Abstract
The attenuated Oka vaccine (V-Oka) strain of varicella-zoster virus (VZV) effectively reduces disease produced by primary infection and virus reactivation. V-Oka was developed by propagation of the Oka parental (P-Oka) strain of VZV in guinea pig and human embryo fibroblasts. Complete DNA sequencing of both viruses has revealed 63 sites that differ between P-Oka and V-Oka, 37 of which are located within 21 unique open reading frames (ORFs). Of the ORFs that differ, ORF 62 contains the greatest number (10) of mutated sites. ORF 62 encodes IE 62, the major immediate-early transactivator of virus genes, and is essential for lytic virus replication. To determine whether a disproportionate number of mutations in ORF 62 might account for virus attenuation, we compared the global pattern of V-Oka gene expression to that of P-Oka. Transcription of ORFs 62, 65, 66, and 67 was suppressed, whereas ORF 41 was elevated in V-Oka-infected cells compared to P-Oka-infected cells (P < 0.01; z test). Suppression of ORF 62, 65, and 66 transcription was confirmed by quantitative dot blot and Western blot analyses. Transient-transfection assays to determine whether mutations within V-Oka-derived IE 62 affected its ability to transactivate VZV gene promoters revealed similar IE 62 transactivation of VZV gene 20, 21, 28, 29, 65, and 66 promoters in both P-Oka and V-Oka. Together, our results indicate that mutations in V-Oka IE 62 alone are unlikely to account for vaccine virus attenuation.
Varicella-zoster virus (VZV) is a ubiquitous human pathogen. Primary infection causes varicella (chicken pox), after which virus becomes latent in cranial nerve, dorsal root, and autonomic ganglia. Virus reactivation, primarily in elderly individuals whose cell-mediated immune response to VZV is reduced, produces zoster (shingles). The Oka vaccine strain of VZV was developed by limited propagation in human (11 passages) and guinea pig (6 passages) embryo fibroblasts (32). Vaccination with the Oka vaccine strain of VZV effectively reduces disease produced by primary infection by approximately 85% (31). In the decade before vaccine licensure, an annual average of 94 deaths associated with primary varicella was reported in the United States (23). After vaccination was approved, there were three varicella-related deaths in 1997 and nine in 2002. Recently, a multicenter, randomized, double-blind, placebo-controlled trial showed that both the incidence of zoster and the burden of illness due to virus reactivation were reduced >50% by vaccination of adults over age 60 years (mean age, 69 years) (27). In light of the vaccine success, investigators have begun to determine the molecular basis of virus attenuation. Comparison of the Oka parental (P-Oka) and vaccine (V-Oka) DNA sequences has revealed 63 sites that differ between P-Oka and V-Oka, including 27 sites where the nucleotide sequence could not be unambiguously determined. Also, sequence polymorphism is present in different vaccine preparations (29). Thus, V-Oka is not a pure virus strain but consists of a mixture of closely related viruses.
The VZV genome contains regions of inverted repeated DNA sequences; consequently, 3 open reading frame (ORF) pairs (ORF 62 and ORF 71, ORF 63 and ORF 70, and ORF 64 and ORF 69) are diploid. Thus, 37 of the 63 mutated sites between P-Oka and V-Oka genomic DNA are unique mutations located within 21 ORFs. Ten mutations are found within ORF 62, which encodes IE 62, the major immediate-early transactivator of VZV gene transcription. Previous transient-transfection-based studies suggested that IE 62 derived from V-Oka is a less potent transactivator of VZV genes than the P-Oka-derived IE 62 (7-9). Here, we used well-characterized PCR-based macroarrays (5) to compare the global virus gene transcriptional activity of both the parental and vaccine strains of VZV during lytic infection. Array data were confirmed at the RNA level by quantitative cDNA dot blot analysis and at the protein level by Western blot analysis. The ability of IE 62 derived from P-Oka and V-Oka to transactivate selected VZV gene promoters was also determined.
MATERIALS AND METHODS
Virus and cells.
P-Oka and V-Oka were kind gifts from M. Takahashi. V-Oka was from a Japanese vaccine lot and was subsequently passaged 9 times in MRC-5 cells, and P-Oka was passaged 14 times in MRC-5 cells prior to use in this study. The array of mutations in V-Oka, which demonstrates 27 sites of sequence polymorphism (9), indicates that the virus preparation is not a pure virus strain but consists of a mixture of closely related viruses. Equal titers of P-Oka or V-Oka were used to infect T75 flasks of MRC-5 cells (human diploid fibroblasts). Infected cells from each T75 flask were passaged onto three T175 flasks of MRC-5 cells and harvested 3 days later. Cells were washed twice with phosphate-buffered saline, scraped from tissue culture flasks, collected by low-speed centrifugation, and frozen at −80°C.
