Abstract
Mice infected with reovirus develop abnormalities in glucose homeostasis. Reovirus strain type 3 Abney (T3A) was capable of systemic infection of nonobese diabetic (NOD) mice, an experimental model of autoimmune diabetes. Reovirus antigen was detected in pancreatic islets of T3A-infected mice, and primary cultures of pancreatic islets from NOD mice supported T3A growth. Significantly fewer T3A-infected animals compared to uninfected controls developed diabetes. However, despite the alteration in diabetes penetrance, insulitis was evident in T3A-infected mice. These results suggest that viral infection of NOD mice alters autoimmune responses to β-cell antigens and thereby delays development of diabetes.
Type 1 diabetes mellitus is an autoimmune disease resulting in destruction of pancreatic β cells. The initiating event in the process of β-cell destruction leading to autoimmune diabetes is not known, but current theories center on a viral, bacterial, or environmental stimulus that initiates an autoimmune process directed against β-cell antigens (12, 26). Nonobese diabetic (NOD) mice are an experimental model of autoimmune diabetes (13, 25). However, infections of NOD mice by some viruses, such as group B coxsackieviruses (CVB) (32), encephalomyocarditis virus (8), lactate dehydrogenase-elevating virus (29), lymphocytic choriomeningitis virus (18), and mouse hepatitis virus (38), prevent the development of diabetes. These findings suggest that viral infections of NOD mice alter autoimmune responses against β-cell antigens, with subsequent suppression of autoimmune β-cell destruction.
Mammalian reoviruses are nonenveloped viruses that contain a genome of 10 double-stranded RNA segments (16). Reoviruses replicate in the cytoplasm of host cells (16) and produce cell death by apoptosis (1, 17, 34). Virtually all mammals serve as hosts for reovirus infection, but disease is limited to the very young (33). Reovirus is capable of establishing persistent infections in cultured cells (2, 37) but not in immunocompetent animals (15). Mice infected with reovirus develop endocrine abnormalities, including growth hormone deficiency (23), hypothyroidism (19), and diabetes mellitus (20, 23).
After passage in primary cultures of pancreatic β cells, each of the three reovirus serotypes can infect the murine pancreas (20, 22, 23). Newborn SJL/J mice infected with reovirus develop an acute inflammatory response within pancreatic islets, and viral antigen can be detected by immunofluorescence in β cells (20, 23). Infected mice produce autoantibodies against a variety of endocrine tissues and hormones, including insulin (7, 23). Serum insulin levels decrease, and mice develop abnormalities in glucose homeostasis. However, in most cases, these abnormalities resolve within 6 weeks postinfection. Therefore, in SJL/J mice, reovirus does not evoke a host response that leads to autoimmune diabetes.
Reovirus strain T3A can infect NOD mice.
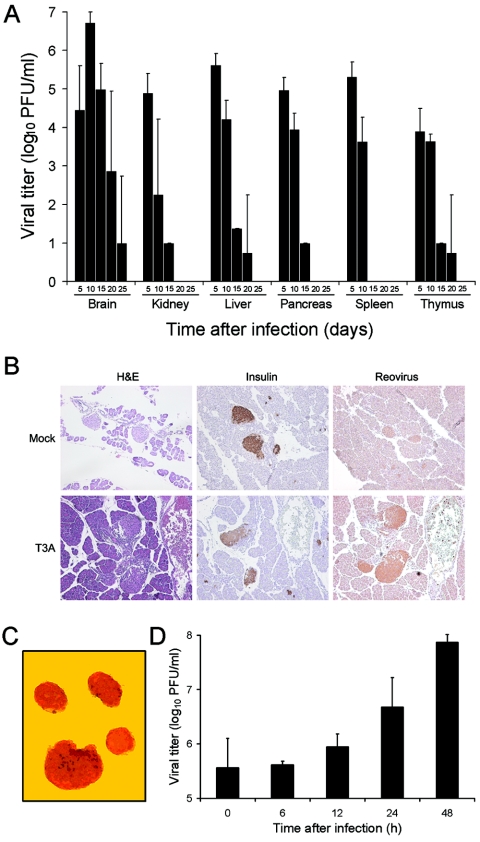
To determine whether reovirus alters the pathogenesis of autoimmune diabetes in a mouse model of the disease, we first tested whether reovirus can productively infect NOD mice. Two-day-old NOD mice, born to dams purchased from Taconic (Germantown, N.Y.), were inoculated intraperitoneally with 1 × 105 PFU of reovirus strain type 3 Abney (T3A) in a volume of 10 μl using a 30-gauge needle and a Hamilton syringe (BD Biosciences, San Jose, Calif.). Strain T3A is virulent in NIH SW mice, producing bile duct injury (39) and myocarditis (28). At days 5, 10, 15, 20, and 25 after inoculation, NOD mice were euthanized, and brain, kidney, liver, pancreas, spleen, and thymus were collected into 1.0 ml of gelatin-saline. Organs were homogenized by three cycles of freezing (−70°C) and thawing (37°C), followed by sonication, and virus titers in tissue homogenates were determined by plaque assay using mouse L929 cells (35). T3A was capable of infecting each of the organs examined, including the pancreas (Fig. 1A). Virus titers in most organs peaked at 5 days after inoculation and became undetectable in all organs tested except the brain by 25 days after inoculation. These findings show that NOD mice are permissive hosts for reovirus infection.
