Abstract
Herpes simplex virus 1 (HSV-1) entry into cells and cell-cell fusion mediated by HSV-1 glycoproteins require four glycoproteins, gD, gB, gH, gL. Of these, gH is the only one that so far exhibits structural-functional features typical of viral fusion glycoproteins, i.e., a candidate fusion peptide and, downstream of it, a heptad repeat (HR) segment able to form a coiled coil, named HR-1. Here, we show that gH carries a functional HR-2 capable of physical interaction with HR-1. Specifically, mutational analysis of gH aimed at increasing or decreasing the ability of HR-2 to form a coiled coil resulted in an increase or decrease of fusion activity, respectively. HSV infection was modified accordingly. A mimetic peptide with the HR-2 sequence inhibited HSV-1 infection in a specific and dose-dependent manner. Circular dichroism spectroscopy showed that both HR-2 and HR-1 mimetic peptides adopt mainly random conformation in aqueous solution, while a decrease in peptide environmental polarity determines a conformational change, with a significant increase of the α-helical conformation content, in particular, for the HR-1 peptide. Furthermore, HR-1 and HR-2 mimetic peptides formed a stable complex, as revealed in nondenaturing electrophoresis and by circular dichroism. The mixture of HR-1 and HR-2 peptides reversed the inhibition of HSV infection exerted by the single peptides. Complex formation between HR-1 and HR-2 was independent of the presence of adjacent gH sequences and of additional glycoproteins involved in entry and fusion. Altogether, HR-2 adds to the features typical of class 1 fusion glycoproteins exhibited by HSV-1 gH.
Viral fusion glycoproteins are responsible for execution of fusion between the viral envelope and cell membranes. Key structural elements of class 1 viral fusion glycoproteins (e.g., the influenza virus hemagglutinin, the paramyxovirus F protein, human immunodeficiency virus gp41, Ebola virus, and severe acute respiratory syndrome coronavirus [CoV] glycoproteins) are the fusion peptide and heptad repeat (HR) sequences (12, 23). The fusion peptide is a hydrophobic α-helix able to penetrate the membrane of the target cell and destabilize it. Through it, the fusion glycoproteins form a bridge between the virion envelope and the cell membrane, an event that initiates fusion pore formation (12, 23). Following receptor binding, or induced by low pH, the trimeric fusion glycoproteins undergo a series of conformational changes and switch from the fusion-inactive to the fusion-active state. Critical for these changes are the HRs, usually two, termed HR-1 and HR-2, located downstream of the fusion peptide and upstream of the transmembrane segment, respectively. HR-1 forms a three α-helical structure termed a coiled coil, which is further modified to form a trimer of hairpins, also designated a six-helix bundle. In this structure, three HR-1 helices form a central coiled coil surrounded by three HR-2 helices in an oblique antiparallel orientation (23).
Entry of herpes simplex virus (HSV) into the cell requires the concerted action of several glycoproteins, four of which, gD, gB, gH, and gL, play essential roles for the fusion of the virion envelope to the cell membranes (5, 7, 14, 27, 46, 49). The number of glycoproteins involved in this process differentiates the fusion induced by HSV, and by herpesviruses in general, from the fusion induced by most viruses, which require one or, at most, two glycoproteins.
gD serves two functions. It acts as the receptor binding glycoprotein able to interact with two alternative protein receptors, herpesvirus entry mediator and nectin 1 (10, 17, 28, 35, 52). It also triggers fusion, as inferred by the findings that soluble forms of gD encompassing at least residues 1 to 285, but not shorter forms, were sufficient to rescue the infectivity of a noninfectious gD-null HSV mutant. Structurally, the gD ectodomain is organized in two regions. The N terminus (amino acids [aa] 1 to 260 or 1 to 250), with an immunoglobulin (Ig)-folded core, carries the receptor-binding sites (8, 25, 56). The C terminus (aa 260 to 310) functions as the pro-fusion domain, required for infectivity and fusion but not for receptor binding (9). Biochemical and structural studies indicate that, at or around aa 260, gD folds back over the N terminus and that the receptor-unbound gD adopts a “closed” conformation, where the pro-fusion domain interacts with the N terminus. By contrast, the receptor-bound gD adopts an “opened” conformation, where the N and C termini are released from reciprocal interactions and enabled to trigger fusion (15, 26).
The trio of gB, gH, and gL is required for downstream events that culminate in virus fusion with cell membranes. While the roles of gB and gL are unclear, accumulating evidence points to gH as the executor of fusion. Specifically, gH exhibits a hydrophobic α-helix (αH-1) (aa 377 to 397) with attributes of an internal fusion peptide, located in a loop made of two cysteines (6, 19). Mutational disruption of αH-1 abolished virus infectivity and cell-cell fusion. Its substitution with the fusion peptide of human immunodeficiency virus gp41 or of vesicular stomatitis virus G partially rescued the infection and cell-cell fusion, whereas its substitution with the candidate fusion peptide of varicella-zoster virus fully rescued HSV infectivity and cell-cell fusion (19). Additional sequences potentially able to interact with membranes may be present in HSV type 1 (HSV-1) gH. They include a region located membrane-proximally, at aa 626 to 644 and αH-2. The aa 624-644 region exhibits a tendency to partition at the membrane interface. A mimetic peptide with this sequence adopts an α-helix conformation upon lowering the polarity of the solvent, is capable of interacting with nude lipid vesicles, and enhances the fusion activity of a synthetic peptide that carries the fusion peptide sequence (16). Further yet, αH-2, located at aa residues 512 to 531, exhibits the ability to interact with membranes, although at lower extent than αH-1 (19).
