Abstract
Natural Killer (NK) cells are important in the immune response to a number of viruses; however, the mechanisms used by NK cells to discriminate between healthy and virus-infected cells are only beginning to be understood. Infection with vaccinia virus provokes a marked increase in the susceptibility of target cells to lysis by NK cells, and we show that recognition of the changes in the target cell induced by vaccinia virus infection depends on the natural cytotoxicity receptors NKp30, NKp44, and NKp46. Vaccinia virus infection does not induce expression of ligands for the activating NKG2D receptor, nor does downregulation of major histocompatibility complex class I molecules appear to be of critical importance for altered target cell susceptibility to NK cell lysis. The increased susceptibility to lysis by NK cells triggered upon poxvirus infection depends on a viral gene, or genes, transcribed early in the viral life cycle and present in multiple distinct orthopoxviruses. The more general implications of these data for the processes of innate immune recognition are discussed.
Natural killer cells are important components of the immune responses to many viruses, yet with the exception of NK recognition of cytomegalovirus-infected cells (17, 25), the mechanisms used by NK cells to distinguish between healthy and virus-infected cells are not well understood. The behavior of an NK cell confronted by a target cell is thought to depend on a delicate balance of signals transduced by activating and inhibitory receptors (36). Major histocompatibility complex (MHC) class I molecules are key ligands transmitting inhibitory signals to NK cells, while NKG2D and the natural cytotoxcity receptors (NCR), NKp46, NKp44, and NKp30, seem to be the major receptors driving activation of NK cells. The importance of the different activating receptors for NK cell recognition of different types of target cells varies, and it is clear that the basis of this phenomenon is differential ligand expression by target cells (43, 58). These observations are obviously important but derive mainly from studies using tumor cell targets (36). It is thus important to explore the mechanisms controlling NK cell recognition of virus-infected cells, since it seems likely that the mechanisms used by NK cells for target cell recognition have evolved in response to the pressure of pathogens, such as viruses, rather than in response to cancer. The few NK cell-deficient humans identified have suffered from recurrent virus infections (8), while in various murine systems, loci associated with resistance to infection with cytomegalovirus, herpes simplex virus, and ectromelia have been mapped to the NK gene complex on chromosome 6 (12, 31, 44). Thus, we decided to explore the mechanisms used by NK cells to discriminate between uninfected targets and cells infected with poxviruses.
Vaccinia virus infection elicits NK activation, proliferation, and accumulation at the site of infection (14, 18, 21, 40), and NK cells have long been proposed to mediate an important element in the protective response against this poxvirus (14, 49), especially in situations of T-cell deficiency (27). However, while it is known that vaccinia virus infection can provoke increased susceptibility to NK cell lysis (6, 13), the receptors and ligands important for NK cell recognition of vaccinia virus-infected cells are not known.
Our data confirm that infection with multiple distinct orthopoxviruses provokes a marked increase in the susceptibility of target cells to lysis by NK cells, and they demonstrate that the changes in the target cell recognized by the NK cell depend on a gene or genes transcribed early in the viral life cycle. Vaccinia virus infection does not alter expression of ligands for the activating NKG2D receptor, nor does downregulation of MHC class I molecules appear to be of critical importance in the altered target cell susceptibility to NK lysis induced by vaccinia virus infection; rather, the NCR NKp30, NKp44, and NKp46 appear to be the most important receptors for NK cell recognition of the vaccinia virus-infected target cell. These observations suggest that recognition by NK cell activating receptors involves the detection of changes in the target cells induced by conserved processes important for virus replication complementing other components of the innate immune system, where conserved structural elements of pathogens directly interact with conserved germ line-encoded receptors (57).
MATERIALS AND METHODS
Cells and antibodies.
The human foreskin fibroblast (HFF) cell lines Hs27 [HLA-A3 and -A23, -B8 and -B71(70), and Cw7] and HFFF2 [HLA-A11 and -A24(9), -B61(40) and B35, and Cw2 and Cw4] were obtained from the ECACC and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics.
NK cell lines were prepared from healthy adult donors as previously described (33) and were monitored periodically for the presence of T cells. The NK cell lines used in the experiments presented were over 95% CD3− CD56+. All of the NK cells expressed the activating receptors NKG2D, NKp46, NKp44, and NKp30, but in general, the NKp44 receptor was expressed at lower levels than the other NCR or NKG2D.
The anti-HLA class I (HLA-A-, -B-, and -C-reactive) antibodies used were HP-1F7 (45) (a gift from M. López-Botet, Universitat Pompeu Fabra, CEXS, Barcelona, Spain) and PA2.6 (41). Monoclonal antibodies (MAbs) to CD3, CD56, and CD58 were purchased from BD Pharmingen. MAbs to NKG2D, MICA/B, and ULBP-1, -2, and -3 were purchased from R&D systems. MAbs to NKp30, NKp44, and NKp46 were purchased from Beckman Coulter. MAb 138, an immunoglobulin M (IgM) antibody raised against NKp30 ligand-expressing tumor cells and shown to block the binding of soluble NKp30 to these cells (N. Mavaddat and H. T. Reyburn, unpublished data), was purified on mannan-binding protein columns (Pierce). Isotype control monoclonal antibodies were purchased from Sigma.
Viruses.
The various poxvirus isolates used in these experiments were a gift from A. Alcami (CNB, UAM, Spain) and have been described previously (1). Vaccinia virus deletion mutants (39, 51) were a gift of G. Smith (Department of Virology, Faculty of Medicine, Imperial College London). The vaccinia virus strain MVA (VR 1508) was obtained from the ATCC. Virus stocks were prepared in BSC-1 or TK-143B cells and titered in the human fibroblast cell lines Hs27 and HFFF2, except for MVA, which was grown and titered in BHK cells. Inactivation of vaccinia virus was achieved by treatment with Trioxsalen (4,5′,8-trimethylpsoralen; Sigma) and long-wave UV light. Virus-containing supernatants were brought to a Trioxsalen concentration of 2 μg/ml, incubated at room temperature for 10 min, and then exposed to UV light for 5 min. These conditions inactivate viral replication without abolishing the ability of the virus to infect cells (54).
In some experiments, target cells were pretreated with cycloheximide (CHX) at a concentration of 20 μg/ml for 1 h before the cells were infected with virus. The CHX was kept at this final concentration in the medium throughout the killing assay. The efficiency of cycloheximide treatment was verified by metabolic labeling with [35S]methionine and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of whole-cell lysates. For experiments involving cytosine-d-arabinofuranoside (AraC), an inhibitor of DNA replication that allows early protein expression but prevents synthesis of late viral proteins, cells were treated with 50 μg/ml AraC (Sigma) 30 min after infection. The efficiency of AraC treatment in the inhibition of viral-DNA replication was assessed by measurement of the inhibition of incorporation of [3H]thymidine.
Expression of recombinant proteins.
The plasmids for expression of NKp30, NKp44, NKp46 (32) (kind gifts of Ofer Mandelboim, Hebrew University-Hadassha Medical School, Jerusalem, Israel), and NKG2D (55) as fusion proteins with the Fc portion of human IgG1 have been described. Recombinant proteins were expressed by transfection of 293T cells with 47 μg of plasmid DNA/5 × 106 cells/175-cm2 flask using calcium phosphate. After removal of the precipitate at 16 h, the cells were incubated for 48 h to 72 h in X-Vivo-10 serum-free medium (BioWhittaker, Walkersville, MD). The supernatant was then recovered and concentrated in Centricon-YM10 spin concentrators, and the concentration of Ig fusion protein was determined by enzyme-linked immunosorbent assay. Negative controls for the Ig fusion protein staining were purified human IgG1 (Sigma) and an Fc fragment of human IgG1 (47) expressed in the same way as the IgG1 fusion proteins.