FIG. 1.
Reovirus infects pancreatic β cells of NOD mice. (A) Virus titers in brain, kidney, liver, pancreas, spleen, and thymus following inoculation of NOD mice with T3A. Two-day-old NOD mice were inoculated intraperitoneally with 1 × 105 PFU of T3A. Mice were euthanized at the times shown, organs were collected, and virus titers in tissue homogenates were determined by plaque assay. Each time point represents the mean for two to four animals. Error bars indicate standard deviations. (B) Presence of viral antigen in sections of pancreatic tissue dissected from T3A-infected NOD mice. Two-day-old mice were inoculated intraperitoneally with either 1 × 105 PFU of T3A or phosphate-buffered saline (mock). Pancreatic tissue was collected 8 days after inoculation, and serial sections were stained with hematoxylin and eosin (H&E), insulin-specific antiserum, or reovirus-specific antiserum. Antibody-stained sections were counterstained with hematoxylin. Brown staining indicates insulin or reovirus protein. Original magnifications, ×100. (C) Pancreatic islets were prepared from NOD mice and incubated with dithizone, which stains endocrine cells. Shown is a representative field of view. Original magnification, ×100. (D) Growth of reovirus strain T3A in primary cultures of pancreatic islets. Pancreatic islets were prepared from NOD mice and inoculated with 10 PFU per cell of T3A. After incubation at 37°C for the times shown, virus titers in islet homogenates were determined by plaque assay. The results are presented as the means of 2 to 10 independent experiments. Error bars indicate standard deviations.
We next used immunohistochemistry to localize reovirus antigen in the pancreas of animals infected with T3A. Two-day-old mice were inoculated intraperitoneally with 1 × 105 PFU of T3A, and pancreatic tissue was collected 8 days after inoculation. Tissues were fixed in 10% buffered formalin, equilibrated in 70% ethanol, embedded in paraffin blocks, and sectioned using a microtome. Viral antigen was detected by using a 1:600 dilution of a polyclonal reovirus-specific antiserum (37) and an immunoperoxidase staining assay (DakoCytomation, Carpinteria, CA). Sections without primary antibody served as negative controls. Immunostaining of the pancreas from infected animals but not mock-infected controls showed viral antigen in regions that also stain for insulin and exhibit islet morphology (Fig. 1B). Thus, T3A infects pancreatic islets of NOD mice.
To directly test whether T3A can productively infect pancreatic islet cells, primary cultures of pancreatic islets were prepared from NOD mice by collagenase treatment of pancreatic tissue (36) and infected with 10 PFU per cell of T3A. Islets prepared using this technique contain <15% acinar cells (36) (Fig. 1C). After virus adsorption at 4°C for 1 h, the inoculum was removed, fresh medium was added, and the islets were incubated at 37°C for 0, 6, 12, 24, and 48 h. Cells were frozen and thawed three times, and virus titers in cell lysates were determined by plaque assay (35). Yields of T3A in islet cultures were approximately 20-fold at 24 h and 100-fold at 48 h (Fig. 1D). These findings corroborate results obtained using immunoperoxidase staining of pancreatic tissue from NOD mice infected with reovirus. Furthermore, since the majority of explanted islet cells are β cells (36), these results suggest that reovirus strain T3A infects pancreatic β cells in NOD mice.
Reovirus strain T3A inhibits diabetes in infected NOD mice.