Bioinformatic search of HR motifs in HSV-1 gH by means of the Lupas advanced algorithm identified two HR motifs downstream of the fusion peptide, designated HR-1 and HR-2 (20). HR-1 exhibited a high predicted propensity to form a coiled coil. The simultaneous substitution of two amino acids in HR-1, predicted to abolish the coiled coil, abolished the ability of gH to complement the infectivity of a gH-null HSV mutant. When coexpressed with gB, gD, and gL, the mutant gH was unable to promote cell-cell fusion. A 25-amino-acid-long synthetic peptide with the sequence of HR-1 (pep.gH-HR125) specifically inhibited HSV replication if present at the time of virus entry into the cell (20).
The purpose of this work was to ascertain whether the predicted HR-2, located at aa residues 556 to 585, serves as a functional HR and whether it can physically interact with HR-1.
MATERIALS AND METHODS
Cells and viruses.
The J1.1-2 cell line (J cells), a derivative of BHKtk− cells with high resistance to HSV infection, and their derivative J-nectin 1, stably expressing human nectin1, were described previously (10). BHK, COS, F6, and J-nectin 1 cells were grown in Dulbecco's modified Eagle's (DME) medium supplemented with 5% to 10% fetal calf serum (FCS). F6 cells are a stably transformed Vero cell line that expresses HSV-1 gH under the control of the HSV-1 gD promoter (14). In the gH deletion mutant (ΔgH HSV) SCgHZ, the gH gene was replaced with a lacZ gene. SCgHZ was grown and titrated in the complementing F6 cells (14). R8102, a recombinant HSV-1 carrying a LacZ gene under the α27 promoter, and a pseudorabies virus (PrV) carrying a LacZ gene were described previously (3, 32).
Bioinformatic analysis.
The bioinformatic search for coiled coils was conducted by means of the optimized Lupas algorithm (30, 31), with window widths of 14, 21, and 28 and the MTIDK (myosins, tropomyosins, intermediate filaments type I to V, desmosomal proteins, and kinesins) matrix (optimized for a better resolution between the scores of globular and coiled-coil proteins). Weighted and unweighted scans were run in parallel, and the outputs were compared.
Plasmids.
Expression plasmids for HSV-1 gD, gB, gH, and gL were described previously (2). Plasmid pcagt7 contains the T7 RNA polymerase gene under control of the CAG promoter, and the pt7emcluc plasmid expresses firefly luciferase under the T7 promoter (39, 45).
Constructs.
gHHR2-up, carrying the A573L, H574A, and K577L substitutions in HR-2, was derived by site-directed mutagenesis with oligonucleotide 5′-CGG ACC GCG CTG GCC CGG CTC GAC GCC CAG CTG ATA TCC TTT TGG CTT CCG GAC CAC-3′, containing a EcoRV site that introduced TL578-579IS substitutions for easiness of screening, as described previously (32). gHHR2-down1, carrying the L566I and L570A substitutions in HR-2, was derived by site-directed mutagenesis with oligonucleotide 5′-GCA GCG ACC AAC GCC GAT ATC CGG ACC GCG GCG GGC CGG GCC GAC CAC CAG-3′, containing an EcoRV site that introduced the A572G substitution for screening. gHHR2-down2, carrying the LARA570-573AVPQ substitutions in HR-2, was derived by site-directed mutagenesis with oligonucleotide 5′-GCC GAC CTC CGG ACC GCG GCG GTA CCG CAG GAC CAC CAG AAA ACC CTC-3′, containing a silent Asp718 site for screening.
Indirect immunofluorescence assay (IFA).
COS or BHK cells were grown on glass coverslips, transfected with the indicated plasmids by means of Polyfect (QIAGEN), and fixed 48 h later with 4% paraformaldehyde for 10 min at room temperature. Samples were incubated with monoclonal antibodies (MAbs) 52S and 53S to gH (43, 47) and fluorescein isothiocyanate-conjugated anti-mouse IgG (Jackson Immunoresearch) and observed with a Zeiss microscope. Micrographs were taken with a Kodak DC290 digital camera, as described previously (19).
CELISA.
Cell enzyme-linked immunosorbent assay (CELISA) was performed as described previously (45). Briefly, subconfluent cultures of COS cells in 24-well plates were transfected with 250 ng of gL plasmid and plasmids encoding wild-type (wt) gH or mutant gH. After 8 h, the cells were replaced in 96-well plates and, after incubation for 18 h, were reacted with MAb 52S. Subsequently, cells were fixed with 4% formaldehyde in phosphate-buffered saline, followed by biotinylated anti-mouse IgG conjugate, streptavidin-β-galactosidase conjugate, and ONPG (o-nitrophenyl-β-d-galactopyranoside) substrate. The optical density at 405 nm was read.
Cell-cell fusion assay.
The luciferase-based cell-cell fusion assay was performed as detailed previously (33, 45) using the luciferase assay system from Promega (Florence, Italy). The β-galactosidase (β-Gal) fusion assay in BHK cells was described previously (1). All assays were run three times and in triplicate.
Infectivity complementation assay.
The infectivity complementation assay was performed as detailed previously (9). Briefly, cells in T25 flasks were transfected with the appropriate gH plasmid. Four hours later, cells were infected with a gH−/+ stock of SCgHZ (7 PFU/cell). Unpenetrated virions were inactivated by acidic wash for 1 min with 40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3 (4). Progeny virus was titrated in F6 cells.
Inhibition of infection by mimetic peptides.