Flow cytometry.
For flow cytometry, 105 cells were preincubated in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), 0.1% sodium azide (Sigma), and either 10% normal goat serum, for staining with NCR-Ig, or 10% human serum, for staining with mouse MAbs. Cells stained with mouse MAbs were visualized, after being washed, by incubation with phycoerythrin-labeled F(ab′)2 fragments of goat anti-mouse Ig (Dako).
Staining with the Ig fusion proteins was done after immobilization of the chimeric proteins on fluorescent Covaspheres (Spherotech, Inc., Libertyville, IL). This experimental procedure was carried out essentially as described previously (11), except that the Ig fusion proteins (1 to 2 μg/sample) were mixed with 10 to 20 μl protein A-coated fluorescent beads/sample. Small volumes were shaken in round-bottom microtiter plates. Where volume adjustment was necessary, the beads were centrifuged and resuspended in PBS containing 0.2% BSA.
In all experiments, cells were stained 16 to 18 h after infection, and the efficiency of infection was monitored by staining with polyclonal rabbit anti-vaccinia virus antisera (gifts of A. Alcami). Dead/apoptotic cells were eliminated from the analysis by propidium iodide staining. Samples were analyzed using a FACScan II flow cytometer (Becton Dickinson).
Cytotoxicity assays.
The cytolytic activities of NK cell lines and clones against virus-infected and uninfected target cell lines were assessed in 4-h 51Cr release assays. In these assays, 5 ×103 target HFFs were plated in flat-bottom 96-well microtiter plates at 37°C, allowed to adhere, and then infected with the indicated virus strain or mutant. After adsorption of the virus for 1 to 2 h at 37°C, the inoculum was removed and medium containing Na51Cr2O4 was added (1 μCi/well). Mock-infected targets were prepared in parallel. At the indicated times after infection, effector cells at various concentrations were added to the infected and mock-infected targets, and the assay mixture was incubated at 37°C.
In experiments with antibody blocking, NK cells were preincubated with saturating amounts of the indicated MAbs (10 μg/ml) for 1 h before addition to the target cells.
Assays were performed in triplicate, and data values differed by <10% (average, ∼5%) of the mean. In all the cytotoxicity assays presented, the spontaneous release of 51Cr was <25% (average, ∼10%) of the maximal release. All cytotoxicity assays were performed 6 to 8 days after restimulation of the NK cells.
RESULTS
Vaccinia virus-infected cells are highly susceptible to lysis by primary human NK cells.
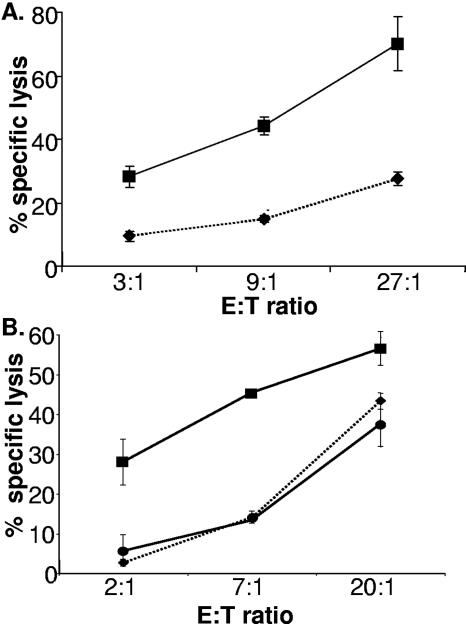
Human foreskin fibroblasts infected with vaccinia virus are markedly more sensitive to NK lysis than uninfected fibroblasts (Fig. 1). Study of Epstein-Barr virus-transformed B-cell lines (DBB and JY) showed that increased susceptibility to NK lysis upon vaccinia virus infection is seen in cell types other than fibroblasts (not shown), while time course experiments revealed that infection for only 2 h was sufficient to produce this altered sensitivity (not shown). Exposure of fibroblasts to inactivated virus did not trigger increased target cell susceptibility to NK lysis (Fig. 1B). Vaccinia virus inactivated by treatment with psoralen and long-wave UV is still able to bind and infect cells, but transcription and translation of many viral early genes and all viral late genes are blocked (54). Thus, these data indicate that increased susceptibility to NK lysis is not triggered by virion proteins or by cellular signaling events induced by virion binding.
FIG. 1.
Vaccinia virus-infected cells become highly susceptible to NK lysis. (A) Human fibroblast cells (HFFF2, passage 12) were either mock infected or infected with vaccinia virus (strain WR; multiplicity of infection [MOI] = 5) before being labeled overnight with 51Cr. NK cells were added to the cultures at the indicated effector-to-target cell (E:T) ratios, and the assay mixture was incubated at 37°C for 4 h. The fibroblasts were either uninfected (⧫) or infected with vaccinia virus WR (▪). The data are representative of at least three experiments done with polyclonal NK lines generated from more than eight donors. The error bars indicate standard deviations. (B) Human fibroblast cells (HFFF2, passage 12) were mock infected or infected with either vaccinia virus (strain WR; MOI = 5) or psoralen/UV-inactivated vaccinia virus (strain WR; MOI = 5) before being labeled overnight with 51Cr. NK cells were added to the cultures at the indicated E:T ratios, and the assay was incubated at 37°C for 4 h. ⧫, uninfected fibroblasts; ▪, fibroblasts infected with vaccinia virus WR; •, fibroblasts infected with psoralen/UV-inactivated vaccinia virus WR. The data are representative of at least three experiments done with polyclonal NK lines generated from more than four donors.
The altered target cell susceptibility to lysis by NK cells is provoked by expression of an early viral gene or genes.