RNA extraction.
Cell pellets were thawed in Tri-Reagent (Molecular Research Center, Cincinnati, OH) and sonicated on ice three times for 10 s each, and 1-bromo,3-chloropropane was added to initiate phase separation. RNA was precipitated with a 0.5 volume of isopropanol for 20 min at room temperature, collected by centrifugation at 12,500 × g for 30 min at 4°C, washed in 70% ethanol, and dissolved in water. Residual DNA was digested with RNase-free DNase (DNA-free; Ambion, Austin, TX) at 37°C for 20 min. poly(A)+ RNA was extracted by oligo(dT) cellulose chromatography (NucleoTrap; Clontech, Palo Alto, Calif.). Lack of PCR amplification using VZV DNA-specific primers (5) confirmed the absence of DNA from the poly(A)+ RNA. poly(A)+ RNA (1 μg) was primed with oligo(dT) for cDNA synthesis (SuperScript II; Invitrogen, Carlsbad, Calif.). After incubation at 37°C for 90 min, enzyme was inactivated at 65°C for 5 min and cDNA was cleaned by microfiltration (Microcon-PCR; Millipore, Bedford, Mass.).
VZV macroarrays.
Construction and validation of PCR-based VZV macroarrays has been described previously (5). Briefly, 300- to 600-bp segments of the VZV genome mapping to the 5′ and 3′ ends of all predicted VZV ORFs were PCR amplified, inserted into pGEM3zf −, and used to generate (by PCR amplification with universal vector-specific primers) target DNA. Target DNA was manually spotted on nylon-based membranes, alkaline denatured, UV cross-linked, and stored under a vacuum until use.
cDNA probe synthesis and hybridization.
For each sample, 100 ng cDNA was labeled with [32P]dCTP by nick translation (Rediprime II; Amersham Pharmacies Biotech, Piscataway, N.J.). Individual labeling reaction mixtures containing 25 ng cDNA and 10 μCi [32P]dCTP were incubated at 37°C for 60 min. Unincorporated isotope was removed by gel filtration (Micro Bio-Spin P30; Bio-Rad, Hercules, Calif.). Probes were heat denatured, quenched on ice, and added to prehybridized arrays in 20 ml Perfecthyb (Sigma-Aldrich, St. Louis, Mo.). Hybridization was for 48 h at 62°C, after which the arrays were washed at 62°C three times at low stringency (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% sodium dodecyl sulfate [SDS]), twice at high stringency (0.5× SSC, 0.1% SDS), and once at ultra-high stringency (0.1× SSC, 0.1% SDS). Arrays were air dried and exposed for uniform times to phosphorimager screens (Molecular Dynamics, Piscataway, N.J.).
Array data analysis.
The signal intensity, reflecting the amount of 32P-labeled cDNA that bound to each array target, was quantitated by densitometric scanning (ImageQuant; Molecular Dynamics). The formula used to determine the transcriptional activity for each ORF (5) was modified as follows to reflect the expression level for each P-Oka ORF relative to the same V-Oka ORF: RE = (P̄i/P̄c)/(V̄i/V̄c) where RE is the relative expression, P̄i is the average intensity of VZV ORF target (i) probed with 32P-labeled P-Oka cDNA, P̄c is the average intensity of control targets (actin, glyceraldehyde 3-phosphate dehydrogenase [GAPdH], no DNA, and plasmid DNA) probed with 32P-labeled P-Oka cDNA, V̄i is the average intensity of VZV target (i) probed with the 32P-labeled V-Oka cDNA, and V̄c is the average intensity of control targets (actin, GAPDH, no DNA, and plasmid DNA) probed with the 32P-labeled V-Oka cDNA.
Dot blot analysis.
First-strand cDNA was synthesized from mRNA extracted from either P-Oka- or V-Oka-infected MRC-5 cells, spotted onto nylon-based charged membranes (Zeta probe; Bio-Rad), air dried, and UV cross-linked. Recombinant plasmids containing cloned inserts specific to cellular beta-actin and VZV ORFs 41, 62, and 66 were propagated, and the inserts were excised, gel purified, and radiolabeled with 32P by nick translation. Quadruplicate cDNA spots containing cDNA from each sample were probed with the respective plasmid-derived insert, washed, and exposed to phosphorimager screens, and the amount of 32P-labeled probe hybridizing to each individual target was determined by densitometric scanning (ImageQuant; Molecular Dynamics).
Western blots.