Lyophilized peptides, pep.gH-HR225, pep.gH-HR214, pep-gHwt25 (herein designated pep.gH-HR125), pep-gHscr25 (herein designated pep.gH-HR1scr25), and pep-gD265-289 (synthesized by Primm, San Raffaele Biomedical Science Park, Milan, Italy, or by the Peptide Synthesis Facility, University of Wisconsin Biotechnology Center, Madison) were dissolved in DME medium without serum at 3 mM concentration; the solution was adjusted to neutral pH by addition of 1 M Tris-HCl, pH 10. The experiments were performed in 96-well plates with extracellular R8102 or PrV LacZ virions at an input multiplicity of infection of 7 PFU/cell. For cell coexposure, the cells were exposed to increasing concentrations of the peptides and the viral inoculum for 90 min at 37°C. After removal of the inoculum and rinsing with DME containing 1% FCS, the cells were incubated with the peptides for 16 h. For cell preexposure, cells were incubated with a single concentration of peptides (500 μM) for 30 min at 4°C, rinsed twice with phosphate-buffered saline, and infected with R8102 for 90 min at 37°C. Following infection, unpenetrated virus was inactivated by means of an acid wash as above, and the cells were incubated for a further 16 h in the absence of the peptide. For attachment, the cells were infected with R8102 (14 PFU/cell) in the presence or absence of 500 μM peptides for 90 min at 4°C. Unbound virus was inactivated by acidic wash. In all cases, infection was measured as β-Gal activity using ONPG as a substrate (35). To measure the effect of peptide mixtures on virus infection, a mixture of 500 μM (each) pep.gH-HR125 and pep.gH-HR225, pep.gH-HR125 and pep-gD265-289, or pep.gH-HR225 and pep-gD265-289 or 500 μM pep-gD265-289 was incubated with the viral inoculum and cells for 90 min at 37°C. After removal of the inoculum and rinsing of the cells with DME containing 1% FCS, the cells were overlaid with medium containing the same peptide mixtures for 16 h. Infection was quantified as β-Gal activity using ONPG.
CD.
For circular dichroism (CD) spectroscopy, a CD Jasco J-810 spectropolarimeter (Jasco, Japan) was used. Individual peptides (pep.gH-HR125 [HR-1], pep.gH-HR225 [HR-2], or pep-gD265-289 [pep-gD]), and mixtures (HR-1/HR-2, HR-1/pep-gD, and HR-2/pep-gD) were analyzed at 50 μM concentrations in 10 mM phosphate buffer, pH 7.4, and in the same buffer containing up to 15% (2.5, 5, 7.5, 10, 12.5, and 15%) trifluoroethanol (TFE). The CD spectra were recorded in the spectral range of 185 to 260 nm using a 1-mm cell at room temperature. Spectra were recorded at 0.5-nm intervals. Stock solutions (200 μM) were prepared by dissolving the lyophilized peptides in 10 mM phosphate buffer, pH 7.4.
Native polyacrylamide gel electrophoresis (PAGE).
Pep.gH-HR125 (15 nmol) was mixed with increasing amounts of pep.gH-HR225, ranging from 3.75 to 15 nmol, for 15 min at 25°C. The peptides were subjected to electrophoresis in 12% polyacrylamide gels containing Tris-glycine, pH 8, without boiling, in the absence of sodium dodecyl sulfate and β-mercaptoethanol. The migration positions were detected by Coomassie blue staining.
RESULTS
Mutational analysis of HR-2.
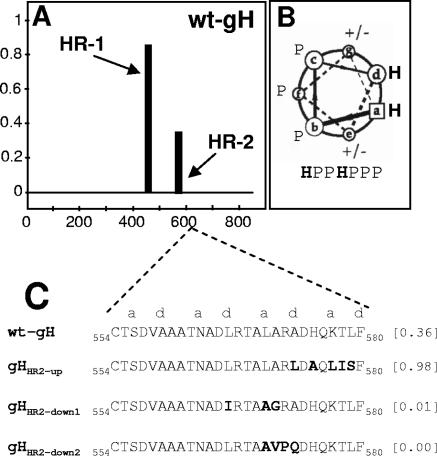
Probability plots for HRs in HSV-1 gH were generated by means of the Lupas algorithm (31, 30), with window widths of 14, 21, and 28, and the MTIDK matrix (optimized for a better resolution between the scores of globular and coiled-coil proteins). Weighted and unweighted scans were run in parallel, and the outputs were compared. This identified HR-2, located at aa residues 556 to 585 (Fig. 1 A). Its predicted probability to form a coiled coil was 0.4, i.e., below the suggested cutoff value of 0.5 (30). Three gH mutants in HR-2 were generated, named gHHR2-up, gHHR2-down1, and gHHR2-down2, whose propensities to form a coiled coil were either increased (0.98) or decreased (0.01 and 0.0), respectively. The relevant substitutions and their locations in the helix are reported in Fig. 1C. gHHR2-up carried the A573L and K577L substitutions (at the d and a positions) aimed at increasing the hydrophobicity. gHHR2-down1 carried three substitutions. Two of them, L566I and L570A (at the d and a positions), predicted a decrease in hydrophobicity. gHHR2-down2 carried three substitutions in addition to the L570A substitution. The A573Q substitution (position d) was designed to decrease the hydrophobicity, whereas the A571V and R572P substitutions (b and c positions) affected the solvent-exposed surface of the helix. In particular, the R-P exchange was chosen because it was one of the least conservative substitutions and, at the same time, disrupted the α-helix. The mutant forms of gH were tested in three functional assays, i.e., (i) for proper folding and ability to traffic to the cell surface, (ii) for ability to induce cell fusion when cotransfected with plasmids encoding gD, gB, and gL, and (iii) for ability to complement the infectivity of a gH deletion HSV mutant (infectivity complementation assay).
FIG. 1.
(A) Bioinformatic prediction of HR motifs in HSV-1 gH performed by means of Lupas algorithm (30, 31), as reported in reference 20. The abscissa shows the number of gH residues. The ordinate shows the predicted probability scores to form a coiled coil. (B) Helical wheel representation of heptad repeat with identification of the relative positions of hydrophobic (H) and polar (P) residues. (C) Sequence of HR-2 in wt gH and in the mutants. The values in brackets are predicted probabilities to form coiled coils.
