Abstract
Initiation of reverse transcription and nucleocapsid assembly in hepatitis B virus (HBV) depends on the specific recognition of an RNA signal (the packaging signal, ɛ) on the pregenomic RNA by the viral reverse transcriptase (RT). Using an in vitro reconstitution system whereby the cellular heat shock protein 90 chaperone system activates recombinant HBV RT for specific ɛ binding, we have defined the protein and RNA sequences required for specific HBV RT-ɛ interaction in vitro. Our results indicated that approximately 150 amino acid residues from the terminal protein domain and 230 from the RT domain were necessary and sufficient for ɛ binding. With respect to the ɛ RNA sequence, its internal bulge and, in particular, the first nucleotide (C) of the bulge were specifically required for RT binding. Sequences from the upper portion of the lower stem and the lower portion of the upper stem also contributed to RT binding, as did the base pairing of the upper portion and the single unpaired U residue of the upper stem. Surprisingly, the apical loop of ɛ, known to be required for RNA packaging, was entirely dispensable for RT binding. A comparison of the requirements for in vitro RT-ɛ interaction with those for in vivo pregenomic RNA (pgRNA) packaging clearly indicated that RT-ɛ interaction was necessary but not sufficient for pgRNA packaging. In addition, our results suggest that recognition of some ɛ sequences by the RT may be required specifically for viral DNA synthesis.
Hepatitis B virus (HBV) infection is a major global public health problem, with over 300 million chronically infected patients worldwide (34). Chronic HBV infection carries a great risk of developing severe liver diseases, including cirrhosis and liver cancer, which result in a million deaths annually (4, 10). HBV is a member of the Hepadnaviridae family, a group of small hepatotropic DNA viruses that also includes the related animal viruses, such as the duck hepatitis B virus (DHBV) and the woodchuck hepatitis virus. All hepadnaviruses carry a small (ca. 3.2-kb), relaxed circular, partially double-stranded DNA genome and replicate this DNA genome through an RNA intermediate, the pregenomic RNA (pgRNA), by reverse transcription (45, 46).
Hepadnaviruses encode a novel, multifunctional reverse transcriptase (RT). Like its retroviral counterparts, the hepadnavirus RT catalyzes RNA- and DNA-dependent DNA polymerization and has an intrinsic RNase H activity (12, 40, 49). Thus, the central catalytic RT domain and carboxyl (C)-terminal RNase H domain of the hepadnavirus RT are homologous to the corresponding domains of retroviral RTs. However, all hepadnavirus RTs share an amino (N)-terminal domain called the terminal protein (TP) (2, 12, 40), which is absent from retroviral RTs and unrelated to any other known proteins. TP is separated from the RT domain by a nonessential and nonconserved spacer (tether) region. The unique TP domain is used as a protein primer to initiate reverse transcription catalyzed by the conserved RT domain (54, 59, 60). In addition to having this dual role as a primer and a polymerase in viral DNA synthesis, the RT is also essential for the packaging of the pgRNA into viral nucleocapsids (1, 12, 17), the locale of reverse transcription.
Critical to both protein-primed initiation of reverse transcription and assembly of replication-competent nucleocapsids is the ability of the RT to specifically recognize and form a ribonucleoprotein (RNP) complex with a short RNA signal located at the 5′ end of the pgRNA, called ɛ (21, 39, 55). Initially identified as the RNA packaging signal (28) that directs the specific encapsidation of the pgRNA into nucleocapsids, ɛ was subsequently found to also be the origin of reverse transcription (36, 48, 53). Binding of the RT to ɛ thus triggers two critical early steps in hepadnavirus replication, the initiation of reverse transcription, via protein priming (14, 31, 36, 42, 53, 55), and nucleocapsid assembly, leading to the selective incorporation of both the RT and pgRNA into the viral cores (1, 3, 39).
Detailed biochemical studies of RT-ɛ interaction, using the DHBV model system, have been made possible by the development of cell-free systems in recent years (19, 22). The first breakthrough came in 1992, when Wang et al. succeeded in expressing a DHBV RT active in ɛ binding and protein priming using a rabbit reticulocyte lysate in vitro translation system (54, 55). More recently, we have been able to obtain purified recombinant DHBV RT proteins using the bacterial expression system and truncated mini RT constructs that proved to be more readily expressed (20, 25, 56-58). Studies using these in vitro systems, together with genetic studies with cell cultures, have demonstrated that RT-ɛ interaction in DHBV requires sequences from both the TP and RT domains of the RT protein (39, 44, 55) and both sequence and structural determinants of the ɛ RNA (6, 9, 39, 55). Furthermore, these studies demonstrated that the DHBV RT protein requires the assistance of a cellular molecular chaperone complex consisting of heat shock protein 90 (Hsp90) and several cofactors in order to interact specifically with ɛ (7, 20, 23, 25, 26, 56).
The ɛ RNA sequences from all hepadnaviruses bear two inverted repeats and can form a conserved stem-loop structure. The predicted structure, which is partially supported by enzymatic and chemical mapping studies (5, 28, 29, 38), features a lower and an upper stem, an apical loop, and an internal bulge. In vitro RNA binding studies, again using the DHBV system, suggest that the RT may recognize mainly structural features of ɛ, with only limited direct recognition of specific nucleotide sequence (6, 9, 38, 39, 43; for a review, see reference 24).
In contrast to DHBV, in vitro assays to measure specific RT-ɛ interaction in HBV were not available until very recently; as a result, the sequence and structural determinants of the HBV RT and ɛ important for RNP formation have not been clearly defined. In vivo experiments in cell cultures have shown that an HBV ɛ stem-loop structure similar to that of the DHBV ɛ may also be important for its interaction with the HBV RT, as inferred from the requirements for pgRNA packaging and DNA synthesis in cells (3, 14, 28, 36). However, studies using DHBV have clearly shown that pgRNA packaging and DNA synthesis in vivo have overlapping but distinct requirements compared with those for RT-ɛ interaction in vitro (11, 13, 17, 18, 37).