MRC-5 cells in T75 flasks were infected with equal titers of P-Oka or V-Oka, passaged onto uninfected MRC-5 cells in T175 flasks (1 infected cell: 6 uninfected cells), and harvested 3 days later. After treatment with trypsin and washing with complete medium followed by a phosphate-buffered saline wash, cells were lysed in RIPA buffer (10 mM Tris-Cl, pH 8, 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 0.5% SDS) with TLCK [l-1-chloro-3-(4-tosylamido)-7-amino-2-heptanone hydrochloride, N-α-tosyl-l-lysine chloromethyl ketone], TPCK [l-1-chloro-3-(4-tosylamido)-4-phenyl-2-butanone tosyl-l-phenylalanine chloromethyl ketone], and Roche Complete protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, Ind.) and boiled in SDS loading buffer. Proteins were resolved by electrophoresis on SDS-polyacrylamide gels and transferred to Nytran membranes (Schleicher & Schuell Biotechnology, Keene, N.H.), and the blots were probed with rabbit antiserum directed against VZV ORF 65 (4) or ORF 66 (11) proteins, IE 62 (16) or gE (Chemicon International, Temecula, Calif.). Blots were developed using enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford, Ill.), and bands corresponding to VZV proteins were quantified using a phosphorimager (Molecular Dynamics). Multiple levels of standardization were used in the Western blot analysis. First, equal numbers of cells were analyzed. Second, to take into account any slight differences in virus spread in culture, the amount of gE present in the protein samples was determined. Controlling for the level of infection is more important than controlling for the amount of protein per se, since not all cells in the culture are infected. Finally, all Western blot analyses were done in replicate, and the results are presented as average (± standard deviation) relative protein expressions.
Promoter activity assay.
The transactivational activity of P-Oka- and V-Oka-derived IE 62 on six VZV gene promoters was determined in transient-transfection assays (7, 8). VZV gene promoters were directionally inserted into the luciferase reporter plasmid, pGL3basic (Promega). All promoter regions reflect the intergenic segment separating the opposing ORFs. Thus, the promoters for ORFs 20 and 21 are contained within the same 284-bp DNA segment mapping from 30,476 to 30,758 bp on the VZV genome (Dumas strain, accession number NC_001348), ORF 28 and 29 promoters map to the 221 bp between 50,637 and 50,856, and ORF 65 and 66 promoters are contained within 397 bp between 112,641 and 113,036. DNA sequence analysis of P-Oka (accession number AB097932), V-Oka (accession number AB097933), and the Dumas strain of VZV indicates complete sequence homology between these three genomes in the promoter regions for VZV genes 20, 21, 28, 29, 65, and 66. The effector plasmids consisted of ORF 62 with cognate promoter from either P-Oka or V-Oka inserted into pUC-19. Thus, the effector plasmids expressed IE 62 derived from P-Oka (P-IE 62) or from V-Oka (V-IE 62). The 10 mutations found in V-Oka ORF 62 are also present in V-Oka ORF 71 (9), and three of the 10 V-Oka ORF 62/71 mutations display sequence polymorphism, emphasizing the polyclonal nature of V-Oka. The V-Oka ORF62 expression plasmid used in transient-transfection assays was selected to contain all 10 nucleotide and 8 amino acid changes within the IE 62 protein coding segment (7). For transient transfections, various amounts (0 to 2 μg) of effector plasmid were transfected into CV1 cells together with one of the reporter plasmids. The transfection efficiency was normalized to total protein concentration of each cell lysate. The relative induction for each VZV promoter construct was calculated compared to controls consisting of reporter plasmid alone. All promoter assays were done in duplicate on independent samples with similar results. P-Oka- or V-Oka-derived IE 62 differentially activates transcription (see Fig. 2 below), resulting in different levels of IE 62 protein (see Fig. 3 below), which could result in autoactivation of IE 62 during transfection. However, to be consistent with previously published results concerning P-Oka- and V-Oka-derived IE 62 activation of gene transcription (7, 8), transfection experiments were performed with increasing amounts of effector plasmid showing a dose response activation of all VZV gene promoters. For transient transfections, various amounts (0 to 2 μg) of effector plasmid were transfected into CV1 cells together with one of the reporter plasmids. In initial experiments, MRC-5 cells were used as targets for transfections; however, the efficiency of DNA uptake in these cells was very low. Thus, to be consistent with previous data (7), CV1 cells were used for transfections. In addition, 293 cells were used previously for transient transfection and VZV gene promoter induction with P-Oka- and V-Oka-derived IE 62 with similar results (9).
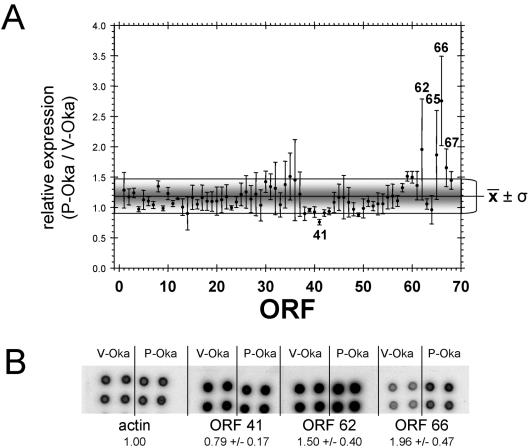
FIG. 2.