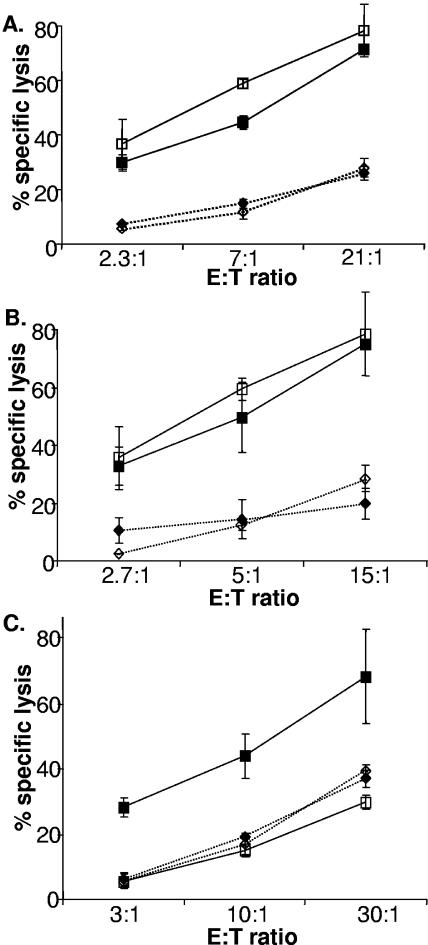
Although psoralen/UV treatment blocks replication of vaccinia virus, transcription of late genes, and induction of cytopathic effect, transcription and translation of some, but not all, early genes still occur (54). These observations suggest that the triggering of susceptibility to lysis depends on viral genes expressed early rather than late. To test this idea, infection was carried out in the presence of AraC, an inhibitor of poxviral DNA replication and thus an inhibitor of late-viral-gene expression. If only viral early-gene products were required, then AraC should not affect the increased susceptibility to NK lysis on infection. As indicated in Fig. 2A, treatment with AraC had virtually no effect on the vaccinia virus-induced increased susceptibility to NK lysis, indicating that this effect depends principally on early-viral-gene expression. Addition of AraC to a cytotoxicity assay had no effect on the ability of NK cells to kill susceptible targets (Fig. 2B); thus, these experiments confirm that one or more early-gene products of vaccinia virus participate in the process that results in the markedly increased susceptibility to NK lysis provoked by infection with the virus. The increase in susceptibility to NK lysis produced by infection was not seen when cells were infected with vaccinia virus in the presence of the protein synthesis inhibitor CHX (Fig. 2C) at concentrations which block viral, as well as cellular, protein synthesis but do not interfere with virion uptake or early viral mRNA synthesis (10). In the aggregate, therefore, these data demonstrate that viral entry, viral early-gene transcription, and protein synthesis are required to provoke the cellular response. However, since the cycloheximide treatment blocks both cellular- and viral-protein synthesis, these observations do not distinguish between NK cell recognition of viral proteins or host cell ligands induced by viral RNAs or proteins.
FIG. 2.
Viral early-gene expression is required to provoke altered susceptibility to lysis by NK cells. Human fibroblasts (HFFF2, passage 12) were either mock infected (⧫) or infected with vaccinia virus (strain WR; multiplicity of infection = 5) (▪) and labeled with 51Cr. NK cells were added to the various cultures at the indicated effector-to-target cell (E:T) ratios, and the assay mixture was incubated at 37°C for 4 h. The data are representative of at least three experiments done with polyclonal NK cell lines generated from more than five donors. (A) Target cells were left untreated (filled symbols) or AraC was added to a concentration of 50 μg/ml 1 h after infection (open symbols) and maintained in the medium at that concentration throughout the killing assay. The error bars indicate standard deviations. (B) Treatment with AraC does not affect target cell susceptibility to NK lysis. Where indicated, AraC was added to a concentration of 50 μg/ml and maintained in the medium at that concentration throughout the killing assay. NK cells were added to the various cultures at the indicated E:T ratios, and the assay mixture was incubated at 37°C for 4 h. The data are representative of three experiments done with polyclonal NK lines. ⧫, Hs27 fibroblasts; ⋄, Hs27 fibroblasts treated with AraC; ▪, K562 cells; □, K562 cells treated with AraC. (C) Target cells were untreated (filled symbols) or pretreated with CHX at a concentration of 20 μg/ml for 1 h before the cells were infected with virus (open symbols). The CHX was kept at this final concentration in the medium throughout the killing assay.
Increased susceptibility to NK lysis depends on a viral gene or genes present in multiple poxvirus isolates.
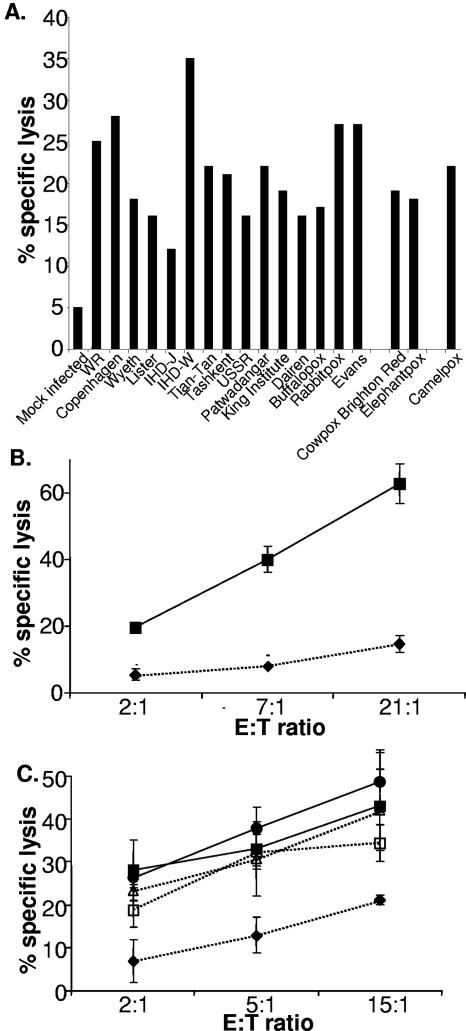
We next tested whether the ability to provoke increased susceptibility to NK lysis was common among multiple orthopoxviruses, including three distinct species (vaccinia, cowpox, and camelpox viruses). Figure 3A shows that infection with any of 12 strains of vaccinia, rabbitpox, and buffalopox viruses (considered vaccinia virus strains), cowpox virus (Brighton Red strain), elephantpox virus (considered a cowpox virus strain), or camelpox virus triggered susceptibility to NK cytotoxicity, implying that the changes in the infected cell recognized by NK cells for lysis are provoked by expression of a gene or genes found in a wide range of poxviruses. Genes that are conserved among poxviruses tend to be centrally located in the genome and involved with common molecular functions, such as replication or virion assembly. In contrast, terminally located genes tend to be more variable and are often involved in host range restriction or immune subversion (37). We therefore tested whether infection with a set of mutant vaccinia viruses, with deletions in the left- and right-hand ends of the genome (39, 51), could trigger increased susceptibility to NK lysis. In these experiments, we also included the highly attenuated vaccinia virus strain MVA, which has large deletions in both the left and right termini of the genome and some fragmented genes even in the central conserved region (3). NK cells were able to recognize and kill cells infected with any of the four viruses (Fig. 3B and C), indicating that the genes inactivated in these mutant viruses are not necessary to render infected cells susceptible to NK cell-mediated lysis. Thus, although the gene(s) triggering NK susceptibility has not been located, these observations are consistent with the hypothesis that conserved viral genes located centrally in the genome are important for this effect.
FIG. 3.
Increased susceptibility to NK lysis is due to a viral gene or genes present in multiple poxvirus isolates. (A) HFFF2 cells were either mock infected or infected with the indicated viruses and labeled overnight with 51Cr. NK cells were then added, and the assay mixture was incubated at 37°C for 4 h. The data are representative of three experiments done with polyclonal NK lines generated from two donors. (B) Infection, 51Cr labeling, and killing assays were carried out as described for panel A; cells were mock infected (⧫) or infected with MVA (▪). The error bars indicate standard deviations. (C) Infection, 51Cr-labeling, and killing assays were carried out as for panel A; cells were either mock infected (⧫) or infected with WR (▪), with v6/2 (□), with vGS100 (•), or with vSS/K2 (▵). The data are representative of six experiments done with polyclonal NK lines generated from three donors.
Alterations in inhibitory signaling via MHC class I molecules do not have a major effect on the increased susceptibility of vaccinia virus-infected cells to NK cell attack.