In order to study the HBV RT-ɛ interaction directly, we have recently developed an in vitro reconstitution system for HBV RNP formation, using purified HBV RT proteins expressed in bacteria and components of the Hsp90 chaperone complex, similar to what we had done previously using the DHBV system. Using this reconstitution system, we reported recently that similar to DHBV, HBV RT requires both its TP and RT domains for ɛ binding (21), but the boundaries of these domains required for ɛ recognition were undefined. Similarly, we reported that deletion of the internal bulge, but not the apical loop, of the HBV ɛ RNA abolished RT binding, but the exact sequence and structural determinants of ɛ recognized by the RT remained to be elucidated. To better understand the molecular basis of specific HBV RT-ɛ interactions, we have now carried out a detailed mapping study of the protein and RNA sequence requirements for specific RT-ɛ interaction in HBV. A comparison of the requirements for RT-ɛ interaction in vitro with those required for pgRNA packaging and DNA synthesis in cells revealed that distinct RT-ɛ interactions may be required specifically for pgRNA packaging or viral DNA synthesis, and both RNA packaging and DNA synthesis entail additional requirements beyond RNP formation.
MATERIALS AND METHODS
Plasmids.
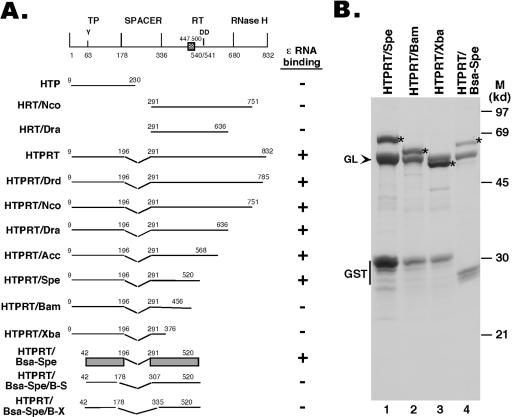
The HBV (strain ayw) RT coding sequences were derived from pCMVHBV (14), kindly provided by Christoph Seeger. The starting and ending amino acid positions of the different segments of the RT that were expressed are as indicated in Fig. 1. Deletions and truncations were generated using appropriate restriction enzyme digestions and are denoted by the appropriate restriction sites. DNA coding sequences for these fragments were subcloned into pGEX-KT (16) so that the glutathione S-transferase (GST) was fused in-frame to the N terminus of the RT sequences in the resulting fusion proteins, as described before (21). pCI-HE was constructed by subcloning the HBV sequence from nucleotides (nt) 1803 to 1988, encompassing direct repeat 1 (DR1) 5′ of ɛ, ɛ, and 80 nt 3′ of ɛ, into the vector pCI (Promega), downstream of the T7 promoter.
FIG. 1.
Expression of HBV RT truncation and deletion mutants. (A) Schematic diagram of the HBV RT proteins and domains expressed. Shown on the top is the domain structure of the HBV RT, with the primer tyrosine (Y63) and the double aspartate (D540/D541) in the RT active site denoted. The cross-hatched box denotes an insertion sequence (residues 447 to 500) in the HBV RT (and other mammalian RTs) relative to the DHBV RT, based on sequence alignment. The ends of the truncations and deletions are indicated. The ɛ binding activities of the RT mutants are summarized to the right. The shaded bars represented in the diagram for construct HTPRT/Bsa-Spe denote the boundaries of the TP and RT domains required for ɛ binding in vitro. (B) The truncated HBV RT proteins and domains were expressed as GST fusion proteins in bacteria, purified using glutathione resins, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected by Coomassie blue staining. The intact fusion proteins are indicated by asterisks. The bacterial chaperone protein GroEL (GL), copurifying with the RT proteins, is indicated by an arrowhead. The major degradation product, GST, is also indicated. M, molecular mass markers. Note that the first seven constructs in panel A have been reported recently (21).
Protein expression and purification.
GST-tagged HBV RT fusion proteins were expressed in Escherichia coli and purified by using the glutathione affinity resin as described before (20, 21). Chaperone proteins were purified as previously described (21).
RNA binding.
The HBV and DHBV ɛ RNAs were made from a synthetic DNA template by in vitro transcription using the MegaScript kit (Ambion) as described previously (20, 21). The HE RNA was made from the pCI-HE plasmid by linearizing it immediately after the HBV sequence, followed by in vitro transcription using the T7 promoter. To make labeled RNAs, [α-32P]UTP was included in the transcription reaction mixture. The wild-type, or “long,” HBV ɛ RNA corresponded to that described before (24, 28). The short (shorter lower stem), dB (bulge deletion), and dL (loop deletion) HBV ɛ RNAs have been described recently (21). Other deletion and substitution mutants of ɛ are as described in Fig. 7. Binding of the GST-HBV RT fusion proteins to the ɛ RNA was measured by a reconstituted in vitro RNA gel mobility shift assay, as described recently (21). Briefly, approximately 20 ng purified GST-RT fusion proteins were incubated in 10-μl reaction mixtures with approximately 20 ng of 32P-labeled RNA, together with Hsp90 (360 ng), Hsp70 (3 μg), Hdj1 (200 ng), Hop (370 ng), and p23 (100 ng). After incubation for 2 h at 30°C, the reaction mixtures were resolved on 5% native polyacrylamide gels. Autoradiography of the dried gels was then used to detect the labeled RNA and RNA-protein complexes. Where indicated, cold, unlabeled RNAs were added at 100-fold molar excess relative to the concentrations of labeled RNAs as competitors.
FIG. 7.
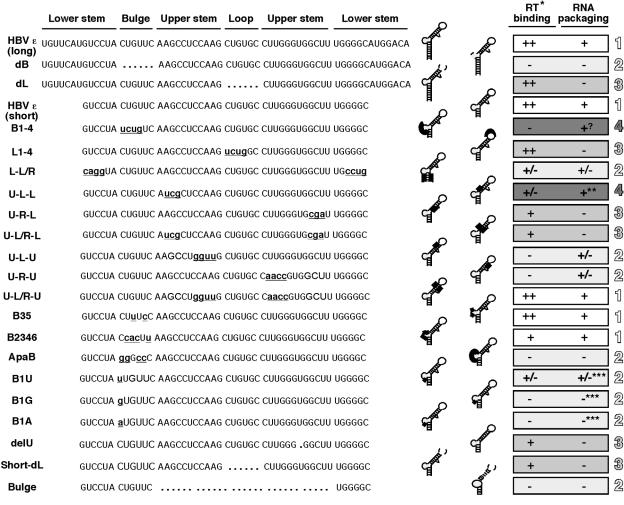
Summary of RT binding activities of HBV ɛ RNA mutants. Left. The wild-type (long) HBV ɛ sequences are shown at the top, with the lower-stem, bulge, upper-stem, and loop structural elements indicated. Deletions are denoted by the dots at the appropriate positions, and substitutions are indicated by lowercase, bold, and underlined lettering. Middle. The RNA mutants are schematized in the secondary-structure model. Deletions are denoted by dotted lines and substitutions by thick lines. Right. RT binding activities of the ɛ RNAs are summarized. The following symbols are used to indicate the RT binding activity: ++, 50 to 100% of wild-type activity; +, 10 to 50%; +/−, <10%; −, undetectable activity. The RNA packaging activities of the various ɛ mutants are taken from the literature (see Discussion for details). **, DNA synthesis is blocked; ***, deduced from the RNA selection study; ?, no effect on pgRNA packaging though defective in ɛ binding. The different degrees of shading and the numerals 1 to 4 are used to denote the classification of the ɛ mutants into four different groups (see Discussion for details).