Relative expression of each predicted VZV ORF. Panel A shows the steady-state level of each VZV ORF determined by densitometric scanning of the transcriptional macroarray. The ratio (relative expression) of P-Oka ORF expression compared to the corresponding V-Oka ORF expression level indicates that the steady-state levels of VZV ORFs 62, 65, 66, and 67 are significantly higher and that of ORF 41 is significantly lower (P < 0.01) than the overall average relative expression of all VZV ORFs. Panel B shows the dot blot analysis of VZV ORFs 41, 62, and 66. The extent of hybridization was determined and normalized to cellular actin. The relative expression for each ORF in P-Oka- or V-Oka-infected cells was compared. Consistent with the array analysis, ORF 41 was underexpressed and ORFs 62 and 66 were overexpressed in V-Oka-infected MRC-5 cells.
FIG. 3.
Western blot analysis of P-Oka- and V-Oka-infected MRC-5 cells. Protein lysates prepared from P-Oka- and V-Oka-infected MRC-5 cells were analyzed by Western blotting for expression of VZV ORF 62 (62P), 65 (65P), and 66 (66P) protein. Compared with expression of VZV ORF 68 (gE), levels of 62P (1.96), 65P (1.65), and 66P (1.23) were greater in P-Oka-infected cells than in V-Oka-infected cells.
RESULTS
Steady-state levels of VZV gene transcription in P-Oka- and V-Oka-infected cells.
DNA sequence analysis has revealed a disproportionate number of sequence mutations within V-Oka ORF 62/71 (9, 29). Figure 1 shows results of transcriptional array analysis conducted on two sets of independently prepared mRNA from MRC-5 cells infected with either P-Oka or V-Oka; target spot locations for ORFs that were suppressed in V-Oka-infected cells are identified. Table 1 lists the ratio of relative expression for each VZV ORF. Relative expression ratios of >1 indicate that the steady-state amount of the corresponding ORF was greater in P-Oka-infected cells, whereas ratios of <1 indicate greater steady-state levels of the ORF in V-Oka-infected cells. The highest ratio was obtained for VZV ORF 66, with steady-state levels of ORF 66 transcripts 2.75 times higher in P-Oka-infected cells than in V-Oka-infected cells. The lowest ratio was obtained for VZV ORF 41, with steady-state levels in P-Oka-infected cells reduced by a factor of 0.76 compared to V-Oka-infected cells. The average ratio was 1.19 (average standard deviation = 0.29), indicating an overall slightly higher level of virus transcription in P-Oka-infected cells than in V-Oka-infected cells (Table 1), whereas steady-state levels of ORFs 62, 65, 66, and 67 were significantly (P < 0.01; z-test) increased in P-Oka-infected cells (Fig. 2A). Consistent with array analysis, dot blot hybridization (Fig. 2B) revealed enhanced transcription of ORF 41 and diminished transcription of ORFs 62 and 66 in V-Oka-infected MRC-5 cells compared with P-Oka-infected MRC-5 cells.
FIG. 1.
Transcriptional array analysis of P- and V-Oka VZV-infected MRC-5 cells. Two independent sets of mRNA were extracted from P-Oka and V-Oka VZV-infected MRC-5 cells and processed for analysis on PCR-based macroarrays as described in Materials and Methods. Results for 16 arrays (8 for each virus) are shown. Densitometric scanning revealed a significant difference in the steady-state level of ORF 62, 65, 66, and 67 transcription compared to cellular controls. The location of VZV ORF 62, 65, 66, and 67 target spots and cellular actin and GAPdH target spots is shown on representative arrays. Boxed ORF spots indicate transcripts suppressed in V-Oka-infected cells.
TABLE 1.