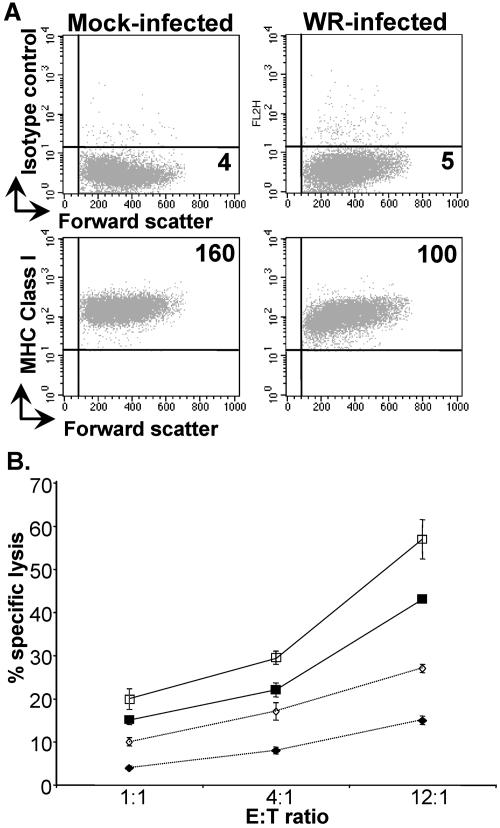
NK cell recognition is controlled by a balance of signals from inhibitory and activating receptors (36, 58). Key ligands transmitting inhibitory signals are MHC class I molecules (28, 34), and so one possible explanation for the increased susceptibility to NK lysis provoked by virus infection was that virus-mediated downregulation of MHC class I molecules made the target cell more susceptible to NK cell attack: the “missing-self hypothesis” (30). To examine this possibility, the levels of cell surface MHC class I molecules expressed on control and vaccinia virus-infected fibroblasts were examined, but only limited downregulation of MHC class I was observed (Fig. 4A). Indeed, when the levels of MHC class I expression of infected and control DBB and JY B-cell lines were compared, no differences were observed (not shown). The significance of this slight reduction in MHC class I expression for NK cell recognition was explored by studying the effect of blocking MHC class I molecules on NK cell lysis of uninfected and vaccinia virus-infected fibroblasts (Fig. 4B). In these experiments, treatment with anti-HLA class I MAbs led to slightly increased NK killing of both uninfected and infected target cells, but it did not affect the specific increased killing of the vaccinia virus-infected targets compared to the uninfected fibroblasts. Further, these experiments showed that blockade with anti-MHC class I MAbs produced only small changes in fibroblast susceptibility to NK lysis, implying that inhibitory signaling via MHC class I molecules is not a major factor protecting fibroblasts from NK cell attack, an observation consistent with previous data from multiple groups (15, 16, 29, 61). Overall, these data suggested that downregulation of MHC class I was of limited significance in specific NK cell recognition and lysis of vaccinia virus-infected target cells.
FIG. 4.
Increased susceptibility of vaccinia virus-infected cells to NK cell attack does not depend on downregulation of MHC class I molecules. (A) HFFF2 fibroblasts were either mock infected or infected with vaccinia virus WR (multiplicity of infection = 5) for 16 h. The cells were recovered with PBS-2 mM EDTA and stained for MHC class I using MAb HP-1F7. Lines indicate quadrants drawn for negative controls; large numbers indicate mean fluorescence intensity of antibody staining. (B) HFFF2 cells were either mock infected (⋄) or vaccinia virus infected (□) and labeled overnight with 51Cr. After being washed, the target cells were incubated with either isotype control MAb (filled symbols) or MAb specific for MHC class I (PA2.6) (open symbols) for 30 min. NK cells were then added, and the assay mixture was incubated at 37°C for 4 h. The data are representative of four experiments. Similar data were obtained using the MAb HP-1F7 (data not shown). The error bars indicate standard deviations.
The increased susceptibility of vaccinia virus-infected cells to NK cell attack does not depend on altered expression of ligands for the activating receptor NKG2D.
An alternative hypothesis to explain the increased sensitivity of virus-infected cells to NK lysis was that virus infection produces increased target cell expression of ligands for activating NK receptors, such as NKG2D (17) or the NCR (35).
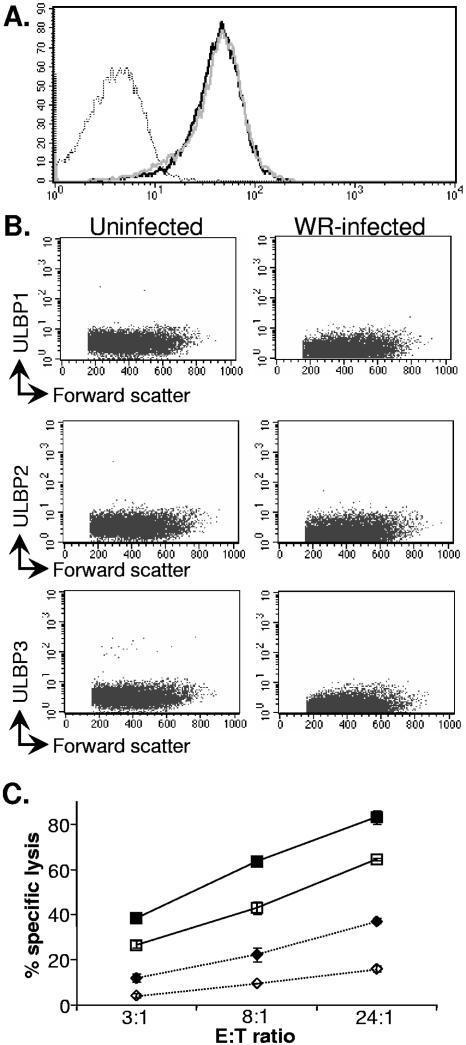
In experiments with flow cytometry, no alteration in levels of expression of NKG2D ligands, such as MICA/B molecules (Fig. 5A) or ULBP-1, -2, or -3 (Fig. 5B), was observed on vaccinia virus-infected cells. Consistent with these observations, vaccinia virus infection did not produce alterations in transcription of the MICA/B or ULBP-1, -2, and -3 genes (data not shown). Still, it was possible that the fluorescence-activated cell sorter binding experiments were not sensitive enough to detect subtle changes in NKG2D ligand expression or that upregulation of an NKG2D ligand not bound by the available MAbs was required for a functional role in NK-mediated killing of infected cells. Therefore, to determine definitively if NKG2D plays any part in NK lysis of vaccinia virus-infected target cells, NK cells were preblocked with saturating amounts of either anti-CD56 MAb, as a control, or anti-NKG2D MAb before incubation with uninfected and vaccinia virus-infected HFFF2 (Fig. 5C) and Hs27 (not shown) target cells in a 51Cr release killing assay.
FIG. 5.
The NKG2D receptor does not play a major role in specific NK cell recognition of vaccinia virus-infected cells. (A) HFFF2 fibroblasts were either mock infected or infected with vaccinia virus WR (multiplicity of infection = 5) for 16 h. The cells were recovered with PBS-2 mM EDTA and stained for MICA/B. Dotted line, infected cells (isotype control); solid line, infected cells (MICA/B); grey line, uninfected cells (MICA/B). (B) Fibroblasts were either mock infected or infected as for panel A, recovered with PBS-2 mM EDTA 16 h after infection, and stained with the indicated ULBP-specific MAb. (C) NK cells were preincubated for 30 min with MAb to CD56 (filled symbols) or blocking MAb specific for the NKG2D receptor (open symbols) before addition to the mock-infected (⋄) or vaccinia virus-infected (□) targets. The data are representative of more than 12 experiments done with polyclonal NK cell lines generated from multiple donors. The error bars indicate standard deviations.