To determine whether infection with T3A during the newborn period alters the development of diabetes in NOD mice, 2-day-old animals were inoculated intraperitoneally with 1 × 105 PFU of T3A in a volume of 10 μl. Control animals were inoculated with an identical volume of an equivalent dilution of a cell lysate prepared from uninfected L929 cells. Glucose homeostasis in infected and control mice was monitored for 30 weeks by weekly assessment of glycosuria, determined by dipstick analysis (Roche, Indianapolis, Ind.) of freshly voided urine. Two consecutive findings of glycosuria at a 1-week interval established the diagnosis of diabetes. Since the penetrance of diabetes in female NOD mice is significantly greater than in males (13, 25), the analysis was restricted to females. Two experiments (I and II), spaced 1 year apart, were performed using independent groups of T3A-infected and mock-infected mice (Table 1). In experiment I, 7 of 8 mock-infected animals and 3 of 14 T3A-infected animals developed diabetes during the 30-week observation period. In experiment II, 19 of 23 mock-infected animals and 11 of 17 T3A-infected animals developed diabetes during the observation interval.
TABLE 1.
Development of diabetes in NOD mice infected with reovirus strain T3Aa
| Inoculum | No. of mice with diabetes/no. tested (%) in expt
|
|
|---|---|---|
| I | II | |
| Control | 7/8 (88) | 19/23 (83) |
| T3A | 3/14 (21) | 11/17 (65) |
Two-day-old NOD mice were inoculated intraperitoneally with 1 × 105 PFU of T3A; control animals were inoculated intraperitoneally with an equivalent volume of L929 cell lysate diluted in pyrogen-free normal saline. Glucose homeostasis in infected and control mice was monitored for 30 weeks. Only female mice were included in the analysis.
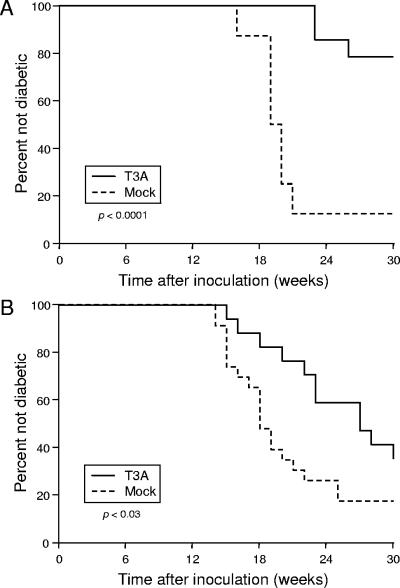
We used the log rank test to compare the development of diabetes in T3A-infected NOD mice versus that in mock-infected control animals (Fig. 2). The log rank test is a statistical method for comparing times until occurrence of an event among study groups. The null hypothesis of the log rank test is that similar event rates occur among study groups. Rejection of the null hypothesis indicates that the event rates differ among the groups at one or more time points during the study. Although the frequencies of diabetes among T3A-infected mice in the two experiments differed at the study end point, application of the log rank test indicates that, in both experiments, onset of diabetes in T3A-infected animals was significantly delayed in comparison to that in mock-infected animals (Fig. 2). It is possible that quantitative differences in diabetes blockade between the two experimental groups resulted from changes in animal husbandry in the interval between experiments or natural variation in the susceptibility of these animals to reovirus infection (note the large standard deviations in Fig. 1A). Interexperimental differences aside, these results indicate that infection with reovirus T3A inhibits the development of diabetes in NOD mice.
FIG. 2.
Development of diabetes in female NOD mice infected with T3A. Two-day-old NOD mice were inoculated intraperitoneally with either 1 × 105 PFU of T3A or an equivalent dilution of L929 cell lysate in a volume of 10 μl. Glucose homeostasis in infected and control female mice was monitored for 30 weeks by weekly assessment of glycosuria. The diagnosis of diabetes was established by two consecutive findings of glycosuria at a 1-week interval. P values were determined by using the log rank test. (A) Experiment I. (B) Experiment II.
Reovirus strain T3A does not prevent insulitis in infected NOD mice.