Relative expression of VZV ORFs
| ORF | Relative expression (P/V ratio ± SD)a | nb |
|---|---|---|
| 1 | 1.28 ± 0.29 | 16 |
| 2 | 1.17 ± 0.13 | 32 |
| 3 | 1.24 ± 0.08 | 32 |
| 4 | 0.97 ± 0.04 | 32 |
| 5 | 1.12 ± 0.16 | 32 |
| 6 | 1.10 ± 0.07 | 32 |
| 7 | 1.04 ± 0.06 | 32 |
| 8 | 1.35 ± 0.09 | 32 |
| 9 | 0.98 ± 0.04 | 32 |
| 10 | 1.23 ± 0.10 | 32 |
| 11 | 1.06 ± 0.05 | 32 |
| 12 | 1.14 ± 0.00 | 32 |
| 13 | 1.01 ± 0.14 | 32 |
| 14 | 0.90 ± 0.27 | 32 |
| 15 | 1.17 ± 0.20 | 32 |
| 16 | 1.05 ± 0.08 | 32 |
| 17 | 1.16 ± 0.21 | 32 |
| 18 | 1.10 ± 0.15 | 32 |
| 19 | 1.10 ± 0.16 | 32 |
| 20 | 1.10 ± 0.23 | 32 |
| 21 | 1.12 ± 0.21 | 32 |
| 22 | 1.18 ± 0.20 | 32 |
| 23 | 0.99 ± 0.04 | 32 |
| 24 | 1.09 ± 0.09 | 32 |
| 25 | 1.21 ± 0.19 | 32 |
| 26 | 1.25 ± 0.27 | 32 |
| 27 | 1.14 ± 0.22 | 32 |
| 28 | 1.22 ± 0.36 | 32 |
| 29 | 1.03 ± 0.26 | 32 |
| 30 | 1.42 ± 0.18 | 32 |
| 31 | 1.34 ± 0.21 | 32 |
| 32 | 1.31 ± 0.43 | 32 |
| 33 | 1.04 ± 0.20 | 32 |
| 34 | 1.38 ± 0.39 | 32 |
| 35 | 1.51 ± 0.39 | 32 |
| 36 | 1.45 ± 0.67 | 32 |
| 37 | 1.22 ± 0.37 | 32 |
| 38 | 0.90 ± 0.11 | 32 |
| 39 | 0.96 ± 0.03 | 32 |
| 40 | 0.92 ± 0.09 | 32 |
| 41 | 0.76 ± 0.05 | 32 |
| 42 | 0.91 ± 0.05 | 32 |
| 45 | 1.17 ± 0.22 | 32 |
| 43 | 0.94 ± 0.06 | 32 |
| 44 | 1.08 ± 0.17 | 32 |
| 46 | 1.17 ± 0.36 | 32 |
| 47 | 1.08 ± 0.24 | 32 |
| 48 | 0.97 ± 0.11 | 32 |
| 49 | 0.88 ± 0.03 | 16 |
| 50 | 0.98 ± 0.16 | 32 |
| 51 | 1.10 ± 0.09 | 32 |
| 52 | 1.02 ± 0.08 | 32 |
| 53 | 1.06 ± 0.18 | 32 |
| 54 | 1.06 ± 0.16 | 32 |
| 55 | 1.17 ± 0.19 | 32 |
| 56 | 1.19 ± 0.20 | 32 |
| 57 | 1.11 ± 0.09 | 16 |
| 58 | 1.32 ± 0.07 | 32 |
| 59 | 1.51 ± 0.08 | 32 |
| 60 | 1.50 ± 0.10 | 32 |
| 61 | 1.36 ± 0.23 | 32 |
| 62/71 | 1.96 ± 0.83 | 160 |
| 63/70 | 1.06 ± 0.09 | 32 |
| 64/69 | 0.96 ± 0.23 | 32 |
| 65 | 1.87 ± 0.73 | 16 |
| 66 | 2.75 ± 0.74 | 32 |
| 67 | 1.65 ± 0.31 | 32 |
| 68 | 1.44 ± 0.15 | 32 |
| Totalc | 1.19 ± 0.29 |
Ratio of relative expression of each P-OKA ORF to V-OKA ORF ± standard deviation.
Number of individual data points used to determine relative expression ratio.
Average and standard deviation for all ORFs.
ORF protein expression in P-Oka- and V-Oka-infected cells.
Western blot analysis showed that proteins encoded by ORFs 62, 65, and 66 were more abundant in P-Oka-infected cells than in V-Oka-infected cells (Fig. 3). The amount of specific VZV protein synthesized was determined by densitometry and normalized to the amount of glycoprotein E (gE). The ratio of protein synthesized in P-Oka-infected cells compared to V-Oka-infected cells was 1.96 for ORF 62 protein, 1.65 for ORF 65 protein, and 1.23 for ORF 66 protein, indicating higher steady-state amounts of these proteins in P-Oka-infected cells than in V-Oka-infected cells.
Transactivation of specific VZV promoters by P- and V-Oka-derived IE 62.
Transient-transfection assays were performed to compare the response of the VZV ORF 65 and 66 promoters to P-IE 62 and V-IE 62. Reporter plasmids were constructed to place luciferase synthesis under the control of VZV ORF 20, 21, 28, 29, 65, and 66 promoters. ORF 20, 21, 28, and 29 promoters were used as controls, since they are of lengths similar to those of the ORF 65 and ORF 66 promoters and because the steady-state levels of ORF 20, 21, 28, and 29 transcripts in both P-Oka- and V-Oka-infected cells are similar (Table 1). All promoters showed an increase in activity with increasing amounts of IE 62 and a slight, but not statistically significant, increase in activity when transactivated by P-IE 62 compared to V-IE 62 (Fig. 4).