In these experiments, treatment with anti-NKG2D led to reduced NK cell killing of both uninfected and infected target cells, but it did not reduce the increased killing of the virus-infected targets compared to the uninfected targets. Therefore, although NKG2D is clearly involved in the killing of the fibroblasts in this assay system, NKG2D/NKG2D ligand interactions do not appear to be the mechanism by which NK cells distinguish between uninfected and vaccinia virus-infected fibroblasts.
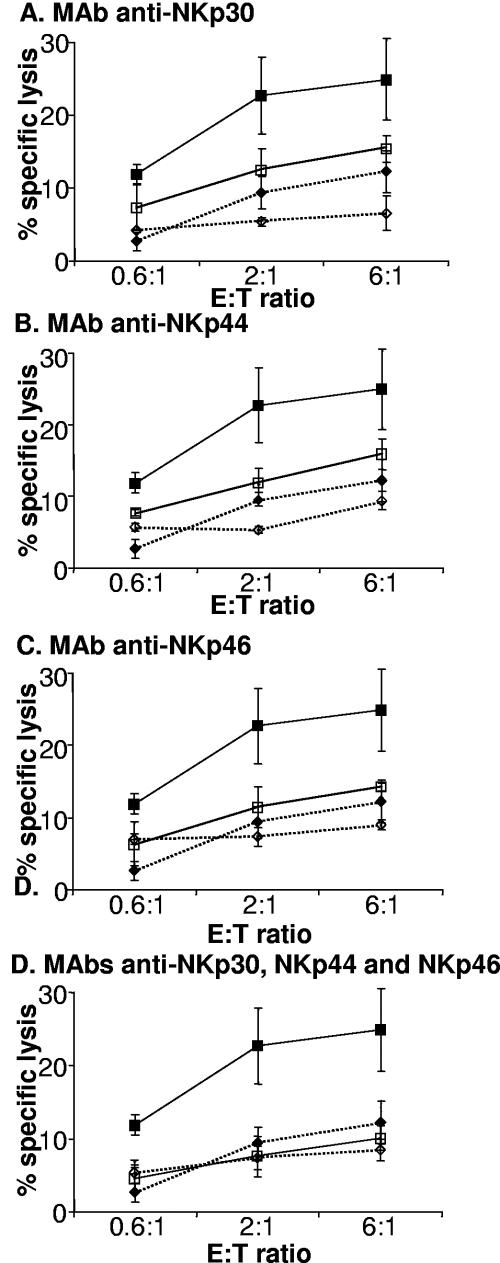
NK cell recognition of vaccinia virus-infected cells involves all three NCR.
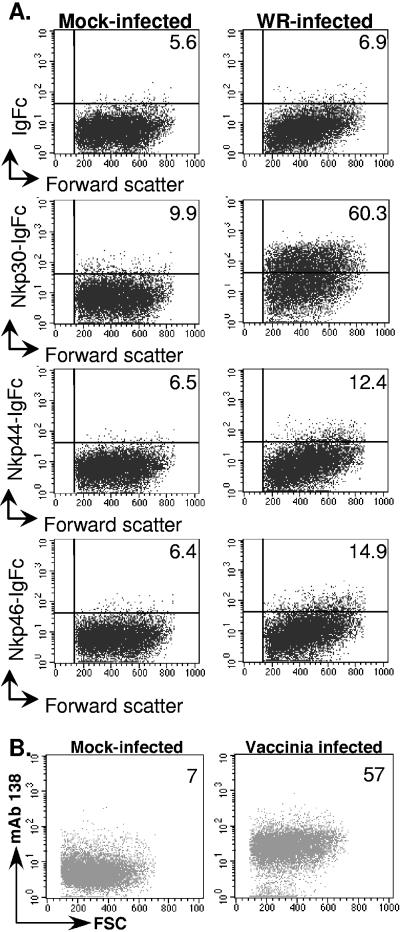
The role of the NCR in NK-mediated killing of vaccinia virus-infected target cells was investigated by pretreating NK cells with MAbs specific for either NCR or CD56 (as a control) to block NCR-mediated NK cell activation. Inclusion of MAbs specific for any of the three NCR could block, at least partially, NK cell lysis of vaccinia virus-infected target cells (Fig. 6A, B, and C), while pretreatment of NK cells with a cocktail of MAbs specific for all three NCR completely blocked NK recognition of vaccinia virus-infected cells (Fig. 6D). These data demonstrate that ligands for NKp30, NKp44, and NKp46 are expressed in response to vaccinia virus strain WR infection and play a role in the specific killing of vaccinia virus-infected target cells by NK cells. Induction of ligands for the NCR on vaccinia virus-infected cells could be confirmed in flow cytometry experiments, where target cells infected with vaccinia virus were stained with control Ig-Fc protein or NKp30-, -44-, and -46-Ig fusion proteins. A definite increase in binding of NKp30-Ig to the WR-infected cells compared to the uninfected cells was observed, as were small, but consistent, increases in binding of NKp44-Ig and NKp46-Ig to infected cells (Fig. 7). Similar observations were made in staining experiments with Hs27 fibroblasts and HeLa cells infected with vaccinia virus (data not shown). In control experiments, all three NCR-Ig fusion proteins stained the A549 cell line (not shown), which has been reported to express ligands for all three NCR (42). We could confirm that at least some of the NKp30 ligands upregulated upon vaccinia virus infection were cellular proteins, since infected cells showed increased reactivity with a novel MAb, 138, raised against NKp30 ligand-expressing tumor cells and shown to block binding of soluble NKp30 to these cells (Fig. 7B) (Mavaddat and Reyburn, unpublished). We do not yet have similar MAbs specific for NKp44 and NKp46 ligands.
FIG. 6.
NK cell recognition of vaccinia virus-infected cells involves all three NCR. NK cells were preincubated with either MAb to CD56 (filled symbols) or the indicated MAb, or combinations of MAbs, specific for NCR (open symbols) for 30 min before addition to the mock-infected (⋄) or infected (□) targets. The data are representative of 12 experiments done with polyclonal NK lines generated from multiple donors. The error bars indicate standard deviations.
FIG. 7.
Binding of soluble NCR to vaccinia virus-infected cells. (A) HFFF2 fibroblasts were either mock infected or infected with vaccinia virus WR (multiplicity of infection = 5) for 16 h. The cells were recovered with PBS-2 mM EDTA and stained with the indicated Ig fusion protein or control Ig-Fc immobilized on fluorescent microspheres. The data are representative of more than five experiments done with two batches of the various fusion proteins. The mean fluorescence intensity of staining with each NCR-Ig fusion protein is indicated. (B) Fibroblasts were either mock infected or infected, recovered as for panel A, and then stained with the NKp30 ligand-specific MAb, 138.