RESULTS
Mapping of RT sequences required for ɛ binding.
We have recently reported that both the TP and RT domains of the HBV RT protein were required for specific binding to the cognate HBV ɛ RNA, as determined by using an in vitro RNA mobility get shift assay (21) that can directly measure specific HBV RT-ɛ interaction (for details, see Materials and Methods). Using this assay, we have carried out a detailed mapping of the sequences within the TP and RT domain that are required for ɛ binding. As depicted in Fig. 1, truncations were made from both the N and C termini, as were deletions from within the spacer region. The truncated HBV RT proteins were expressed and purified as GST fusion proteins at similar levels (Fig. 1B and data not shown). The ability of the purified RT proteins to bind ɛ was determined by incubating them with radiolabeled HBV ɛ RNA and five purified Hsp90 chaperone components, i.e., Hsp90, Hsp70, Hop (p60), Hdj-1, and p23, as described before (21). These studies showed that the TP domain sequences from residues 42 to 196, together with the RT domain sequences from residues 291 to 520, as represented by the construct HTPRT/Bsa-Spe, were sufficient for specific ɛ binding (Fig. 1 and 2). Thus, the spacer sequences from at least residues 197 to 290, the entire RNase H domain, at least 41 residues from the N-terminal portion of the TP domain, and the C-terminal portion of the RT domain, including the YMDD catalytic motif, were dispensable for ɛ binding, indicating that ɛ binding activity can be genetically separated from any known enzymatic activities of the RT protein.
FIG. 2.
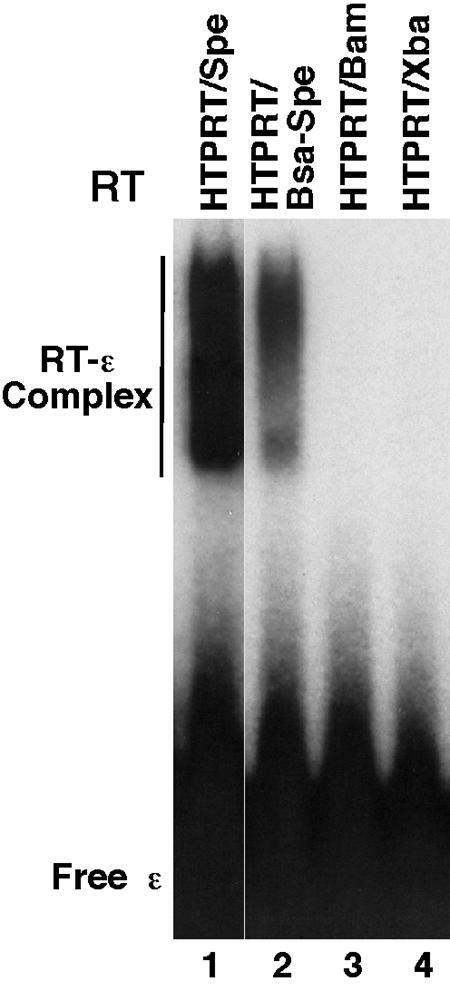
In vitro ɛ RNA binding activity of HBV RT mutants as detected by the gel mobility shift assay. Purified HBV RT proteins, all as GST fusion proteins, were incubated with 32P-labeled HBV ɛ RNA in the presence of the Hsp90 chaperone factors as detailed in Materials and Methods. The binding reaction mixtures were resolved on a native polyacrylamide gel, which was then dried and subjected to autoradiography. The labeled free ɛ RNA and the RT-ɛ complex are indicated.
Mapping of ɛ sequences required for RT binding.
The HBV ɛ RNA is thought to adopt a secondary structure, with a lower and an upper stem, an internal bulge, and an apical loop, which is depicted schematically in Fig. 3 to 8. To dissect the contribution of each of the ɛ structural elements to specific RT binding, we carried out a systematic-mutagenesis study with each of these structural elements. To help correlate the in vitro RT binding activity of the ɛ RNA mutants with their in vivo pgRNA packaging function, we chose to construct most of the ɛ RNA mutants corresponding to those previously tested in the pgRNA packaging assay (see Discussion below). To ascertain whether the different RT proteins would bind the different ɛ RNA mutants differentially, each ɛ mutant was assayed for binding to multiple RT proteins. However, all RT proteins tested bound a given RNA similarly (e.g., Fig. 3), and only the results obtained with one particular RT protein for any given ɛ mutant is thus shown below.
FIG. 3.

In vitro binding of HBV RT proteins to ɛ RNA mutants with the apical loop but not the internal bulge deleted. Two different RT proteins were tested for their binding to the wild-type (long), lower-stem-shortened (short), bulge deletion (dB), or loop deletion (dL) ɛ RNA, as described in the legend to Fig. 2. The RNA mutants are schematized in the secondary-structure model. Deletions are denoted by dotted lines. The symbol * indicates a minor structural isoform detected sometimes in the free RNA, as reported before (21).
FIG. 8.
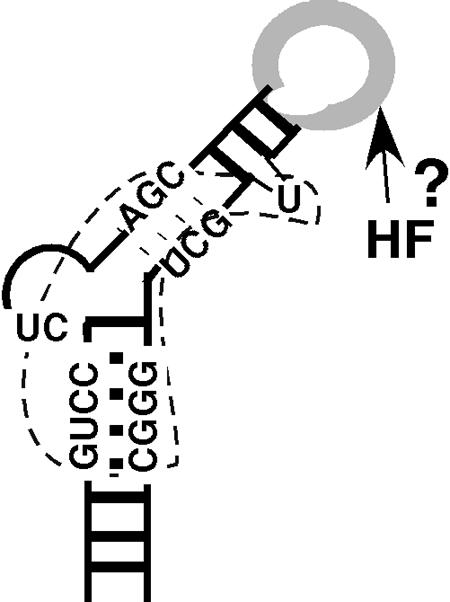
Model of HBV ɛ binding by the RT and putative host factor. The HBV ɛ RNA is depicted as the typical stem-loop structure. Critical sequences for RT binding (bounded by the dotted line) center around the internal bulge, including the first two nucleotides of the bulge, the upper portion of the lower stem, and the lower portion of the upper stem. The apical loop, dispensable for RT binding but critical for pgRNA packaging, may interact with an unknown host factor(s) (HF) in directing pgRNA packaging and possibly protein priming. See the text for detailed discussions of the model.