FIG. 4.
Transactivation of VZV promoters by IE 62 derived from P-Oka or V-Oka VZV. The promoter regions for VZV ORFs 20, 21, 28, 29, 65, and 66 were inserted into the luciferase reporter plasmid, pGL3Basic. Since the promoter regions are contained within intergenic DNA segments separating the opposing ORFs, the promoters for ORFs 20 (pro20) and 21 (pro21) are contained within the same 284-bp DNA segment, the promoters for ORFs 28 (pro28) and 29 (pro29) are both contained within the same 221-bp segment, and the promoters for ORFs 65 (pro65) and 66 (pro66) are within the same 397-bp segment. Transcription originating from all promoter regions was transactivated with increasing amounts (from 0 to 2 μg) of plasmid expressing IE 62 derived from P-Oka (pIE 62) or V-Oka (vIE 62) VZV. The transactivating ability of pIE 62 (solid lines) was slightly greater than that of vIE 62 (dotted lines) for the same promoter but was not statistically significant.
DISCUSSION
Comparison of the steady-state levels of VZV gene transcripts in P-Oka and V-Oka has revealed reduced transcription of VZV ORFs 62, 65, 66, and 67 in V-Oka-infected cells. VZV ORF 62 encodes IE 62, the major immediate-early transactivator of VZV genes, and is able to stimulate transcription from all VZV promoters tested (3, 17). In transfection assays, IE 62 increases infectivity of purified VZV DNA (24). Mutational analysis has shown that IE 62 functional activity is required for efficient virus spread (30). Early in VZV infection, IE 62 localizes to the nucleus; however, at later times, IE 62 is phosphorylated by VZV ORF 66 protein kinase, resulting in cytoplasmic localization and incorporation of IE 62 into progeny virions (6, 15, 16, 18). Because IE 62 is critical during lytic infection, mutations that dampen its function or alter its cellular location might be expected to attenuate virus.
Transient-transfection assays have shown that wild-type P-IE 62 is a better transactivator of selected VZV genes than V-IE 62 (7, 8). We have extended these findings to show that the steady-state level of all VZV genes is increased on average 1.19-fold in P-Oka-infected cells compared to V-Oka-infected cells in tissue culture. Further, the steady-state level of VZV ORF 62 transcription is significantly decreased in V-Oka-infected cells, along with a decrease in IE 62 protein. Thus, with respect to IE 62 transactivation of virus genes, mutations within V-Oka IE 62 result in a slight overall decrease in transcription of all VZV genes, a significant decrease in V-Oka ORF 62 transcription, and a concomitant reduction in IE 62 protein. ORF 66 transcripts and ORF 66 protein levels were also decreased in V-Oka-infected cells, making it likely that less IE 62 is incorporated into infectious V-Oka virions. Together, the reduced amount of IE 62 and ORF 66 protein might contribute to V-Oka attenuation.
Transient-transfection assays were performed to determine whether the reduced amounts of ORF 66 transcripts in V-Oka-infected cells compared to P-Oka-infected cells might reflect a reduced ability of V-IE 62 to activate the ORF 66 promoter. Control reporter constructs included the promoters for ORFs 20, 21, 28, 29, and 65. All promoter regions consisted of the intergenic segment separating the ORF pairs (ORFs 20/21, ORFs 28/29, and ORF 65/66), and DNA sequences in the P-Oka and V-Oka genomes do not differ within these promoter regions. In contrast with a previous report of the reduced ability of V-IE 62 to activate transcription from VZV ORF 28 and 29 promoters (7), we found no statistically significant difference in the ability of P-IE 62 or V-IE 62 to activate transcription from any of the promoter regions tested. In that study, CV-1 cells were transfected with promoter constructs consisting of approximately 750 bp of upstream VZV DNA sequences, and promoter activity was monitored based on chloramphenicol acetyltransferase activity. Since the same effector plasmids (P-IE 62 and V-IE 62) were used in both studies and all assays were performed in the same respective laboratory, the apparent discrepancy in activation of ORF 28 and 29 by V-IE 62 most likely reflects the presence of upstream regulatory sites in the DNA sequences external to the intergenic region.
Transcriptional array and Western blot analyses also revealed reduced expression of VZV ORF 65 in V-Oka-infected cells compared to P-Oka-infected cells. VZV ORF 65 encodes a nonessential virion protein that localizes to the Golgi apparatus (4, 25).