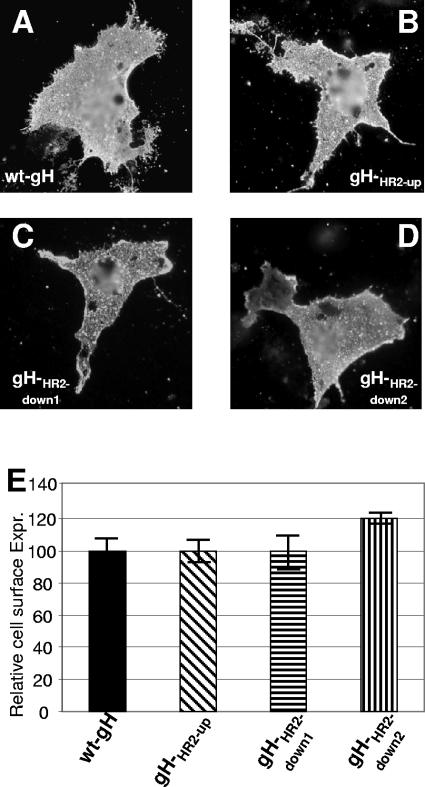
It is well established that gH dimerizes with its chaperone gL to achieve proper folding and be trafficked to the plasma membrane (46). The ability of mutant forms of gH to form a heterodimer with gL and to be trafficked to the plasma membrane was determined by IFA of cells cotransfected with the mutant or wt gH plasmid and a gL expression plasmid. MAb 53S recognizes a discontinuous epitope and strictly requires gL for reactivity. MAb 52S recognizes a gL-independent discontinuous epitope with critical residues at position 536 to 537 (43, 47). All three gH mutants maintained the reactivity to MAb 52S, suggesting no major defect in proper folding (data not shown). The IFA reactivity of paraformaldehyde-fixed cells to MAb 53S indicates no major defect in trafficking to the plasma membranes and in heterodimer formation with gL (Fig. 2A to D). Cell surface expression measured by CELISA was essentially the same as that of cells expressing the wt gH-gL heterodimer (Fig. 2E). Thus, the three mutants were capable of heterodimer formation with gL and of cell surface expression.
FIG. 2.
Cell surface expression of gHHR2-up, gHHR2-down1, and gHHR2-down2 mutants and wt gH. (A to D) Paraformaldehyde-fixed COS cells transfected with wt gH or mutant gH plasmids and a gL-encoding plasmid and reacted with MAb 53S. Positive reactivity denotes gH-gL heterodimer formation and no major defect in trafficking to the plasma membrane. (E) Quantification of cell surface expression (Expr.) of the gH mutants in COS cells cotransfected with a gL plasmid by CELISA. Each assay was performed in triplicate. Bars represent mean percentages relative to wt gH ± standard deviations.
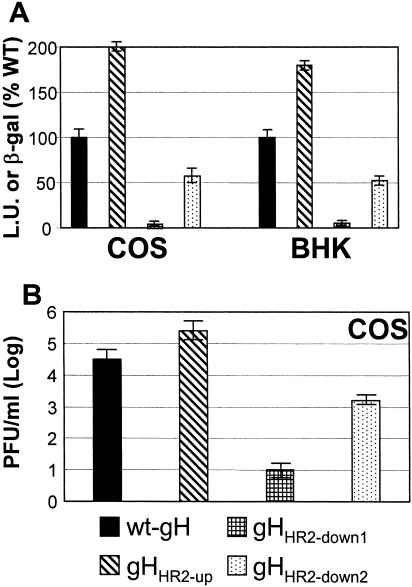
The cell-cell fusion in COS cells was quantified by means of a T7 promoter-driven reporter luciferase gene (45); the cell-cell fusion in BHK cells was quantified by cotransfection of a reporter LacZ gene, as detailed previously (1). As shown in Fig. 3, gHHR2-up exhibited a greatly increased fusion activity in COS cells, whereas both gHHR2-down1 and gHHR2-down2 exhibited a decreased fusion activity (Fig. 3A). The results in BHK cells fully agreed with those in COS cells. Thus, increasing the propensity to form a coiled coil resulted in an actual increase in fusion activity. The converse also held true.
FIG. 3.
Cell-cell fusion (A) and infectivity complementation (B) of mutant forms of gH. (A) Cell-cell fusion of COS or BHK cells transfected with the gH mutant plasmids plus plasmids encoding gL, gB, or gD. Fusion was quantified by means of a T7 promoter-driven reporter luciferase gene and expressed as luciferase units (L.U.) (according to reference 45) in COS cells or quantified as β-Gal activity (1) in BHK cells. Each assay was performed in triplicate. Bars represent means ± standard deviations (SD). (B) COS cells were transfected with mutant or wt gH plasmids and superinfected with the gH deletion mutant virus SCgHZ 4 h later. Virus was harvested 24 h after transfection and titrated in F6 cells in triplicate. Bars represent means of triplicate assays ± SD.
In the infectivity complementation assay, cells were transfected with mutant or wt gH and superinfected with the gH deletion mutant virus SCgHZ (14). The transgenic gH complements the deletion in the virus, and the complemented virions are or are not infectious, depending on whether gH is or is not functional. The results shown in Fig. 3B show that virions complemented with gHHR2-up exhibited an about 10-fold increase in infectivity, whereas virions complemented with gHHR2-down1 and gHHR2-down2 exhibited an about 1,000-fold or 10-fold decrease in infectivity, respectively.
A mimetic HR-2 peptide specifically inhibits HSV-1 infection.