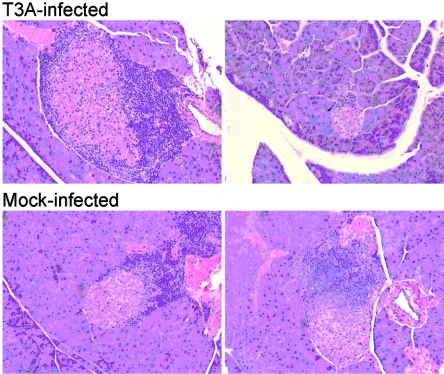
Destruction of pancreatic β cells leading to diabetes results from infiltration of pancreatic islets by autoreactive CD4+ T cells, which in turn recruit CD8+ T cells and macrophages, a process termed insulitis (5, 6, 24). To determine whether the delay in diabetes onset associated with T3A infection is associated with blockade of insulitis, infected and control mice that had not developed glycosuria after 30 weeks of observation were euthanized and pancreatic tissue was resected and processed for histological analysis. Insulitis was evident in pancreatic tissue sections from both infected and uninfected animals (Fig. 3). Thus, the decrease in diabetes penetrance in NOD mice following reovirus infection is not associated with prevention of insulitis.
FIG. 3.
Histopathology in sections of pancreatic tissue dissected from NOD mice 30 weeks after reovirus T3A infection. Pancreatic tissue from infected and control animals that had not developed glycosuria after 30 weeks of observation was fixed, sectioned, and stained with hematoxylin and eosin. Shown are sections from independent animals. Original magnifications, ×200.
Reovirus and diabetes in NOD mice.
In this study, we show that reovirus infection of newborn NOD mice delays the onset of overt diabetes. Infection by reovirus in the newborn period may alter autoreactive lymphocyte populations, resulting in active or passive tolerance to β-cell antigens. Reovirus can infect the murine thymus (21) (Fig. 1A), and it is possible that infection at that site, or perhaps in the periphery, leads to removal of β-cell-specific lymphocytes. However, given that insulitis occurs in NOD mice infected with reovirus, we think it unlikely that passive tolerance alone explains the diabetes delay in these animals. Reovirus also can infect the murine pancreas (20, 22, 23), and our results suggest that reovirus is capable of infecting pancreatic β cells in NOD mice (Fig. 1B and D). Therefore, release of β-cell antigens as a by-product of reovirus infection may lead to active tolerance by induction of regulatory T cells. Of note, blockade of diabetes in NOD mice infected with encephalomyocarditis virus can be adoptively transferred to uninfected mice using splenocytes (8), suggesting that active tolerance is involved in some cases of virus-induced diabetes blockade in NOD mice. There is precedent for this idea in the NOD mouse model of autoimmune diabetes. Intravenous (10) or intrathymic (31) administration of glutamic acid decarboxylase to NOD mice prevents the development of diabetes, as does adoptive transfer of certain glutamic acid decarboxylase-specific T cells (11).
Similar to the effects of reovirus infection reported here, CVB infection of newborn NOD mice blocks the development of diabetes (32). However, in contrast to reovirus, CVB is incapable of infecting pancreatic islets in newborn NOD mice (32). Interestingly, CVB accelerates diabetes progression following infection of older NOD mice and is capable of infecting pancreatic islets in these animals (3). Since the capacity of mice to support systemic infection by reovirus declines rapidly with age (14, 30), it is not possible to determine whether reovirus infection of adult NOD mice alters diabetes onset. Even so, our findings, coupled with previous studies of CVB infections of NOD mice, suggest that the immunologic environment of newborn animals is conducive to attenuation of autoimmune β-cell attack following viral infection, although the precise mechanisms of this effect may differ among viruses.
It is not known whether virus-induced protection against autoimmune disease occurs in humans. However, it is noteworthy that the prevalence of type 1 diabetes is greater in more-industrialized countries with higher levels of sanitation (4, 9, 27, 40), suggesting that viral infections in childhood, perhaps acquired in the presence of maternal antibodies (41), protect against subsequent development of diabetes. Studies of viruses that block diabetes in NOD mice will enhance an understanding of mechanisms by which viral infections alter cytotoxic responses to β-cell antigens and contribute new information about the pathogenesis of autoimmune diabetes. Such information may establish a framework for intervention in persons at risk for development of diabetes prior to the onset of disease.
Acknowledgments
We thank Mary Ann Deathridge and the Vanderbilt University Medical Center Histopathology Laboratory for tissue embedding and sectioning, Pamela Wirth and the Vanderbilt Mouse Metabolic and Phenotyping Core for immunohistochemical staining, Bashar Shakhtour for statistical analysis, and Greg Hanley and Joan Richerson for expert veterinary care. We thank members of our laboratory for many helpful discussions and Luc Van Kaer and Bryan Youree for careful review of the manuscript.