DISCUSSION
Vaccinia virus infection is known to drive NK activation, proliferation, and accumulation at the site of infection (14, 18, 21, 40), and it is thought that NK cells mediate an important element in the protective response against this poxvirus (14, 49). However, while the importance of NK cells in the immune response to poxvirus infection has been known for some time, very little information is known about how NK cells recognize target cells infected by these viruses. It has been reported that vaccinia virus-infected cells show an increased susceptibility to NK lysis that coincides with a limited reduction in expression of H-2 class I molecules (13). Interpretation of this study is complicated, however, since apart from the well-documented differences in NK receptor repertoire and function between human and murine NK cells (24), the function of the MHC class I molecules in NK recognition was not tested directly in this study. Indeed, the peak of susceptibility to NK lysis was also the moment of maximal target cell sensitivity to lysis by vaccinia virus-specific cytotoxic T lymphocytes. Our data (Fig. 4) clearly demonstrate, first, that vaccinia virus infection provokes only a modest decrease in MHC class I expression, consistent with previous observations (10), and second, that inhibitory signaling by MHC class I molecules is of only limited importance in protecting fibroblasts from NK cell lysis, an observation in agreement with previous data from multiple groups both in vitro (15, 16, 29) and in vivo (61). It might be argued that uninfected HFFs lack positive signals needed to trigger NK cells and that MHC class I-dependent inhibitory signaling could have a stronger effect in the context of infected cells, which have upregulated ligands for the “triggering” receptors. However, blocking MHC class I on vaccinia virus-infected HFFs did not enhance the susceptibility to NK lysis of infected cells to a greater degree than that seen with uninfected cells. Overall, therefore, these data indicate that while MHC class I expression confers a low level of protection against NK cell attack, alterations in this inhibitory signaling are not the molecular basis of specific NK cell recognition of vaccinia virus-infected fibroblasts.
In this context, an alternative hypothesis to explain the increased sensitivity to NK lysis of virus-infected cells was that virus infection produces increased target cell expression of ligands for activating NK receptors, such as NKG2D (17) or the NCR (35). The activating receptor NKG2D, which is expressed by NK cells, T cells, some cytolytic CD8+ T cells and NK T cells, and a minor subset of CD4+ T cells (46), is believed to be important in immunosurveillance of multiple tumor types (17, 46), as well as cells infected with viruses, such as cytomegalovirus, and certain bacteria (19, 23, 52). Surprisingly, however, while the interaction of NKG2D with MICA/B is clearly a component of NK recognition and lysis of fibroblasts, either mock infected or vaccinia virus infected, it is also clear that this interaction is not the basis on which NK cells discriminate between vaccinia virus-infected and uninfected cells (Fig. 5).
The major factor provoking specific NK lysis of poxvirus-infected target cells is the induction, on the target cell, of ligands for the NCR (Fig. 6 and 7). Inclusion of MAbs specific for all three NCR was required for a complete block of NK lysis (Fig. 6), demonstrating, first, that infection induces or upregulates the expression of ligands for the three NCR and, second, that the NCR are of critical importance in NK-mediated lysis of poxvirus-infected cells. These observations also show that the concept that expression of ligands for NK-activating receptors is induced rather than constitutive is relevant not only to the interactions of NKG2D with its ligands (36, 58), but also the interaction of the NCR with their ligands.
At present, little is known about the ligands of the NCR. It has been suggested that proteoglycans posttranslationally modified by addition of heparan-sulfate may be components of the structures bound by NKp30 and NKp46 (9), but others have failed to reproduce these observations (59). It has also been reported that the hemagglutinin and neuraminidase proteins of influenza and parainfluenza viruses can bind to sialic acid moieties on the NKp44 and NKp46 receptors, but not NKp30, to activate NK cells (5, 32). However, for the experiments presented here, it seems clear that vaccinia virus hemagglutinin (HA) or neuraminidase protein is not required, since vaccinia virus lacks detectable neuraminidase activity (7, 22) and infection with HA-negative viruses, such as IHD-W, which has no detectable hemadsorption activity and no HA protein on the cell surface (26, 48), or MVA (4), still provokes increased susceptibility to NK lysis (Fig. 3).
We have not yet identified a specific vaccinia virus gene or genes whose expression provokes the changes in the target cell recognized by NK cells, but the data clearly indicate that this effect depends principally on a gene or genes present in multiple distinct species of orthopoxviruses (Fig. 3A). The experiments with psoralen/long-wave UV light-inactivated virus are particularly interesting, since this treatment targets viral nucleic acid and introduces chemical cross-linking in the viral genome, generating noncytopathic viruses able to infect cells and express small, but not large, viral early genes (54). Expression of viral late genes is abolished in cells infected with these viruses; thus, the lack of susceptibility to NK lysis of cells infected with psoralen/UV-treated virus (Fig. 1B) demonstrates, first, that binding and entry of virus is not sufficient to trigger susceptibility to NK cell attack and, second, that late-gene expression and viral replication are also not required. These observations strongly suggest that expression of viral early genes is the key step in triggering NK susceptibility, and the results of the AraC experiments, in which late-gene expression is blocked but early-gene expression is allowed, are consistent with this interpretation (Fig. 2A). We cannot absolutely exclude the possibility that other factors may have some minor role in provoking susceptibility to NK lysis (e.g., induced cellular signaling events at the level of virus entry could help to initiate a cascade of events leading up to the synthesis of the ligands for the NCR), but our working hypothesis is that the ligands for the NCR that appear on infection are cellular proteins upregulated, or altered, by some viral early gene or genes after infection with vaccinia virus. In support of this idea, cells infected with vaccinia virus showed increased reactivity with a novel NKp30 ligand-specific MAb raised against NCR ligand-expressing tumor cells and shown to block binding of soluble NKp30 to these cells (Fig. 7B). Other evidence to support this hypothesis comes from bioinformatics analyses of known and putative early genes (defined by the presence of conserved upstream promoter sequences and downstream transcription termination sequences [20, 60; E. Lefkowitz, personal communication]) common to all the orthopoxviruses and able to trigger susceptibility to NK cell lysis. None of these open reading frames encoded proteins with a signal sequence and a transmembrane domain, implying that they are expressed at the surface of the infected cell, although with the caveat that there could be a direct interaction of the NCR with some poxvirus protein, since certain poxviral proteins that lack a transmembrane domain may still associate with the cell surface, e.g., B18R, the viral type I interferon receptor (2). Overall, however, these observations suggest that some NK cell recognition may be based on monitoring changes in the target cells induced by conserved processes involved in virus survival or replication. This would be an interesting parallel to other components of the innate immune system where conserved structural elements of pathogens directly interact with conserved germ line-encoded receptors (57). Molecular characterization of the ligands for the NCR and the factors controlling their expression will allow determination of the validity of this hypothesis.