The steady-state level of VZV ORF 67 transcripts was significantly reduced in V-Oka-infected cells compared to P-Oka-infected cells. VZV ORF 67 encodes glycoprotein I (gI), one of seven glycoproteins present in VZV-infected cells. VZV gI is homologous to herpes simplex virus type 1 gI (10). In both viruses, this small (∼40 kDa) protein forms a complex with the neighboring gene product (gE for VZV and gE for herpes simplex virus type 1). This complex binds the Fc fragment of immunoglobulin and is involved in correct sorting of the virus to tight junctions for cell-to-cell spread (12, 13, 28). Reduced VZV gI reduces the spread of V-Oka in tissue culture cells (2, 20). The external domains of gI and gE associate, resulting in heterodimer formation (14). The gI:gE complex present in the trans-Golgi network (TGN) cycles to the cell membrane and returns to the TGN via endocytosis (1, 26). VZV gE is the most abundant VZV glycoprotein and is essential for virus propagation (10, 21). In the absence of gI, normal gE trafficking is disrupted, resulting in the formation of abnormal polykaryocytes with adherent TGN and reduced syncytium formation, significantly reducing yields of infectious virus (19, 26). While gI null VZV can grow in melanoma and human embryonic lung fibroblast cells, albeit with reduced yields of infectious virus (2, 19, 21, 33), gI is required for virus growth in human skin and thymus/liver (T-cell) implants (22). Thus, down-regulation of gI in V-Oka-infected cells may limit gI:gE complex formation and result in reduced delivery of gE to the membrane, which could be especially important as a mechanism by which V-Oka is attenuated
The common function of the VZV genes whose expression is diminished in V-Oka-infected cells (ORFs 62, 65, 66, and 67) is related to assembly and spread of infectious virus. Thus, attenuation of V-Oka is not solely the result of mutational events clustered within IE 62 but may include reduced expression in VZV ORFs 65, 66, and 67 that together reduce assembly and spread of virus. Such a notion is supported by recent studies in which overlapping cosmid sets were used to generate recombinant VZV; chimeric VZV containing P-Oka-derived sequences covering ORFs 30 to 55 on a V-Oka background displayed wild-type infectivity in skin xenografts (34). Together, the data suggest that diminished vaccine virus virulence reflects a combination of mutations acting to reduce V-Oka replication.
Acknowledgments
This work was supported in part by Public Health Service grants AG 06127 and NS 32623 from the National Institutes of Health and was supported by the intramural program of the National Institute of Allergy and Infectious Diseases (J.I.C.).
We thank Paul Kinchington for IE62 antibody, Marina Hoffman for editorial assistance, and Cathy Allen for manuscript preparation.
REFERENCES
- 1.Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, J. I., and H. Nguyen. 1997. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 71:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lipincott-Williams & Wilkins, Philadelphia, Pa.
- 4.Cohen, J. I., H. Sato, S. Srinivas, and K. Lekstrom. 2001. Varicella-zoster virus (VZV) ORF65 virion protein is dispensable for replication in cell culture and is phosphorylated by casein kinase II, but not by the VZV protein kinases. Virology 280:62-71. [DOI] [PubMed] [Google Scholar]
- 5.Cohrs, R. J., M. P. Hurley, and D. H. Gilden. 2003. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella-zoster virus. J. Virol. 77:11718-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forghani, B., R. Mahalingam, A. Vafai, J. W. Hurst, and K. W. Dupuis. 1990. Monoclonal antibody to immediate early protein encoded by varicella-zoster virus gene 62. Virus Res. 16:195-210. [DOI] [PubMed] [Google Scholar]
- 7.Gomi, Y., T. Imagawa, M. Takahashi, and K. Yamanishi. 2000. Oka varicella vaccine is distinguishable from its parental virus in DNA sequence of open reading frame 62 and its transactivation activity. J. Med. Virol. 61:497-503. [DOI] [PubMed] [Google Scholar]
- 8.Gomi, Y., T. Imagawa, M. Takahashi, and K. Yamanishi. 2001. Comparison of DNA sequence and transactivation activity of open reading frame 62 of Oka varicella vaccine and its parental viruses. Arch. Virol. Suppl. 2001(17):49-56. [DOI] [PubMed] [Google Scholar]
- 9.Gomi, Y., H. Sunamachi, Y. Mori, K. Nagaike, M. Takahashi, and K. Yamanishi. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 76:11447-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grose, C. 1991. Glycoproteins of varicella-zoster virus and their herpes simplex virus homologs. Rev. Infect. Dis. 13(Suppl. 11):S960-S963. [DOI] [PubMed] [Google Scholar]
- 11.Heineman, T. C., K. Seidel, and J. I. Cohen. 1996. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J. Virol. 70:7312-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura, H., S. E. Straus, and R. K. Williams. 1997. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology 233:382-391. [DOI] [PubMed] [Google Scholar]
- 15.Kinchington, P. R., and S. E. Turse. 1998. Regulated nuclear localization of the varicella-zoster virus major regulatory protein, IE62. J. Infect. Dis. 178(Suppl. 1):S16-S21. [DOI] [PubMed] [Google Scholar]
- 16.Kinchington, P. R., K. Fite, and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinchington, P. R., and J. I. Cohen. 2000. Viral proteins, p. 74-104. In A. M. Arvin and A. A. Gershon (ed.), Varicella-zoster virus. Cambridge University Press, New York, N.Y.