This research was supported by Public Health Service awards T32 HL07751 (J.D.C.), T32 GM07347 (G.S.B. and S.E.R.), F31 GM17208 (M.M.-G.), and R01 AI38296, the National Science Foundation (E.S.B), the Vanderbilt University Research Council (E.S.B.), a Merit Review award from the VA Research Service (A.C.P.), and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt-Ingram Cancer Center, DK20593 for the Vanderbilt Diabetes Research and Training Center, and DK59637 for the Vanderbilt Mouse Metabolic and Phenotyping Core.
REFERENCES
- 1.DeBiasi, R., C. Edelstein, B. Sherry, and K. Tyler. 2001. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J. Virol. 75:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dermody, T. S., M. L. Nibert, J. D. Wetzel, X. Tong, and B. N. Fields. 1993. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J. Virol. 67:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drescher, K. M., K. Kono, S. Bopegamage, S. D. Carson, and S. Tracy. 2004. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381-394. [DOI] [PubMed] [Google Scholar]
- 4.EURODIAB ACE Study Group. 2000. Variation and trends in incidence of childhood diabetes in Europe. Lancet 355:873-876. [PubMed] [Google Scholar]
- 5.Gepts, W. 1965. Pathology and anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14:619-633. [DOI] [PubMed] [Google Scholar]
- 6.Hanninen, A., S. Jalkanen, M. Salmi, S. Toikkanen, G. Nikolakaros, and O. Simell. 1992. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J. Clin. Investig. 90:1901-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haspel, M. V., T. Onodera, B. S. Prabhakar, M. Horita, H. Suzuki, and A. L. Notkins. 1983. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science 220:304-306. [DOI] [PubMed] [Google Scholar]
- 8.Hermitte, L., B. Vialettes, P. Naquet, C. Atlan, M.-J. Payan, and P. Vague. 1990. Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur. J. Immunol. 20:1297-1303. [DOI] [PubMed] [Google Scholar]
- 9.Karvonen, M., M. Viik-Kajander, E. Moltchanova, I. Libman, R. LaPorte, J. Tuomilehto, et al. 2000. Incidence of childhood type 1 diabetes worldwide. Diabetes Care 23:1516-1526. [DOI] [PubMed] [Google Scholar]
- 10.Kauffman, D. L., M. J. Clare-Salzler, J. Tian, T. Forsthuber, G. S. P. Ting, P. Robinson, M. A. Atkinson, E. E. Seroarz, A. J. Tobin, and P. V. Lehman. 1993. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366:69-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, S. K., K. V. Tarbell, M. Sanna, M. Vadeboncoeur, T. Warganich, M. Lee, M. Davis, and H. O. McDevitt. 2004. Prevention of type I diabetes transfer by glutamic acid decarboxylase 65 peptide 206-220-specific T cells. Proc. Natl. Acad. Sci. USA 101:14204-14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knip, M., and H. K. Akerblom. 1999. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107(Suppl. 3):S93-S100. [DOI] [PubMed] [Google Scholar]
- 13.Makino, S., K. Kunimoto, Y. Muraoka, Y. Mizushima, K. Katagiri, and Y. Tochino. 1980. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 29:1-13. [DOI] [PubMed] [Google Scholar]
- 14.Mann, M. A., D. M. Knipe, G. D. Fischbach, and B. N. Fields. 2002. Type 3 reovirus neuroinvasion after intramuscular inoculation: direct invasion of nerve terminals and age-dependent pathogenesis. Virology 303:222-231. [DOI] [PubMed] [Google Scholar]
- 15.Morrison, L. A., B. N. Fields, and T. S. Dermody. 1993. Prolonged replication in the mouse central nervous system of reoviruses isolated from persistently infected cultures. J. Virol. 67:3019-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nibert, M. L., and L. A. Schiff. 2001. Reoviruses and their replication, p. 1679-1728. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Oberhaus, S. M., R. L. Smith, G. H. Clayton, T. S. Dermody, and K. L. Tyler. 1997. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J. Virol. 71:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldstone, M. B. 1988. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science 239:500-501. [DOI] [PubMed] [Google Scholar]
- 19.Onodera, T., and A. Awaya. 1990. Anti-thyroglobulin antibodies induced with recombinant reovirus infection in BALB/c mice. Immunology 71:581-585. [PMC free article] [PubMed] [Google Scholar]