A reasonable prediction of the hypothesis that the NCR ligands are cellular factors whose expression is induced by virus infection is that infection with other viruses might also induce expression of ligands for the NCR, and although this does not appear to be the case for cytomegaloviruses, it has recently been reported that human immunodeficiency virus (HIV) infection can lead to expression of a ligand for NKp44. NK cell recognition of human cytomegalovirus-infected fibroblasts seems to be based on the binding of the NKG2D, DNAM-1, and CD96 receptors to their ligands, whereas no changes are seen in the expression of ligands for NCR, such as NKp30 and NKp46 (25, 53). NKG2D/NKG2D ligand interactions are also important for NK cell recognition of murine cytomegalovirus-infected cells, but subsets of murine NK cells can also be activated by direct recognition of viral glycoproteins by activating Ly-49 receptors (25). HIV type 1 infection has been reported to induce expression of a cellular ligand for NKp44, but not NKp46, and CD4+ T cells expressing this ligand become highly sensitive to lysis by NKp44+ NK cells (56). In these experiments, HIV infection also produced a slight increase in reactivity with NKp30-Ig, but the functional significance of this observation was not tested. Vaccinia virus infection produced an increased expression of ligands for all three NCR (Fig. 6), but experiments with flow cytometry showed that the NCR ligands were not upregulated to the same extent (Fig. 7): vaccinia virus-infected target cells consistently stained much more brightly with soluble NKp30 than with soluble NKp44 or NKp46 protein. The significance of these observations is not clear, but one simple explanation of these data would be that the binding of NKp30 to its ligand(s) is of much higher affinity than NKp44 or NKp46 ligand binding and so is easier to visualize. An alternative hypothesis is that the ligands for the various NCR are upregulated differentially in response to different stimuli, so that the different NCR would be variably important in NK cell recognition of different target cells. The available data do not distinguish between these possibilities; indeed, in vitro, even low-level expression of NCR ligand appears sufficient to trigger NK cell cytotoxicity (Fig. 6 and 7); however, it is at least conceivable that this may not be the case in vivo.
Finally, the fact that infection with MVA produces increased target cell susceptibility to NK lysis is interesting from another point of view. This highly attenuated virus has lost around 15% of the parental viral genome, and its ability to replicate in human cells is extremely limited, yet in mammals, infection with MVA recombinants induces protective immunity against a wide spectrum of pathogens and the use of MVA as a vector for the generation of live vaccines against infectious diseases and in cancer therapy has been widely explored due to its safety and its ability to evoke protection (38, 50). The data here show that despite the extensive genetic lesions of this virus, MVA-infected cells are efficiently recognized and lysed by NK cells. It is possible that this phenomenon could affect the duration and/or efficiency of antigen challenge to the immune system, and it would be interesting to explore the effect of NK cell depletion on MVA-based vaccination strategies.
Acknowledgments
We thank E. Lefkowitz for sharing unpublished data; A. Alcamí for the gift of viruses and antisera and helpful discussions; M. Lopez-Botet, O. Mandelboim, and G. L. Smith for gifts of antibodies, plasmids, and viruses; and Tony Nash, Mark Wills, Mar Valés-Gómez, Pedro Roda-Navarro, and Robin Cassady-Cain for critical reading of the manuscript.
This work was supported by grants to H.T.R. from the Wellcome Trust and the Medical Research Council.
REFERENCES
- 1.Alcami, A., A. Khanna, N. L. Paul, and G. L. Smith. 1999. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J. Gen. Virol. 80:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Alcami, A., J. A. Symons, and G. L. Smith. 2000. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 74:11230-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 4.Antoine, G., F. Scheiflinger, G. Holzer, T. Langmann, F. G. Falkner, and F. Dorner. 1996. Characterization of the vaccinia MVA hemagglutinin gene locus and its evaluation as an insertion site for foreign genes. Gene 177:43-46. [DOI] [PubMed] [Google Scholar]
- 5.Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. [DOI] [PubMed] [Google Scholar]
- 6.Baraz, L., E. Khazanov, R. Condiotti, M. Kotler, and A. Nagler. 1999. Natural killer (NK) cells prevent virus production in cell culture. Bone Marrow Transplant. 24:179-189. [DOI] [PubMed] [Google Scholar]
- 7.Bik, T., I. Sarov, and A. Livne. 1982. Interaction between vaccinia virus and human blood platelets. Blood 59:482-487. [PubMed] [Google Scholar]
- 8.Biron, C. A. 1997. Activation and function of natural killer cell response during viral infections. Curr. Opin. Immunol. 9:24-34. [DOI] [PubMed] [Google Scholar]
- 9.Bloushtain, N., U. Qimron, A. Bar-Ilan, O. Hershkovitz, R. Gazit, E. Fima, M. Korc, I. Vlodavsky, N. V. Bovin, and A. Porgador. 2004. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 173:2392-2401. [DOI] [PubMed] [Google Scholar]
- 10.Boshkov, L. K., J. L. Macen, and G. McFadden. 1992. Virus-induced loss of class I MHC antigens from the surface of cells infected with myxoma virus and malignant rabbit fibroma virus. J. Immunol. 148:881-887. [PubMed] [Google Scholar]
- 11.Brown, M. H., S. Preston, and A. N. Barclay. 1995. A sensitive assay for detecting low-affinity interactions at the cell surface reveals no additional ligands for the adhesion pair rat CD2 and CD48. Eur. J. Immunol. 25:3222-3228. [DOI] [PubMed] [Google Scholar]
- 12.Brownstein, D. G. 1998. Comparative genetics of resistance to viruses. Am. J. Hum. Genet. 62:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brutkiewicz, R. R., S. J. Klaus, and R. M. Welsh. 1992. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat. Immunol. 11:203-214. [PubMed] [Google Scholar]
- 14.Bukowski, J. F., B. A. Woda, S. Habu, K. Okumura, and R. M. Welsh. 1983. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J. Immunol. 131:1531-1538. [PubMed] [Google Scholar]
- 15.Carr, W. H., A. M. Little, E. Mocarski, and P. Parham. 2002. NK cell-mediated lysis of autologous HCMV-infected skin fibroblasts is highly variable among NK cell clones and polyclonal NK cell lines. Clin. Immunol. 105:126-140. [DOI] [PubMed] [Google Scholar]
- 16.Cerboni, C., M. Mousavi-Jazi, A. Linde, K. Soderstrom, M. Brytting, B. Wahren, K. Karre, and E. Carbone. 2000. Human cytomegalovirus strain-dependent changes in NK cell recognition of infected fibroblasts. J. Immunol. 164:4775-4782. [DOI] [PubMed] [Google Scholar]
- 17.Cerwenka, A., and L. L. Lanier. 2001. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 1:41-49. [DOI] [PubMed] [Google Scholar]
- 18.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das, H., V. Groh, C. Kuijl, M. Sugita, C. T. Morita, T. Spies, and J. F. Bukowski. 2001. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity 15:83-93. [DOI] [PubMed] [Google Scholar]
- 20.Davison, A. J., and B. Moss. 1989. Structure of vaccinia virus early promoters. J. Mol. Biol. 210:749-769. [DOI] [PubMed] [Google Scholar]
- 21.Dokun, A. O., S. Kim, H. R. Smith, H. S. Kang, D. T. Chu, and W. M. Yokoyama. 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2:951-956. [DOI] [PubMed] [Google Scholar]
- 22.Els, M. C., W. G. Laver, and G. M. Air. 1989. Sialic acid is cleaved from glycoconjugates at the cell surface when influenza virus neuraminidases are expressed from recombinant vaccinia viruses. Virology 170:346-351. [DOI] [PubMed] [Google Scholar]