Lower stem.
Since we have previously shown that the lower portion of the lower stem was dispensable for RT binding in the DHBV system (20), we were interested in determining if this was also the case for the HBV ɛ RNA. As depicted in Fig. 3, 4, and 7, removal of the bottom seven base pairs from the lower stem of the HBV ɛ did not significantly impair the ability of the deletion mutant (short RNA) to bind the RT, as determined by both a direct binding assay, where the mutant ɛ was labeled as the probe (Fig. 3 and 4B), and a competition assay, where the mutant ɛ was added as a competitor in a binding reaction mixture with the labeled, wild-type (long) ɛ serving as the probe (Fig. 4A). In addition, we tested a longer HBV RNA, called HE (Fig. 4), encompassing not only the ɛ but also the DR1 sequence upstream and 80 nt downstream. This longer RNA did not compete any better than the short HBV ɛ RNA in the competition assay, indicating that the short HBV ɛ RNA contained all the essential sequences for RT binding in vitro. Thereafter, we chose to construct most of the ɛ mutations in the shorter ɛ RNA background for convenience.
FIG. 4.
Binding of HBV RT to ɛ mutants. (A) HTPRT/Drd was incubated with 32P-labeled wild-type HBV ɛ RNA, as described for Fig. 2, in the presence of the indicated unlabeled RNA competitors (Compet). The RNA mutants are schematized in the secondary-structure model. Deletions are denoted by dotted lines and substitutions by thick lines. Refer to Fig. 7 for the exact deletions/substitutions in each of the ɛ mutants tested. HE is a 190-nt-long HBV RNA, encompassing DR1 5′ of ɛ, ɛ, and 80 nt 3′ of ɛ (see Materials and Methods for details). The DHBV ɛ and tRNA, which cannot bind to the HBV RT (21), were used as nonspecific competitors. All competitor RNAs were used at a 100-fold molar excess. Note that all reactions also contained an additional 300-fold molar excess of tRNA over the labeled probe (21). (B) HTPRT/Drd (lanes 1 to 7) or HTPRT/Spe (lanes 8 to 11) was tested for binding to the 32P-labeled HBV ɛ RNA mutants, as described for Fig. 2.
To test the role of the upper portion of the lower stem in RT binding, we changed the nucleotide sequence but maintained the base pairing in this region. The resulting ɛ mutant (L-L/R) was severely impaired in its ability to bind the RT in both the direct binding and the competition assays (Fig. 4 and 7), indicating that base pairing here alone was not sufficient for RT binding and that some specific sequences in the lower stem contributed to the RT interaction.
Internal bulge.
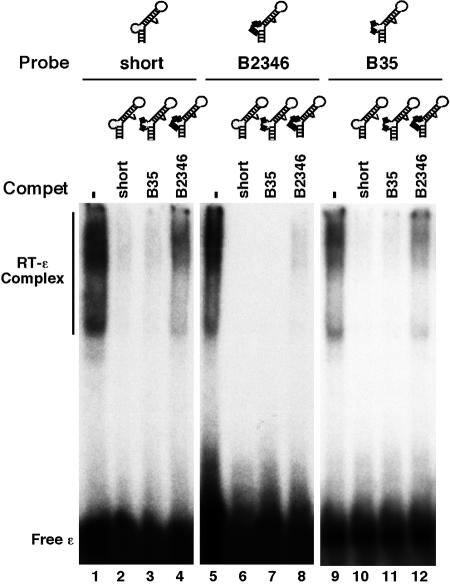
We have recently reported that deletion of the entire bulge of the HBV ɛ RNA (dB ɛ) abolished RT binding (21) (Fig. 3 and 7). To determine if the internal bulge acted only as a structural element or if its specific nucleotide sequence was required for RT binding, we made selected base substitutions in this region, using mainly mutants that had previously been tested in the pgRNA packaging assay. The mutant B1-4 had the first (5′) 4 nucleotides of the bulge replaced and completely lost the ability to bind the RT (Fig. 4 and 7), indicating that at least some of the bulge sequences were specifically required for RT binding. To further ascertain the base-specific contribution of the bulge to RT binding, further substitutions were made in the bulge region. Changes atthe third and fifth positions of the bulge (B35) did not affect RT binding at all, while changes at positions 2, 3, 4, and 6 (B2346) significantly decreased RT binding (Fig. 5, 6B, and 7). Thus, while the B2346 mutant still displayed specific RT binding activity, in competition experiments, it was less effective in competing with either the short ɛ or the B35 mutant for RT binding. Another bulge mutant, ApaB, having substitutions at positions 1, 2, 4, and 5 lost RT binding activity completely (Fig. 6B and 7). These results suggested that the first two nucleotides, particularly the 5′-most nucleotide of the bulge, may be critical for RT binding. To test this further, we changed the first position of the bulge from C to all three other nucleotides. In support of our hypothesis, the single nucleotide substitution from C to G (B1G) or to A (B1A) (both transversions) completely abolished RT binding, while the less drastic transition mutation (B1U) retained a low residual RT binding activity (Fig. 6A and 7).
FIG. 5.
Binding of HBV RT to ɛ bulge mutants. HTPRT/Drd was tested for binding to the 32P-labeled short (lanes 1 to 4), B2346 (lanes 5 to 8), or B35 (lanes 9 to 12) ɛ RNA, as described for Fig. 2. Where indicated, unlabeled RNAs (in 100-fold molar excess of the labeled RNAs) were added to the binding reaction mixtures as competitors (Compet). The RNA mutants are schematized in the secondary-structure model. Substitutions are denoted by thick lines. Refer to Fig. 7 for the exact bulge substitutions.
FIG. 6.