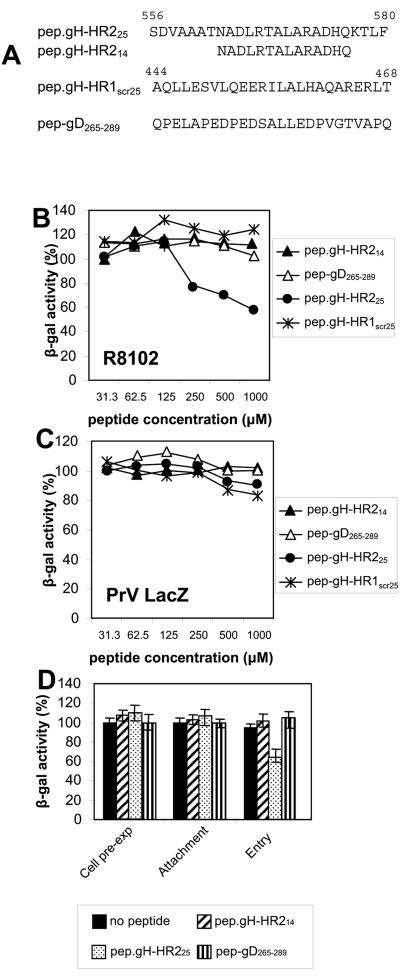
Lopper and Compton and our laboratory showed that synthetic peptides with the sequence of human cytomegalovirus gB and gH HRs and of HSV-1 gH HR-1 inhibit virus infections (20, 29). Here, we tested the effect of two synthetic peptides with the HR-2 sequence. pep.gH-HR214 and pep.gH-HR225 are 14 and 25 residues long, respectively (Fig. 4A). The negative controls were a scrambled peptide from HSV-1 gH HR-1 (pep.gH-HR1scr25, aa 444 to 468) and pep-gD265-289. Cells were infected with the recombinant R8102, which carries a LacZ gene under the α-27 promoter. β-Gal activity was a direct measure of the extent of virus infection. Figure 4B shows that pep.gH-HR225, but not pep.gH-HR214, reduced virus infection in a dose-dependent manner. A 40% reduction was observed at a 1 mM peptide concentration. The requirement for such a high concentration is not surprising and was reported previously with mimetic peptides to HSV-1 gH and human cytomegalovirus as well as to other viruses (20, 29, 53). Irrespective of this, the inhibition was specific, based on the two criteria. First, pep.gH-HR214, the scrambled peptide pep.gH-HR1scr25, and pep-gD265-289 were ineffective. Second, pep.gH-HR214 and pep.gH-HR225 did not reduce PrV infection, ruling out that the reduction was due to a toxic effect (Fig. 4C). Of note, the extent of inhibition attained with pep.gH-HR225 was lower than that attained with a 25-mer mimetic peptide to HR-1, pep.gH-HR125 (20).
FIG. 4.
Inhibition of HSV-1 infection by synthetic peptides. (A) Sequence and coordinates of synthetic peptides to HSV-1 gH HR-2, HSV-1 gD, and gH HR-1. (B, C) Effect of peptides on infection by the HSV-1 recombinant R8102 carrying LacZ under the α-27 promoter (B) or of a PrV recombinant carrying LacZ (C). J-nectin1 cells were exposed to the indicated peptides from 30 min prior to infection until harvest. Infection was quantified as β-Gal activity. The abscissas show μM peptide concentrations. (D) Effect on R8102 infection of pep.gH-HR225 administered to cells prior to infection (cell pre-exp), during virus attachment (4°C for 90 min) (attachment), or during the time interval of virus infection (90 min at 37°C) (entry).
To verify the step in HSV entry inhibited by pep.gH-HR225, cells were exposed to pep.gH-HR225 only during the interval of virus attachment to cells at 4°C. Alternatively, they were exposed to the peptide only during the interval of virus entry or prior to virus exposure (preexposure). Figure 4D shows that pep.gH-HR225 inhibited R8102 infection only if present during the interval of virus entry.
Complex formation between HR-1 and HR-2 mimetic peptides.
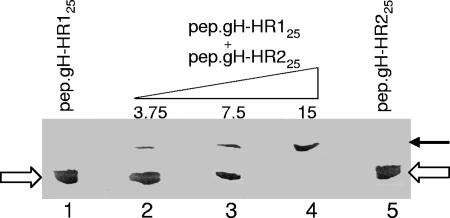
To explore the possibility that HR-1 and HR-2 mimetic peptides interact physically with each other, a fixed amount (15 nmol) of pep.gH-HR125 was mixed with increasing amounts of pep.gH-HR225 for 15 min at 25°C. The peptides were then subjected to nondenaturing PAGE and detected by Coomassie blue staining. As shown in Fig. 5, the single peptides exhibited the same migration position, in accordance with their equivalent Mr. A mixture of the two peptides resulted in a slower-migrating complex and concomitant disappearance of the bands corresponding to the single peptides. At a 1:1 molar ratio, the single-peptide species were not detectable, the peptides were in complex, suggesting that the stoichiometry of the complex was 1:1.
FIG. 5.
Complex formation between pep.gH-HR125 and pep.gH-HR225 as seen in nondenaturing PAGE. Fifteen nanomoles of pep.gH-HR125 was mixed with the indicated increasing nanomole quantities of pep.gH-HR225 for 15 min at 25°C. The complexes were subjected to nondenaturing PAGE. At a 1:1 molar ratio (lane 4), the single peptides species (white arrows) were not detectable, and only the slower-migrating complex (black arrow) was detected.
Secondary structures of HR-1 and HR-2 synthetic peptides singly or in combination.