- 23.Groh, V., R. Rhinehart, J. Randolph-Habecker, M. S. Topp, S. R. Riddell, and T. Spies. 2001. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2:255-260. [DOI] [PubMed] [Google Scholar]
- 24.Gumperz, J. E., and P. Parham. 1995. The enigma of the natural killer cell. Nature 378:245-248. [DOI] [PubMed] [Google Scholar]
- 25.Hamerman, J. A., K. Ogasawara, and L. L. Lanier. 2005. NK cells in innate immunity. Curr. Opin. Immunol. 17:29-35. [DOI] [PubMed] [Google Scholar]
- 26.Ichihashi, Y., and S. Dales. 1971. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology 46:533-543. [DOI] [PubMed] [Google Scholar]
- 27.Karupiah, G., R. V. Blanden, and I. A. Ramshaw. 1990. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J. Exp. Med. 172:1495-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanier, L. L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359-393. [DOI] [PubMed] [Google Scholar]
- 29.Leong, C. C., T. L. Chapman, P. J. Bjorkman, D. Formankova, E. S. Mocarski, J. H. Phillips, and L. L. Lanier. 1998. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatbility complex and a viral class I homologue. J. Exp. Med. 187:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljunggren, H.-G., and K. Karre. 1990. In search of the ‘missing self’: MHC molecules and NK recognition. Immunol. Today 11:237. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg, P., P. Welander, H. Openshaw, C. Nalbandian, C. Edwards, L. Moldawer, and E. Cantin. 2003. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J. Virol. 77:11661-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055-1060. [DOI] [PubMed] [Google Scholar]
- 33.Mandelboim, O., H. T. Reyburn, M. Vales-Gomez, L. Pazmany, M. Colonna, G. Borsellino, and J. L. Strominger. 1996. Protection from lysis by natural killer cells of group 1 and 2 specificity is mediated by residue 80 in human histocompatibility leukocyte antigen C alleles and also occurs with empty major histocompatibility complex molecules. J. Exp. Med. 184:913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moretta, A., C. Bottino, M. Vitale, D. Pende, R. Biassoni, M. C. Mingari, and L. Moretta. 1996. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 14:619-648. [DOI] [PubMed] [Google Scholar]
- 35.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 36.Moretta, L., and A. Moretta. 2004. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 38.Moss, B., M. W. Carroll, L. S. Wyatt, J. R. Bennink, V. M. Hirsch, S. Goldstein, W. R. Elkins, T. R. Fuerst, J. D. Lifson, M. Piatak, N. P. Restifo, W. Overwijk, R. Chamberlain, S. A. Rosenberg, and G. Sutter. 1996. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 397:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss, B., E. Winters, and J. A. Cooper. 1981. Deletion of a 9,000-base-pair segment of the vaccinia virus genome that encodes nonessential polypeptides. J. Virol. 40:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natuk, R. J., and R. M. Welsh. 1987. Accumulation and chemotaxis of natural killer/large granular lymphocytes at sites of virus replication. J. Immunol. 138:877-883. [PubMed] [Google Scholar]
- 41.Parham, P., and W. F. Bodmer. 1978. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature 276:397-399. [DOI] [PubMed] [Google Scholar]
- 42.Pende, D., S. Parolini, A. Pessino, S. Sivori, R. Augugliaro, L. Morelli, E. Marcenaro, L. Accame, A. Malaspina, R. Biassoni, C. Bottino, L. Moretta, and A. Moretta. 1999. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 190:1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pende, D., P. Rivera, S. Marcenaro, C. C. Chang, R. Biassoni, R. Conte, M. Kubin, D. Cosman, S. Ferrone, L. Moretta, and A. Moretta. 2002. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 62:6178-6186. [PubMed] [Google Scholar]
- 44.Pereira, R. A., A. Scalzo, and A. Simmons. 2001. Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J. Immunol. 166:5869-5873. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Villar, J. J., I. Melero, F. Navarro, M. Carretero, T. Bellon, M. Llano, M. Colonna, D. E. Geraghty, and M. Lopez-Botet. 1997. The CD94/NKG2-A inhibitory receptor complex is involved in natural killer cell-mediated recognition of cells expressing HLA-G1. J. Immunol. 158:5736-5743. [PubMed] [Google Scholar]
- 46.Raulet, D. H. 2004. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat. Immunol. 5:996-1002. [DOI] [PubMed] [Google Scholar]
- 47.Routledge, E. G., I. Lloyd, S. D. Gorman, M. Clark, and H. Waldmann. 1991. A humanized monovalent CD3 antibody which can activate homologous complement. Eur. J. Immunol. 21:2717-2725. [DOI] [PubMed] [Google Scholar]
- 48.Seki, M., M. Oie, Y. Ichihashi, and H. Shida. 1990. Hemadsorption and fusion inhibition activities of hemagglutinin analyzed by vaccinia virus mutants. Virology 175:372-384. [DOI] [PubMed] [Google Scholar]
- 49.Stitz, L., J. Baenziger, H. Pircher, H. Hengartner, and R. M. Zinkernagel. 1986. Effect of rabbit anti-asialo GM1 treatment in vivo or with anti-asialo GM1 plus complement in vitro on cytotoxic T cell activities. J. Immunol. 136:4674-4680. [PubMed] [Google Scholar]
- 50.Sutter, G., and C. Staib. 2003. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr. Drug Targets Infect. Disord. 3:263-271. [DOI] [PubMed] [Google Scholar]
- 51.Symons, J. A., A. Alcami, and G. L. Smith. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551-560. [DOI] [PubMed] [Google Scholar]
- 52.Tieng, V., C. Le Bouguenec, L. du Merle, P. Bertheau, P. Desreumaux, A. Janin, D. Charron, and A. Toubert. 2002. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc. Natl. Acad. Sci. USA 99:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomasec, P., E. C. Wang, A. J. Davison, B. Vojtesek, M. Armstrong, C. Griffin, B. P. McSharry, R. J. Morris, S. Llewellyn-Lacey, C. Rickards, A. Nomoto, C. Sinzger, and G. W. Wilkinson. 2005. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat. Immunol. 6:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsung, K., J. H. Yim, W. Marti, R. M. Buller, and J. A. Norton. 1996. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J. Virol. 70:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vales-Gomez, M., H. Browne, and H. T. Reyburn. 2003. Expression of the UL16 glycoprotein of human cytomegalovirus protects the virus-infected cell from attack by natural killer cells. BMC Immunol. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieillard, V., J. L. Strominger, and P. Debre. 2005. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc. Natl. Acad. Sci. USA 102:10981-10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vivier, E., and C. A. Biron. 2002. Immunology. A pathogen receptor on natural killer cells. Science 296:1248-1249. [DOI] [PubMed] [Google Scholar]
- 58.Vivier, E., E. Tomasello, and P. Paul. 2002. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr. Opin. Immunol. 14:306-311. [DOI] [PubMed] [Google Scholar]
- 59.Warren, H. S., A. L. Jones, C. Freeman, J. Bettadapura, and C. R. Parish. 2005. Evidence that the cellular ligand for the human NK cell activation receptor NKp30 is not a heparan sulfate glycosaminoglycan. J. Immunol. 175:207-212. [DOI] [PubMed] [Google Scholar]
- 60.Yuen, L., and B. Moss. 1987. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc. Natl. Acad. Sci. USA 84:6417-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zijlstra, M., H. Auchincloss, Jr., J. M. Loring, C. M. Chase, P. S. Russell, and R. Jaenisch. 1992. Skin graft rejection by beta 2-microglobulin-deficient mice. J. Exp. Med. 175:885-893. [DOI] [PMC free article] [PubMed] [Google Scholar]