Binding of HBV RT to ɛ mutants. (A) HTPRT/Spe was tested for binding to the indicated 32P-labeled HBV ɛ RNAs, as described for Fig. 2. (B) HTPRT/Drd (lanes 1 to 4) or HTPRT/Bsa-Spe (lanes 5 to 10) was incubated with the 32P-labeled HBV short ɛ RNA, as described for Fig. 2. Where indicated, unlabeled RNAs (in 100-fold molar excess of the labeled RNAs) were added to the binding reaction mixtures as competitors (Compet). The RNA mutants are schematized in the secondary-structure model. Deletions are denoted by dotted lines and substitutions by thick lines. Refer to Fig. 7 for the exact ɛ mutations.
Upper stem.
We made substitutions to the lower and upper portions of the upper stem separately due to the interesting differential requirement for these two regions of the HBV ɛ RNA in pgRNA packaging (14, 38). For the lower portion, we replaced three nucleotides on either side of the stem. Interestingly, the left-side substitution (U-L-L) decreased RT binding more severely than the right-side substitution (U-R-L), but both mutants retained some RT binding activity (Fig. 4 and 7) as measured by both direct binding and competition assays, despite the disruption of base pairing. Restoration of base pairing but not the sequence in the third mutant (U-L/R-L) did not restore RT binding completely either. These results indicated that base pairing in this region of ɛ was neither essential nor sufficient for RT binding and, furthermore, that the RT recognized the sequences on either side of the stem differentially. In sharp contrast, we found that base pairing in the upper portion of the upper stem was required and sufficient for RT binding. Both sides of the stem could be replaced without affecting RT binding as long as base pairing was maintained (U-L/R-U), while disruption of base pairing led to a complete loss of RT binding (U-L-U, U-R-U) (Fig. 4 and 7), indicating that the RT recognized this portion of the upper stem as a double-stranded structural element rather than any specific sequences. Finally, deletion of the single unpaired U in the upper stem also severely diminished RT binding (Fig. 6A and 7).
Apical loop.
We have reported recently that the apical loop of the HBV ɛ RNA could be deleted completely (dL), in the context of the long ɛ RNA, without affecting RT binding (21). We made here the same deletion (short-dL) in the short (i.e., shortened lower stem) ɛ, and the resulting mutant still retained RT binding activity (Fig. 4 and 7). To confirm these results, we tested the binding of the ɛ loop deletion mutant to a number of different RT truncations, and it could bind to all the RT proteins that were competent for binding to the wild-type ɛ (Fig. 3 and data not shown). It has been shown previously that deletion of the loop leads to an altered RNA structure that nevertheless maintains the original lower stem and bulge sequence but with a shortened upper stem and a new apical loop with a completely different sequence (29), indicating again that the wild-type loop sequences are not important for RT binding. To further assess the role of the apical loop of ɛ in RT binding, we made the mutant L1-4, which has the first 4 nucleotides of the loop changed and which had previously been shown to abolish pgRNA packaging (38). Again, these substitutions did not affect RT binding at all (Fig. 4). Together, these results indicated that the apical loop of the HBV ɛ did not play a significant role in RT binding.
Finally, we tested whether the lower stem and the bulge sequences alone (Fig. 6 and 7), in the absence of the upper stem and loop sequences, would be sufficient for RT binding, in light of the dispensability of the apical loop and the only limited requirement of the upper stem. However, our results showed that the lower stem plus the internal bulge alone were clearly insufficient for RT binding.
DISCUSSION
Taking advantage of our recently established in vitro reconstitution system for specific HBV RT-ɛ interaction, we have carried out a detailed mapping analysis of the RT and ɛ determinants for specific RNP formation in vitro. The identification of these determinants, together with those mapped previously for pgRNA packaging and DNA synthesis, now provides the opportunity to critically evaluate the role of the HBV RT-ɛ interaction in pgRNA packaging and DNA synthesis.
With regard to the RT, our results indicate that less than 400 residues from the TP domain plus the N-terminal portion of the RT domain are sufficient for specific ɛ binding. The spacer region, the entire RNase H domain, and the C-terminal part ofthe RT domain, including the catalytic YMDD motif (and thusthe RT enzymatic activity), were dispensable for ɛ binding. These results are in good agreement with the previously mapped sequences in the DHBV RT protein that are required for binding to its cognate DHBV ɛ RNA (20, 39, 44, 55). Since pgRNA packaging requires essentially the entire length of the RT sequence, in both HBV and DHBV (1, 17), binding of the RT to ɛ is not sufficient for pgRNA packaging in vivo for these viruses. The C-terminal RT sequences, including the RNase H and part of the RT domain, which are required for pgRNA packaging but dispensable for ɛ binding, may mediate pgRNA packaging by interacting with the assembling core protein subunits, as reported before (35). However, since the RT alone, in the absence of ɛ, cannot be packaged into nucleocapsids (3), the putative RT-core interaction is apparently productive only for RT packaging in the context of the RT-ɛ RNP complex. Interestingly, our results revealed that an “insert” sequence in the HBV RT domain (residues 447 to 500) (Fig. 1) (40), which is conserved in the mammalian hepadnaviruses but absent in the DHBV RT, may play a critical role in ɛ binding.
Recombinant HBV RT proteins have previously been purified from an insect cell expression system and displayed low levels of protein-priming activity in vitro, although this low priming activity could occur independently of the ɛ RNA (30-32, 52). The TP domain sequence (residues 20 to 199) required for this priming activity is similar to that required for ɛ binding mapped here (residues 42 to 196), as are the boundaries for the dispensable spacer region in both assays (residues 200 to 300 in the priming assay versus residues 197 to 290 for ɛ binding). However, protein priming required a much longer sequence from the C-terminal region of the RT, including the entire RT domain and part of the RNase H domain as well, indicating that protein priming requires much more RT sequence than does ɛ binding.