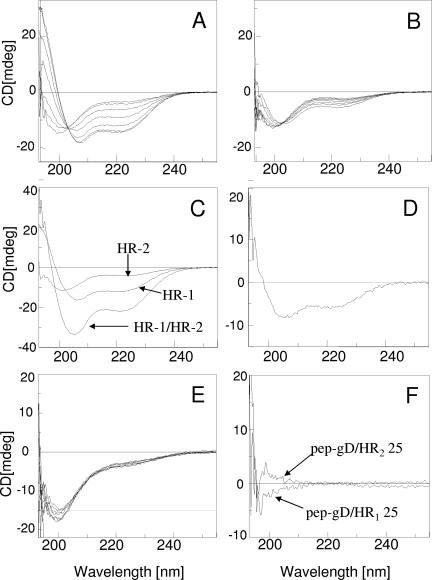
The secondary structure of the HR-1 and HR-2 peptides was analyzed by CD spectroscopy in phosphate buffer and in this buffer containing up to 15% TFE, both for the single peptides and for the 1:1 mixtures. The CD spectrum in buffer solution indicated mainly a random coil conformation for both pep.gH-HR125 and pep.gH-HR225 (Fig. 6A and B). The effect of polarity on peptide conformation was analyzed in aqueous mixtures of TFE. This organic solvent should mimic the decrease in the environmental polarity occurring when the peptide is transferred from water to membrane interfaces. Indeed, even if the oligopeptide or protein amino acid sequence usually gives the propensity to a prevailing secondary structure (34), the conformation is significantly affected by the environment, i.e., the nature of the solvent (54). A significant change in the conformation was observed for pep.gH-HR125 upon increasing the percentage of TFE in the solution (Fig. 6A). Two negative CD bands were present at about 222 and 208 nm; this behavior indicates the adoption of an α-helix structure. By contrast, only a minor change was observed in the CD spectrum of pep.gH-HR225 mimetic peptide in the same experimental conditions (Fig. 6B). This behavior suggests a much lower propensity for the HR-2 mimetic peptide to adopt an α-helix conformation, relative to the HR-1 mimetic peptide. Of note, also mimetic peptides of HSV-1 gH containing the fusion peptide or the aa 626 to 644 membrane to the interface region were shown to adopt an α-helical conformation in TFE-containing buffers but not in aqueous buffers (16).
FIG. 6.
CD spectra of HR-1 and HR-2 mimetic peptides singly and in combination. (A, B) CD spectra of pep.gH-HR125 (HR-1) and pep.gH-HR225. (HR-2). The spectra were carried out in phosphate buffer (10 mM, pH 7.4) at different percentages of TFE (0, 2.5, 5, 7.5, 10, 12.5, 15); the CD intensities at 222 and 208 nm increase upon increasing the percentage of TFE. (C) CD spectra of single HR-1 and HR-2 mimetic peptides and of their 1:1 mixture. (D) Differential CD spectrum [(HR-1/HR-2 mixture) − single (HR-1 + HR-2) CD contribution]. The solvent was phosphate buffer (10 mM, pH 7.4) with 10% TFE; cell size, 1 mm. (E) CD spectra of pep-gD265-289 carried out in the same experimental conditions as for panels A and B. (F) Differential CD spectra {[(HR-1/pep-gD265-289 mixture) − single (HR-1 + pep-gD265-289) CD contribution] and [(HR-2/pep-gD265-289 mixture) − single (HR-2 + pep-gD265-289) CD contribution]}. The solvent was phosphate buffer (10 mM, pH 7.4) with 10% TFE; cell size, 1 mm.
Next, we analyzed the properties of the mixture of HR-1 and HR-2 mimetic peptides. The CD spectrum of the mixture of pep.gH-HR125 and pep.gH-HR225 in a 1:1 molar ratio gave an enhancement of the spectrum intensity (Fig. 6C) compared to that of the single peptides. This change is indicative of heterodimer formation with a significant increase of the α-helix conformation content (Fig. 6D). The effect was specific, as supported by the same experiment carried out using an unrelated peptide, pep-gD265-289, which is not expected to alter its conformation. Indeed the peptide pep-gD265-289 exhibited a very low propensity to adopt an α-helix conformation, as indicated by the minor changes observed in the CD spectrum by increasing the TFE content (Fig. 6E). Furthermore, no significant changes of the CD spectrum, and then of the secondary structure, were observed when the CD spectra of the pep-gD265-289/pep.gH-HR125 or pep-gD265-289/pep.gH-HR225 mixtures were compared to the sum of the single components. This behavior is shown in Fig. 6F, where the negligible differential CD spectra are reported.
The simultaneous presence of HR-1 and HR-2 mimetic peptides reverts the inhibition exerted by the single peptides.
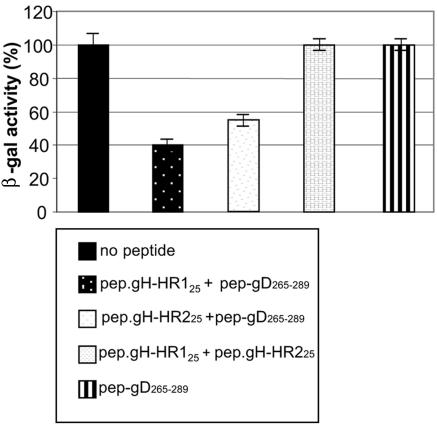
Nondenaturing PAGE and CD spectra revealed a complex formation between pep.gH-HR125 and pep.gH-HR225. We reasoned that complex formation between the two peptides should revert the inhibition of infection exerted by the single peptides. An experiment similar to that described for Fig. 4B was performed, except that a mixture of 500 μM (each) pep.gH-HR125 and pep.gH-HR225 was used instead of the single peptides. As shown in Fig. 7, no inhibition was observed when virus infection was carried out in the presence of a mixture of the two peptides.
FIG. 7.