- 20.Onodera, T., A. B. Jenson, J.-W. Yoon, and A. L. Notkins. 1978. Virus-induced diabetes mellitus: reovirus infection of pancreatic beta cells in mice. Science 201:529-531. [DOI] [PubMed] [Google Scholar]
- 21.Onodera, T., T. Taniguchi, T. Tsuda, et al. 1991. Thymic atrophy in type 2 reovirus infected mice: immunosuppression and effects of thymic hormone. Thymic atrophy caused by reo-2. Thymus 18:95-109. [PubMed] [Google Scholar]
- 22.Onodera, T., T. Taniguchi, K. Yoshihara, S. Shimizu, M. Sato, and T. Hayashi. 1990. Reovirus type 2-induced diabetes in mice prevented by immunosuppression and thymic hormone. Diabetologia 33:192-196. [DOI] [PubMed] [Google Scholar]
- 23.Onodera, T., A. Toniolo, U. R. Ray, A. B. Jenson, R. A. Knazek, and A. L. Notkins. 1981. Virus-induced diabetes mellitus. XX. Polyendocrinopathy and autoimmunity. J. Exp. Med. 153:1457-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pipeleers, D., and Z. Ling. 1992. Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab. Rev. 8:209-227. [DOI] [PubMed] [Google Scholar]
- 25.Prochazka, M., D. H. Leiter, D. V. Serreze, and D. L. Coleman. 1987. Three recessive loci required for insulin-dependent diabetes in nonobese diabetic mice. Science 237:286-289. [DOI] [PubMed] [Google Scholar]
- 26.Robles, D. T., and G. S. Eisenbarth. 2001. Type 1A diabetes induced by infection and immunization. J. Autoimmun. 16:355-362. [DOI] [PubMed] [Google Scholar]
- 27.Schober, E., B. Rami, and T. Waldhoer. 2003. Small area variation in childhood diabetes mellitus in Austria: links to population density, 1989 to 1999. J. Clin. Epidemiol. 56:269-273. [DOI] [PubMed] [Google Scholar]
- 28.Sherry, B., and M. A. Blum. 1994. Multiple viral core proteins are determinants of reovirus-induced acute myocarditis. J. Virol. 68:8461-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takei, I., Y. Asaba, T. Kasatani, T. Maruyana, K. Watanabe, T. Yanagawa, T. Saruta, and T. Ishii. 1992. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J. Autoimmun. 5:665-673. [DOI] [PubMed] [Google Scholar]
- 30.Tardieu, M., M. L. Powers, and H. L. Weiner. 1983. Age-dependent susceptibility to reovirus type 3 encephalitis: role of viral and host factors. Ann. Neurol. 13:602-607. [DOI] [PubMed] [Google Scholar]
- 31.Tisch, R., Y. D. Yang, S. M. Singer, R. S. Libiau, L. Fugger, and H. O. McDevitt. 1993. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366:72-75. [DOI] [PubMed] [Google Scholar]
- 32.Tracy, S., K. M. Drescher, N. M. Chapman, K. S. Kim, S. D. Carson, S. Pirruccello, P. H. Lane, J. R. Romero, and J. S. Leser. 2002. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76:12097-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyler, K. L. 2001. Mammalian reoviruses, p. 1729-1745. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 34.Tyler, K. L., M. K. Squier, S. E. Rodgers, S. E. Schneider, S. M. Oberhaus, T. A. Grdina, J. J. Cohen, and T. S. Dermody. 1995. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J. Virol. 69:6972-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virgin, H. W., IV, R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, T. G., I. Lacik, M. Brissova, A. Prokop, D. Hunkeler, A. V. Anilkumar, R. Green, K. Shahroki, and A. C. Powers. 1997. An encapsulation system for the immunoisolation of pancreatic islets. Nat. Biotechnol. 15:358-362. [DOI] [PubMed] [Google Scholar]
- 37.Wetzel, J. D., J. D. Chappell, A. B. Fogo, and T. S. Dermody. 1997. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J. Virol. 71:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilberz, S., H. J. Partke, F. Dagnaes-Hansen, and L. Herberg. 1991. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34:2-5. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, G. A., L. A. Morrison, and B. N. Fields. 1994. Association of the reovirus S1 gene with serotype 3-induced biliary atresia in mice. J. Virol. 68:6458-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, Z., K. Wang, T. Li, W. Sun, Y. Li, Y. F. Chang, J. S. Dorman, and R. E. LaPorte. 1998. Childhood diabetes in China. Enormous variation by place and ethnic group. Diabetes Care 21:525-529. [DOI] [PubMed] [Google Scholar]
- 41.Zinkernagel, R. M. 2001. Maternal antibodies, childhood infections, and autoimmune diseases. N. Engl. J. Med. 345:1331-1335. [DOI] [PubMed] [Google Scholar]