With regard to the ɛ RNA sequences required for RT binding, our analyses revealed that the main sequence determinants recognized by the RT are (i) the internal bulge, specifically its first and, to a lesser extent, second nucleotides, and (ii) the sequences surrounding the bulge, including the lower part of the upper stem (particularly its left-side sequence) and the upper part of the lower stem, as well as the base pairing of the upper part and the single unpaired base of the upper stem (as summarized in Fig. 7 and 8). A comparison of these requirements with those mapped previously for pgRNA packaging in vivo allowed the classification of the ɛ mutants analyzed here into four groups. The first group, including mutants with the shortened lower stem (short), with some substitutions at the internal bulge (B35), and with the substitutions (but with maintenance of base pairing) at the upper part of the upper stem (U-L/R-U), are fully functional in both RT binding in vitro and pgRNA packaging in vivo. In addition, mutants with other substitutions at the bulge (B2346) retained significant, though decreased, activity in both RT binding and pgRNA packaging. The second group, including mutants with a deletion of the internal bulge (dB), certain substitutions at the bulge (ApaB, involving positions 1, 2, 4, and 5), particularly single substitutions at the first position of the bulge (B1G, B1A, and, to a lesser extent, B1U), disruption of the base pairing at the upper part of the upper stem (U-L-U and U-R-U), and deletion of the upper stem plus the loop (bulge), are defective in both RT binding and pgRNA packaging. In addition, substitutions at the lower stem (L-L/R) also severely decreased both of these activities. These first two groups of ɛ mutants underscore the critical role of RT-ɛ interaction, particularly the beginning sequences of the bulge, in pgRNA packaging. The third group, including mutants with deletions (dL and short dL) and substitutions (L1-4) of the apical loop, substitutions at the right side of the lower part of the upper stem (U-R-L and U-L/R-L), and a deletion of the single unpaired U residue from the upper stem, are fully or partially active in RT binding but defective in RNA packaging, again indicating that RT-ɛ interaction is not sufficient for pgRNA packaging. The last group, including mutants with substitutions at the left side of the lower part of the upper stem (U-L-L) and the B1-4 bulge substitution, are defective in RT binding but retain wild-type-like ability in RNA packaging. The identification of this group of ɛ mutants may suggest that some aspects of RT-ɛ interaction are actually not required for pgRNA packaging but may instead be involved in another stage(s) of viral replication, e.g., DNA synthesis (see below for more discussion on this).
Our result showing a sequence-specific contribution of the lower stem to RT binding agrees well with previous reports implicating both structural and sequence-specific determinants of the lower stem of the HBV ɛ RNA in pgRNA packaging (14, 38, 50, 51). Also, a DHBV-HBV hybrid ɛ RNA, with the HBV lower stem sequence substituting for the corresponding DHBV sequence, was inactive in either DHBV RT binding or protein priming, indicating that at least some specific sequences of the DHBV ɛ lower stem are also required for DHBV RT binding (55). The retention of the RT binding activity of the HBV ɛ mutant with a shortened lower stem is consistent with the dispensability of the beginning portion of the lower-stem pgRNA packaging in vivo (50). We (20) and others (8) also showed that the bottom portion of the lower stem of the DHBV ɛ could be removed without affecting DHBV RT binding or protein priming in vitro.
Our studies have revealed a critical role of the internal bulge sequence in RT binding. Although it was initially thought that the bulge played mainly a structural role in pgRNA packaging in that certain bulge substitutions still allowed efficient pgRNA packaging (14, 29, 38, 50), some specific bulge sequences also appeared to be required for pgRNA packaging. In particular, the first two nucleotides (CU) of the bulge were strongly selected for in a reiterative selection procedure for packaging competent ɛ RNA variants (41). In nature, the entire bulge sequence is highly conserved (15, 33, 38, 41). The only naturally occurring variation reported at the first two positions is the less drastic C-U transition at the first position (33), which we have shown here to retain some residual RT binding activity and which was also selected for, at a lower frequency, in the aforementioned selection study. The conservation of the 3′ four nucleotides of the bulge underscores their role as the template for viral DNA synthesis during protein priming (14, 36). Our results showing a base-specific recognition of the 5′ two nucleotides of the bulge by the RT now provides an explanation for the sequence conservation at these two positions, which, by virtue of their specific interaction with the RT, play a critical role in pgRNA packaging. Surprisingly, a substitution of the first four nucleotides of the bulge (B1-4) was previously reported to be packaging competent (38), although the same mutant was shown here to be completely defective in RT binding. We note that the loss of RT binding by the B1-4 mutant is consistent with the deleterious effect of substitutions at the first and second positions of the bulge on RT binding that we observed here in vitro and the strong selection previously reported for the wild-type sequences at these same positions in the RNA packaging assay (41).
The lower portion of the upper stem has been shown to contribute to pgRNA packaging. In particular, the specific nucleotide sequence at the right side seemed to be critical (38), and restoration of base pairing (but not sequence) could not restore pgRNA packaging (38, 51). The same right-side substitution mutant shown to be defective in pgRNA packaging (38) also displayed decreased RT binding activity, and base pairing here alone also was not sufficient to restore RT binding, indicating that nucleotide-specific recognition of the right side by the RT plays an important role in HBV pgRNA packaging. However, replacement of the left-side sequences here caused an even more severe defect than the right-side substitution in RT binding, although the same left-side substitution mutant was active in pgRNA packaging (38). Intriguingly, other left-side substitutions also showed no defect in pgRNA packaging but instead blocked viral DNA synthesis (14), suggesting that nucleotide-specific recognition of these ɛ sequences by the RT, as detected here in our in vitro binding assay, may play an important role in some aspects of viral reverse transcription. For example, the RT may facilitate the proposed long-range interaction of this region of the ɛ with the sequences near the 3′ DR1 to facilitate template switching from ɛ to DR1 during minus-strand DNA synthesis (47; D.Loeb, personal communication). Our analyses have, thus, begun to reveal that distinct RT-ɛ interactions may be involved in viral DNA synthesis as opposed to RNA packaging. The differential requirements of the two sides of the lower portion of the upper stem, the insufficiency of base pairing alone, and the retention of partial activity without base pairing for RT binding, pgRNA packaging, and DNA synthesis all suggest that this portion of the upper stem contributes to these functions in both structure- and sequence-specific ways, and it may in fact be at least partially molten upon RT binding in the RNP complex.
In contrast, the upper portion of the upper stem seems to play mainly a structural role in RT binding and pgRNA packaging. Thus, we have shown here that base pairing, but not the specific sequence, in this region was required for RT binding, and others have shown this also to be the case for RNA packaging (38). In addition, our results indicate that recognition of the single, unpaired U bulge in the upper stem, which is conserved in all mammalian HBVs, by the RT plays an important role in pgRNA packaging, as its deletion led to defects in both RT binding and pgRNA packaging (38, 51).