- 18.Kinchington, P. R., K. Fite, A. Seman, and S. E. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 75:9106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallory, S., M. Sommer, and A. M. Arvin. 1998. Analysis of the glycoproteins I and E of varicella-zoster virus (VZV) using deletional mutations of VZV cosmids. J. Infect. Dis. 178(Suppl. 1):S22-S26. [DOI] [PubMed] [Google Scholar]
- 21.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 22.Moffat, J., H. Ito, M. Sommer, S. Taylor, and A. M. Arvin. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montalbano, M. A., C. M. Knowles, D. A. Adams, P. A. Hall, R. F. Fagan, K. A. Brendel, H. R. Holden, G. F. Jones, W. J. Anderson, A. Trosclair, S. M. Gilchrist, and F. J. Perry. 1997. Summary of notifiable diseases, United States 1996. Morb. Mortal. Wkly. Rep. 45:1-87. [Google Scholar]
- 24.Moriuchi, M., H. Moriuchi, S. E. Straus, and J. I. Cohen. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297-300. [DOI] [PubMed] [Google Scholar]
- 25.Niizuma, T., L. Zerboni, M. H. Sommer, H. Ito, S. Hinchliffe, and A. M. Arvin. 2003. Construction of varicella-zoster virus recombinants from parent Oka cosmids and demonstration that ORF65 protein is dispensable for infection of human skin and T cells in the SCID-hu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson, J. K., and C. Grose. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 72:1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxman, M. N., M. J. Levin, G. R. Johnson, K. E. Schmader, S. E. Straus, L. D. Gelb, R. D. Arbeit, M. S. Simberkoff, A. A. Gershon, L. E. Davis, A. Weinberg, K. D. Boardman, H. M. Williams, J. H. Zhang, P. N. Peduzzi, C. E. Beisel, V. A. Morrison, J. C. Guatelli, P. A. Brooks, C. A. Kauffman, C. T. Pachucki, K. M. Neuzil, R. F. Betts, P. F. Wright, M. R. Griffin, P. Brunell, N. E. Soto, A. R. Marques, S. K. Keay, R. P. Goodman, D. J. Cotton, J. W. Gnann, Jr., J. Loutit, M. Holodniy, W. A. Keitel, G. E. Crawford, S. S. Yeh, Z. Lobo, J. F. Toney, R. N. Greenberg, P. M. Keller, R. Harbecke, A. R. Hayward, M. R. Irwin, T. C. Kyriakides, C. Y. Chan, I. S. Chan, W. W. Wang, P. W. Annunziato, and J. L. Silber. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352:2271-2284. [DOI] [PubMed] [Google Scholar]
- 28.Polcicova, K., K. Goldsmith, B. L. Rainish, T. W. Wisner, and D. C. Johnson. 2005. The extracellular domain of herpes simplex virus gE is indispensable for efficient cell-to-cell spread: evidence for gE/gI receptors. J. Virol. 79:11990-12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinlivan, M., A. A. Gershon, S. P. Steinberg, and J. Breuer. 2005. An evaluation of single nucleotide polymorphisms used to differentiate vaccine and wild type strains of varicella-zoster virus. J. Med. Virol. 75:174-180. [DOI] [PubMed] [Google Scholar]
- 30.Sato, B., H. Ito, S. Hinchliffe, M. H. Sommer, L. Zerboni, and A. M. Arvin. 2003. Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse in vivo. J. Virol. 77:5607-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi, M. 2004. Effectiveness of live varicella vaccine. Expert. Opin. Biol. Ther. 4:199-216. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi, M., and S. A. Plotkin. 2000. Development of the Oka vaccine, p. 442-459. In A. M. Arvin and A. A. Gershon (ed.), Varicella-zoster virus. Cambridge University Press, New York, N.Y.
- 33.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerboni, L., S. Hinchliffe, M. H. Sommer, H. Ito, J. Besser, S. Stamatis, J. Cheng, D. Distefano, N. Kraiouchkine, A. Shaw, and A. M. Arvin. 2005. Analysis of varicella zoster virus attenuation by evaluation of chimeric parent Oka/vaccine Oka recombinant viruses in skin xenografts in the SCIDhu mouse model. Virology 332:337-346. [DOI] [PubMed] [Google Scholar]