Lack of inhibition of R8102 entry by mixture of HR-1 and HR-2 mimetic peptides. J-nectin1 cells were exposed to the indicated peptides (250 μM each, except pep-gD265-289 in right-hand bar, which is 500 μM) from 30 min prior to infection until harvest. Infection was determined in triplicate assays as β-Gal activity. Bars represent means of results from triplicate assays ± standard deviations.
DISCUSSION
We provide two lines of evidence to support the conclusion that gH HR-2 (aa 556 to 585) constitutes a functional domain for HSV-1 entry into the cell and for fusion. First, a rational mutational analysis designed to either increase or decrease the probability to form a coiled coil indeed resulted in increased or decreased virus infection and cell-cell fusion activity. Second, a mimetic peptide with the sequence of HR-2 inhibited virus infection in a specific manner, although at high concentrations. Examples of functional HRs with probability scores below the suggested cutoff value are infrequent but do exist, as is the case for Newcastle disease virus F glycoprotein (36). Of note, HR-2 is bracketed by two crucial cysteines (cysteines 5 and 6) for gH structure (6).
Two key properties of HSV gH HR-1 and HR-2 were (i) the ability to adopt an α-helical conformation, an important attribute of HR sequences, and (ii) the ability to interact with each other and form a stable complex. (i) Specifically, CD spectroscopy revealed that the HR-1 mimetic peptide adopts mainly a random conformation in aqueous solution; the α-helical structure content increases by decreasing the polarity of the solvent, as obtained by increasing the concentration of TFE in the buffer solution. The secondary structure of HR-2 mimetic peptide was much less affected by the nature of the solvent. (ii) Concerning the ability of HR-1 and HR-2 peptides to interact with each other, three lines of evidence support this conclusion. First, a complex between HR-1 and HR-2 synthetic peptides was revealed in nondenaturing PAGE. Second, the mixture of HR-1 and HR-2 peptides exhibited an α-helical content higher than that of the two single peptides, as shown by the increase of the CD intensities at 222 and 208 nm. Third, a mixture of HR-1 and HR-2 mimetic peptides reversed the inhibition of infection exerted by the single peptides. Our results clearly show that HR-1 and HR-2 peptides interact with each other. Interestingly, complex formation is an intrinsic property of the HR-1 and HR-2 peptides, independent of the presence of adjacent gH sequences and of the additional viral glycoproteins required for fusion.
Cumulatively, previous and current analyses identify in HSV-1 gH the following structural elements: a candidate fusion peptide, located at aa residues 377 to 397, and two HRs with propensity to form a complex and adopt a coiled coil conformation (19, 20). HR-1 and HR-2 are located downstream of the candidate fusion peptide and upstream of the transmembrane segment, in a position canonical for class 1 viral fusion glycoproteins. As mentioned in the introduction, HSV-1 gH may contain further segments able to interact with lipid membranes (16; T. Gianni, C. Bergamini, R. Fato, G. Lenaz, and G. Campadelli-Fiume, unpublished observation), as observed also in a number of viral fusion glycoproteins, e.g., Sendai virus and paramyxovirus F glycoproteins (18, 41, 42). Altogether, these attributes make gH the leading candidate executor of HSV fusion.
Pertinent to the significance of gH as the candidate fusion glycoprotein are the following considerations. The role played by gB in HSV-mediated fusion is still unclear. Viral fusion glycoproteins are dead ends in the fusion process and do not undergo renaturation. The properties of gH make it likely that gH acts as the last link in the chain of events that starts with the formation of the gD-receptor complex and culminates in fusion execution. It follows that gB should act at an intermediate step between gD and gH, a hypothesis that remains to be verified. The alternative possibility is that gB also serves as a fusogenic glycoprotein. If this is the case, it seems reasonable to postulate that gB and gH act in concert to execute fusion; by contrast, the possibility that each of them is fully competent to execute fusion independent of one another appears less tenable. We favor the view that gB acts at an intermediate step between gD and gH, given that bioinformatic and molecular biology analyses of gB so far failed to highlight domains typical of viral fusion glycoproteins.
A further consideration centers on whether the role of gH is conserved in the Herpesviridae family. Bioinformatic analyses coupled with known properties of gH favor this view. Thus, all the examined isoforms of gH carry at least one positionally conserved predicted hydrophobic α-helix and generally two predicted HRs (19, 20). In some cases, they were shown to be functional or could be exchanged with one another (19, 29, 40). Consistent with this view are several attributes of gH. Thus, antibodies to gH exhibit potent neutralizing activity, gH deletion mutant viruses are completely debilitated, and together with gL and gB, gH is an essential component of the cell-cell fusion assays, which have been developed for different herpesviruses (11, 14, 21, 24, 37, 40, 43, 44, 50). The two exceptions are an Epstein-Barr virus gB mutant and the human cytomegalovirus gH-gL heterodimer, each capable of inducing the formation of small syncytia in the absence of additional glycoproteins (24, 40).
Class 1 viral fusion glycoproteins form a broad group of proteins that not only share structural elements but also carry distinctive features. One such feature is the distance between HR-1 and HR-2, that ranges from a few amino acids (5 to 26) to about 250 aa (e.g., influenza virus hemagglutinin, paramyxovirus simian virus 5 F) (12, 42). A major difference is the topological relation that exists between the fusion glycoprotein and the viral domain responsible for receptor binding activity. In some cases, the receptor binding activity lies in a specific domain of the fusion glycoprotein itself (e.g., severe acute respiratory syndrome CoV, vesicular stomatitis virus glycoprotein). In other cases, the receptor binding activity lies in a separate glycoprotein that cross talks with the fusion glycoprotein (e.g., HN for paramyxovirus, gp120 for human immunodeficiency virus, GP1 of Ebola virus, glycoproteins of some retroviruses, and avian and mammalian CoVs) (12, 22, 36, 55). HSV, and herpesviruses in general, differ from both these groups in that they carry a multipartite fusion machinery made of a receptor binding glycoprotein (gD in HSV, gp42 in Epstein-Barr virus, gB in human cytomegalovirus), gB and gL, whose roles in fusion remain to be determined, and gH, with its structural elements (7, 13, 38, 48, 51). Taking into account what is currently known, we propose that gH be considered the candidate executor of fusion with attributes of class one fusion glycoproteins, that herpesvirus fusion be considered a group in itself, classified as a multipartite fusion system, and that HSV be the paradigm for it.