The most intriguing result in our ɛ mapping studies was the finding that the apical loop was entirely dispensable for RT binding. Deletion of the entire 6-nt loop or simultaneous substitutions at the first four positions had no effect on RT binding. Previous mutagenesis studies have shown that the loop is absolutely required in vivo for RNA packaging (29, 38, 51), in particular, this region could tolerate few substitutions without causing a defect in RNA packaging. These results have led to the suggestion that the RT protein may make sequence-specific contacts with the loop in directing pgRNA packaging. A recent nuclear magnetic resonance structure of the HBV ɛ RNA derivative, containing only the upper stem and the apical loop, suggests that the apical loop actually consists of only 3 nucleotides (15), rather than 6 as originally proposed. Regardless, it is clear that these loop sequences could be deleted or replaced without affecting RT binding. We suggest that the requirement for the apical loop in pgRNA packaging does not result from its direct recognition by the RT but rather from its interaction with some yet-to-be-identified cellular factor, both of which, together with the viral RT, are required for RNA packaging (Fig. 8).
It is interesting to contrast the dispensability of the HBV ɛ loop for HBV RT binding with the sequence-specific recognition of the DHBV ɛ loop by its cognate DHBV RT (6, 9). On the other hand, while base pairing of the upper stem near the HBV ɛ loop is required for HBV RT binding, base pairing in the corresponding ɛ region in the avian viruses is not required for RT binding (27, 55). Also, the specific sequences at the beginning of the DHBV ɛ internal bulge do not seem to be important for DHBV RT binding, in contrast to the critical role of the analogous sequences of the HBV ɛ in HBV RT binding observed here. It is possible that some of the differences observed may be a reflection of the inherent difficulty in predicting RNA structures and potentially unanticipated structural alterations of the ɛ RNA by some of the mutations. There also seemed to be some structural flexibility in the ɛ RNA that was compatible with RT binding; either the RT could recognize multiple RNA structures or, upon binding, a common ɛ structure could be induced from the different protein-free RNA structures (6, 9). Clearly, a full understanding of the molecular basis for the specific interactions between the RT and ɛ RNA in hepadnaviruses awaits high-resolution structural studies. Nevertheless, biochemical and mutational studies have helped to define the basic requirements of both the RT protein and the ɛ RNA. They also make it likely that significant differences exist in the interactions between the RT protein and its cognate ɛ RNA in the different hepadnaviruses. These differences in RT-ɛ interactions might be in part responsible for some of the disparities observed with respect to pgRNA packaging and protein priming between these related viruses, which still elude explanations. For example, the DHBV, but not HBV, pgRNA requires a second signal for packaging (11, 18, 24, 37), and the DHBV, but not HBV, RT can carry out ɛ-dependent protein priming under almost identical in vitro conditions (21, 25).
Acknowledgments
We thank David Toft for providing the chaperone proteins, Dafna Flores for technical assistance, and Dan Loeb for a critical reading of the manuscript.
This work was supported by a Public Health Service grant, R01 AI43453, from the National Institutes of Health.
REFERENCES
- 1.Bartenschlager, R., M. Junker-Niepmann, and H. Schaller. 1990. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J. Virol. 64:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager, R., and H. Schaller. 1988. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 7:4185-4192. [DOI] [PMC free article] [PubMed]
- 3.Bartenschlager, R., and H. Schaller. 1992. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 11:3413-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beasley, R. P., C. C. Lin, L. Y. Hwang, and C. S. Chien. 1981. Hepatocellular carcinoma and hepatitis B virus. Lancet ii:1129-1133. [DOI] [PubMed] [Google Scholar]
- 5.Beck, J., H. Bartos, and M. Nassal. 1997. Experimental confirmation of a hepatitis B virus (HBV) ɛ-like bulge-and-loop structure in avian HBV RNA encapsidation signals. Virology 227:500-504. [DOI] [PubMed] [Google Scholar]
- 6.Beck, J., and M. Nassal. 1998. Formation of a functional hepatitis B virus replication initiation complex involves a major structural alteration in the RNA template. Mol. Cell. Biol. 18:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck, J., and M. Nassal. 2001. Reconstitution of a functional duck hepatitis B virus replication initiation complex from separate reverse transcriptase domains expressed in Escherichia coli. J. Virol. 75:7410-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck, J., and M. Nassal. 1996. A sensitive procedure for mapping the boundaries of RNA elements binding in vitro translated proteins defines a minimal hepatitis B virus encapsidation signal. Nucleic Acids Res. 24:4364-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck, J., and M. Nassal. 1997. Sequence- and structure-specific determinants in the interaction between the RNA encapsidation signal and reverse transcriptase of avian hepatitis B viruses. J. Virol. 71:4971-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buendia, M. A. 1992. Hepatitis B viruses and hepatocellular carcinoma. Adv. Cancer Res. 59:167-226. [DOI] [PubMed] [Google Scholar]
- 11.Calvert, J., and J. Summers. 1994. Two regions of an avian hepadnavirus RNA pregenome are required in cis for encapsidation. J. Virol. 68:2084-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, L. J., R. C. Hirsch, D. Ganem, and H. E. Varmus. 1990. Effects of insertional and point mutations on the functions of the duck hepatitis B virus polymerase. J. Virol. 64:5553-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Y., W. S. Robinson, and P. L. Marion. 1994. Selected mutations of the duck hepatitis B virus P gene RNase H domain affect both RNA packaging and priming of minus-strand DNA synthesis. J. Virol. 68:5232-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallows, D. A., and S. P. Goff. 1995. Mutations in the epsilon sequences of human hepatitis B virus affect both RNA encapsidation and reverse transcription. J. Virol. 69:3067-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flodell, S., J. Schleucher, J. Cromsigt, H. Ippel, K. Kidd-Ljunggren, and S. Wijmenga. 2002. The apical stem-loop of the hepatitis B virus encapsidation signal folds into a stable tri-loop with two underlying pyrimidine bulges. Nucleic Acids Res. 30:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakes, D. J., and J. E. Dixon. 1992. New vectors for high level expression of recombinant proteins in bacteria. Anal. Biochem. 202:293-298. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch, R. C., J. E. Lavine, L. J. Chang, H. E. Varmus, and D. Ganem. 1990. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature 344:552-555. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, R. C., D. D. Loeb, J. R. Pollack, and D. Ganem. 1991. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J. Virol. 65:3309-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, J. 2004. Studying DHBV polymerase by in vitro transcription and translation. Methods Mol. Med. 95:259-269. [DOI] [PubMed] [Google Scholar]
