherry leaf roll virus by complementary DNA hybridization analysis. J. Gen. Virol. 71:1169-1172. [Google Scholar]
- 27.Martin, D. P., C. Williamson, and D. Posada. 2005. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21:260-262. [DOI] [PubMed] [Google Scholar]
- 28.Mink, G. I. 1993. Pollen- and seed-transmitted viruses and viroids. Annu. Rev. Phytopathol. 31:375-402. [DOI] [PubMed] [Google Scholar]
- 29.Murant, A. F., M. Taylor, G. H. Duncan, and J. H. Raschke. 1981. Improved estimates of molecular weight of plant virus RNA by agarose gel electrophoresis and electron microscopy after denaturation with glyoxal. J. Gen. Virol. 53:321-332. [Google Scholar]
- 30.Nei, M. 1987. Molecular evolutionary genetics, p. 190-191. Columbia University Press, New York, NY.
- 31.Ohshima, K., Y. Yamaguchi, R. Hirota, T. Hamamoto, K. Tomimura, Z. Tan, T. Sano, F. Azuhata, J. A. Walsh, J. Fletcher, J. Chen, A. Gera, and A. Gibbs. 2002. Molecular evolution of Turnip mosaic virus: evidence of host adaptation, genetic recombination and geographical spread. J. Gen. Virol. 83:1511-1521. [DOI] [PubMed] [Google Scholar]
- 32.Page, R. D. M. 1993. User's manual for COMPONENT, version 2.0. The Natural History Museum. London, England.
- 33.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Applic. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 34.Perriere, G., and M. Gouy. 1996. WWW-query: an online retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 35.Roossinck, M., J. Lee, and K. Hellwald. 1999. Rearrangements in the 5′ untranslated region and phylogenetic analysis of Cucumber mosaic virus RNA 3 indicate radial evolution of three subgroups. J. Virol. 73:6752-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roossinck, M. J. 1997. Mechanisms of plant virus evolution. Annu. Rev. Phytopathol. 25:191-209. [DOI] [PubMed] [Google Scholar]
- 37.Rott, M. E., J. H. Tremaine, and D. M. Rochon. 1991. Comparison of the 5′ and 3′ termini of tomato ringspot virus RNA-1 and RNA-2: evidence for RNA recombination. Virology 185:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyer, S. 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6:526-538. [DOI] [PubMed] [Google Scholar]
- 39.Scott, N. W., J. I. Cooper, Y. Y. Liu, and C. U. Hellen. 1992. A 1.5 kb sequence homology in the 3′-terminal regions of RNA-1 and RNA-2 of a birch isolate of Cherry leaf roll virus is also present, in part, in a rhubarb isolate. J. Gen. Virol 73:481-485. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tóbiás, I. 1995. Characterization of different cherry leaf roll nepovirus (CLRV) isolates. Hortic. Sci. Kertészerti Tudomány 27:69-73. [Google Scholar]
- 42.Tomimura, K., J. Špak, N. Katis, C. E. Jenner, J. A. Walsh, A. J. Gibbs, and K. Ohshima. 2004. Comparisons of the genetic structure of populations of Turnip mosaic virus in West and East Eurasia. Virology 330:408-423. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson, J. A., and D. G. Walkey. 1967. The isolation and identification of rhubarb viruses occuring in Britain. Ann. Appl. Biol. 59:415-427. [Google Scholar]
- 44.Vigne, E., M. Bergdoll, S. Guyader, and M. Fuchs. 2004. Population structure and genetic variability within isolates of Grapevine fanleaf virus from a naturally infected vineyard in France: evidence for mixed infection and recombination. J. Gen. Virol. 85:2435-2445. [DOI] [PubMed] [Google Scholar]
- 45.Wang, S., R. C. Gergerich, S. L. Wickizer, and K. S. Kim. 2002. Localization of transmissible and nontransmissible viruses in the vector nematode Xiphinema americanum. Phytopathology 92:646-653. [DOI] [PubMed] [Google Scholar]
- 46.Wellink, J., O. Le Gall, H. Sanfacon, M. Ikegami, and A. T. Jones. 2000. Genus Nepovirus, p. 697-701. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle and R. B. Wickner (ed.), Virus taxonomy, seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 47.Werner, R., H.-P. Mühlbach, and C. Büttner. 1997. Detection of cherry leaf roll nepovirus (CLRV) in birch, beech and petunia by immunocapture reverse transcription-PCR using a conserved primerpair. Eur. J. Pathol. 27:309-318. [Google Scholar]