Acknowledgments
We thank T. Minson (Cambridge University), B. Roizman (University of Chicago), P. G. Spear (Northwestern University), G. H. Cohen and R. Eisenberg (University of Pennsylvania), and T. Mettenleiter (Friedrich-Loeffler-Institut, Insel Riems, Germany) for gifts of viruses and antibodies. We are indebted to Elisabetta Romagnoli for invaluable assistance.
The work was supported by FIRB autonomous and coordinated project, Cofin-MIUR 40%, University of Bologna 60%, Fondo Pallotti.
REFERENCES
- 1.Avitabile, E., G. Lombardi, and G. Campadelli-Fiume. 2003. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH and gL. J. Virol. 77:6836-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wt-gB or an endocytosis-defective gB mutant, and downmodulates their cell surface expression. J. Virol. 78:8015-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic, N., B. G. Klupp, B. Makoschey, A. Karger, A. Flamand, and T. C. Mettenleiter. 1996. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J. Gen. Virol. 77:2277-2285. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti, C. R., R. L. Burke, B. Hoflack, T. Ludwig, K. S. Dingwell, and D. C. Johnson. 1995. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J. Virol. 69:3517-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [Erratum, 62:4438.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, T. M., D. J. Landsburg, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550-562. [DOI] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 8.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 9.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex viruses 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 12.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 13.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galdiero, S., A. Falanga, M. Vitiello, H. Browne, C. Pedone, and M. Galdiero. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632-28643. [DOI] [PubMed] [Google Scholar]
- 17.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, J. K., S. G. Peisajovich, and Y. Shai. 2000. Sendai virus internal fusion peptide: structural and functional characterization and a plausible mode of viral entry inhibition. Biochemistry 39:11581-11592. [DOI] [PubMed] [Google Scholar]
- 19.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simpex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus 1 gH, located downstream of the α-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gompels, U., and A. Minson. 1986. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology 153:230-247. [DOI] [PubMed] [Google Scholar]
- 22.Ito, H., S. Watanabe, A. Takada, and Y. Kawaoka. 2001. Ebola virus glycoprotein: proteolytic processing, acylation, cell tropism, and detection of neutralizing antibodies. J. Virol. 75:1576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jardetzky, T. S., and R. A. Lamb. 2004. Virology: a class act. Nature 427:307-308. [DOI] [PubMed] [Google Scholar]
- 24.Kinzler, E. R., and T. Compton. 2005. Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 79:7827-7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez, M., F. Cocchi, L. Menotti, E. Avitabile, P. Dubreuil, and G. Campadelli-Fiume. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 74:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 78:8333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupas, A. 1997. Predicting coiled-coil regions in proteins. Curr. Opin. Struct. Biol. 7:388-393. [DOI] [PubMed] [Google Scholar]
- 31.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 32.Menotti, L., R. Casadio, C. Bertucci, M. Lopez, and G. Campadelli-Fiume. 2002. Substitution in the murine nectin1 receptor of a single conserved amino acid at a position distal from herpes simplex virus gD binding site confers high affinity binding to gD. J. Virol. 76:5463-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milne, R. S., S. L. Hanna, A. H. Rux, S. H. Willis, G. H. Cohen, and R. J. Eisenberg. 2003. Function of herpes simplex virus type 1 gD mutants with different receptor-binding affinities in virus entry and fusion. J. Virol. 77:8962-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minor, D. L., Jr., and P. S. Kim. 1994. Measurement of the beta-sheet-forming propensities of amino acids. Nature 367:660-663. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 36.Morrison, T. G. 2003. Structure and function of a paramyxovirus fusion protein. Biochim. Biophys. Acta 1614:73-84. [DOI] [PubMed] [Google Scholar]
- 37.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 38.Mullen, M. M., K. M. Haan, R. Longnecker, and T. S. Jardetzky. 2002. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol. Cell 9:375-385. [DOI] [PubMed] [Google Scholar]
- 39.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 40.Omerovic, J., L. Lev, and R. Longnecker. 2005. The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with epithelial and B cells. J. Virol. 79:12408-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peisajovich, S. G., O. Samuel, and Y. Shai. 2000. Paramyxovirus F1 protein has two fusion peptides: implications for the mechanism of membrane fusion. J. Mol. Biol. 296:1353-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peisajovich, S. G., and Y. Shai. 2003. Viral fusion proteins: multiple regions contribute to membrane fusion. Biochim. Biophys. Acta 1614:122-129. [DOI] [PubMed] [Google Scholar]
- 43.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76:4390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 46.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 49.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe, S., A. Takada, T. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2000. Functional importance of the coiled-coil of the Ebola virus glycoprotein. J. Virol. 74:10194-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterhous, D. V., and W. C. Johnson, Jr. 1994. Importance of environment in determining secondary structure in proteins. Biochemistry 33:2121-2128. [DOI] [PubMed] [Google Scholar]
- 55.Xu, Y., Z. Bai, L. Qin, X. Li, G. Gao, and Z. Rao. 2004. Crystallization and preliminary crystallographic analysis of the fusion core of the spike protein of the murine coronavirus mouse hepatitis virus (MHV). Acta Crystallogr. D 60:2013-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon, M., A. Zago, D. Shukla, and P. G. Spear. 2003. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J. Virol. 77:9221-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]