- 20.Hu, J., and D. Anselmo. 2000. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by Hsp90. J. Virol. 74:11447-11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, J., D. Flores, D. Toft, X. Wang, and D. Nguyen. 2004. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J. Virol. 78:13122-13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, J., and C. Seeger. 1996. Expression and characterization of hepadnavirus reverse transcriptases. Methods Enzymol. 275:195-208. [DOI] [PubMed] [Google Scholar]
- 23.Hu, J., and C. Seeger. 1996. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 93:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu, J., and C. Seeger. 1997. RNA signals that control DNA replication in hepadnaviruses. Semin. Virol. 8:205-211. [Google Scholar]
- 25.Hu, J., D. Toft, D. Anselmo, and X. Wang. 2002. In vitro reconstitution of functional hepadnavirus reverse transcriptase with cellular chaperone proteins. J. Virol. 76:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, J., D. O. Toft, and C. Seeger. 1997. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 16:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu, K., J. Beck, and M. Nassal. 2004. SELEX-derived aptamers of the duck hepatitis B virus RNA encapsidation signal distinguish critical and non-critical residues for productive initiation of reverse transcription. Nucleic Acids Res. 32:4377-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junker-Niepmann, M., R. Bartenschlager, and H. Schaller. 1990. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 9:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knaus, T., and M. Nassal. 1993. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 21:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanford, R. E., Y. H. Kim, H. Lee, L. Notvall, and B. Beames. 1999. Mapping of the hepatitis B virus reverse transcriptase TP and RT domains by transcomplementation for nucleotide priming and by protein-protein interaction. J. Virol. 73:1885-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanford, R. E., L. Notvall, and B. Beames. 1995. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J. Virol. 69:4431-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanford, R. E., L. Notvall, H. Lee, and B. Beames. 1997. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J. Virol. 71:2996-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskus, T. 1994. The stem-loop structure of the cis-encapsidation signal is highly conserved in naturally occurring hepatitis B virus variants. Virology 200:809-812. [DOI] [PubMed] [Google Scholar]
- 34.Lee, W. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 35.Lott, L., B. Beames, L. Notvall, and R. E. Lanford. 2000. Interaction between hepatitis B virus core protein and reverse transcriptase. J. Virol. 74:11479-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassal, M., and A. Rieger. 1996. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J. Virol. 70:2764-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrow, K. M., and D. D. Loeb. 2002. Characterization of the cis-acting contributions to avian hepadnavirus RNA encapsidation. J. Virol. 76:9087-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack, J. R., and D. Ganem. 1993. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J. Virol. 67:3254-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack, J. R., and D. Ganem. 1994. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J. Virol. 68:5579-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radziwill, G., W. Tucker, and H. Schaller. 1990. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J. Virol. 64:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieger, A., and M. Nassal. 1995. Distinct requirements for primary sequence in the 5′- and 3′-part of a bulge in the hepatitis B virus RNA encapsidation signal revealed by a combined in vivo selection/in vitro amplification system. Nucleic Acids Res. 23:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieger, A., and M. Nassal. 1996. Specific hepatitis B virus minus-strand DNA synthesis requires only the 5′ encapsidation signal and the 3′-proximal direct repeat DR1. J. Virol. 70:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaaf, S. G., J. Beck, and M. Nassal. 1999. A small 2′-OH- and base-dependent recognition element downstream of the initiation site in the RNA encapsidation signal is essential for hepatitis B virus replication initiation. J. Biol. Chem. 274:37787-37794. [DOI] [PubMed] [Google Scholar]
- 44.Seeger, C., E. H. Leber, L. K. Wiens, and J. Hu. 1996. Mutagenesis of a hepatitis B virus reverse transcriptase yields temperature-sensitive virus. Virology 222:430-439. [DOI] [PubMed] [Google Scholar]
- 45.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summers, J., and W. S. Mason. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403-415. [DOI] [PubMed] [Google Scholar]
- 47.Tang, H., and A. McLachlan. 2002. A pregenomic RNA sequence adjacent to DR1 and complementary to epsilon influences hepatitis B virus replication efficiency. Virology 303:199-210. [DOI] [PubMed] [Google Scholar]
- 48.Tavis, J. E., S. Perri, and D. Ganem. 1994. Hepadnavirus reverse transcription initiates within the stem-loop of the RNA packaging signal and employs a novel strand transfer. J. Virol. 68:3536-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toh, H., H. Hayashida, and T. Miyata. 1983. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. Nature 305:827-829. [DOI] [PubMed] [Google Scholar]
- 50.Tong, S.-P., J.-S. Li, L. Vitvitski, A. Kay, and C. Trepo. 1993. Evidence for a base-paired region of hepatitis B virus pregenome encapsidation signal which influences the patterns of precore mutations abolishing HBe protein expression. J. Virol. 67:5651-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong, S.-P., L. Vitvitski, and C. Trepo. 1992. Replication capacities of natural and artificial precore stop codon mutants of hepatitis B virus: relevance of pregenome encapsidation signal. Virology 191:237-245. [DOI] [PubMed] [Google Scholar]
- 52.Urban, M., D. J. McMillan, G. Canning, A. Newell, E. Brown, J. S. Mills, and R. Jupp. 1998. In vitro activity of hepatitis B virus polymerase: requirement for distinct metal ions and the viral epsilon stem-loop. J Gen. Virol. 79:1121-1131. [DOI] [PubMed] [Google Scholar]
- 53.Wang, G. H., and C. Seeger. 1993. Novel mechanism for reverse transcription in hepatitis B viruses. J. Virol. 67:6507-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell 71:663-670. [DOI] [PubMed] [Google Scholar]
- 55.Wang, G. H., F. Zoulim, E. H. Leber, J. Kitson, and C. Seeger. 1994. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B viruses. J. Virol. 68:8437-8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, X., N. Grammatikakis, and J. Hu. 2002. Role of p50/CDC37 in hepadnavirus assembly and replication. J. Biol. Chem. 277:24361-24367. [DOI] [PubMed] [Google Scholar]
- 57.Wang, X., and J. Hu. 2002. Distinct requirement for two stages of protein-primed initiation of reverse transcription in hepadnaviruses. J. Virol. 76:5857-5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, X., X. Qian, H.-C. Guo, and J. Hu. 2003. Heat shock protein 90-independent activation of truncated hepadnavirus reverse transcriptase. J. Virol. 77:4471-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber, M., V. Bronsema, H. Bartos, A. Bosserhoff, R. Bartenschlager, and H. Schaller. 1994. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J. Virol. 68:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zoulim, F., and C. Seeger. 1994. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J. Virol. 68:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]