Abstract
A viral CD200 homologue (vCD200) encoded by open reading frame R15 of rhesus rhadinovirus (RRV), a gammaherpesvirus closely related to human herpesvirus 8 (HHV-8), is described here. RRV vCD200 shares 30% and 28% amino acid identity with human CD200 (huCD200) and HHV-8 vCD200, respectively. In vitro analysis indicated that an Fc fusion (vCD200-Fc) is expressed as a glycoprotein with a core molecular mass of 53 kDa. Utilizing monoclonal antibodies raised against vCD200-Fc, vCD200 expression was detected on the surfaces of and within supernatants from infected fibroblasts. Furthermore, in vitro assays demonstrated that vCD200-Fc treatment of monocyte-derived macrophages reduces tumor necrosis factor transcript and protein levels, implying that RRV encodes a functional vCD200.
Human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus (HHV-8/KSHV) is associated with the development of Kaposi's sarcoma (2, 22), multicentric Castleman's disease (18, 25), and primary effusion lymphoma (6, 12, 13, 16, 17, 19) in AIDS patients. A close relative to HHV-8, rhesus macaque rhadinovirus strain 17577 (RRV17577) was isolated from a simian immunodeficiency virus (SIV)-infected rhesus macaque displaying lymphoproliferative disease. Sequence analysis of RRV revealed colinearity of these two genomes, and 67 of 79 RRV open reading frames (ORFs) are similar to those in HHV-8 (23). Additionally, experimental infection of SIV-infected rhesus macaques with RRV17577 results in B-cell hyperplasia and a disease resembling multicentric Castleman's disease, which is often observed in HHV-8+ AIDS patients (3, 27), suggesting that this may serve as a good animal model for some aspects of human immunodeficiency virus/HHV-8 disease development.
RRV proteins likely promoting viral pathogenesis include a viral interleukin-6 homologue and a viral G-protein-coupled receptor, which behave similarly to their counterparts in HHV-8, supporting B-cell proliferation (15) and promoting cellular transformation (10), respectively. R1 of RRV has also been analyzed and was determined to promote transformation and lymphocyte activation (8, 9). Another RRV ORF that likely promotes viral pathogenesis is R15, which encodes a homologue of HHV-8 K14 and human CD200. Human CD200 is a glycoprotein found on the surfaces of many cell types (1, 4, 26) that binds to its receptor, CD200R, which is largely restricted to the surfaces of cells of myeloid lineage (28) and reduces the expression of TH1 cytokines such as tumor necrosis factor (TNF) (14, 21).
Originally, the product of HHV-8 K14, HHV-8 vCD200, was reported to have opposite functions from those of human CD200 (7); however, a more recent study confirmed the function of HHV-8 vCD200 to be similar to that of human CD200 in reducing the secretion of TH1 cytokines from myeloid cells (11). Foster-Cuevas et al. also demonstrated HHV-8 vCD200 expression on the surfaces of BCBL-1 cells following lytic cycle induction (11). Myxomavirus M141R-encoded vCD200 has also been investigated and found to be associated with virulence in infected rabbits (5).
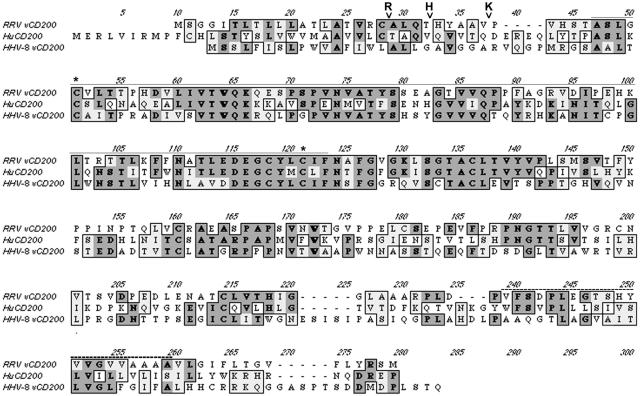
Here we provide a primary characterization of RRV vCD200. At the amino acid sequence level, RRV vCD200 is 30% and 28% identical to human CD200 and HHV-8 vCD200, respectively (Fig. 1). Despite their low sequence identities, the structural organizations of these three proteins are very similar, with all three proteins containing V-like immunoglobulin (Ig) domains.
FIG. 1.
ClustalW protein alignments of huCD200 and the vCD200 homologues RRV vCD200 and HHV-8 vCD200. The huCD200 V-like Ig domain is noted with a fine solid line above the sequences, and the transmembrane domain of huCD200 is noted with a dashed line above the sequences. Cysteine residues important for disulfide bond formation within Ig domains are noted with asterisks. Signal peptide cleavage sites are noted with v's, along with “R” for RRV vCD200, “H” for huCD200, and “K” for HHV-8 vCD200.
To assess its function, the predicted extracellular domain of vCD200 (amino acids 1 to 228) was amplified from RRV17577 (forward EcoRI primer, 5′-GAATTCTCAATTATGTCGGGAGGAA-3′; reverse BstII primer, 5′-GGTGACCGCGTAGTGGCTCGTCC-3′) and fused in frame with the Fc fragment of human IgG1 (Fig. 2A). vCD200-Fc was purified from supernatants of transfected Chinese hamster ovary (CHO) cells (Transit LT1; Mirus, Madison, WI) through binding to protein G-Sepharose 4 (Amersham Biosciences, Piscataway, NJ) and was eluted at a low pH. This purification scheme was also employed for the purification of huCD200-Fc (data not shown).
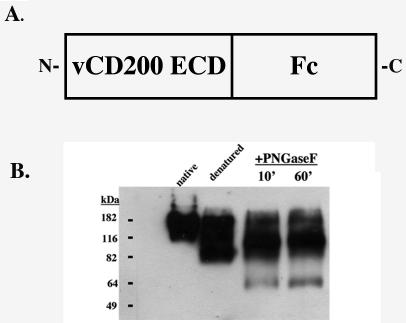
FIG. 2.
Schematic of RRV vCD200-Fc. (A) The predicted extracellular domain (ECD) of vCD200 is fused in frame with an Fc fragment at its C terminus. (B) Purified vCD200-Fc samples were run under native and denaturing conditions to determine if vCD200-Fc can form dimers (lanes 1 and 2). Treatment of the native protein with peptide N-glycosidase F revealed that vCD200-Fc is N-glycosylated, as the core protein size (approximately 53 kDa) was revealed following treatment (lanes 3 and 4).
Purified vCD200-Fc was subjected to Western blot analysis with an Fc-specific antibody (Sigma, St. Louis, MO) to confirm the size of the fusion protein and that the Fc fragment of the fusion protein allows for dimerization of vCD200-Fc, which has been demonstrated to be critical for maintaining the function of soluble CD200 molecules (11). Dimerization of RRV vCD200-Fc was achieved, as observed from a comparison of samples run under native and denaturing conditions (Fig. 2B, lanes 1 and 2). huCD200-Fc also exists as a dimer in our hands (data not shown). RRV vCD200-Fc has a predicted molecular mass of approximately 53 kDa, which can be visualized through treatment with peptide N-glycosidase F (New England Biolabs, Beverly, MA) (Fig. 2B, lanes 3 and 4).
To facilitate expression analysis, vCD200-specific monoclonal antibodies were generated and shown to react with CHO cells transfected with a full-length vCD200 expression vector by an immunofluorescence assay (Fig. 3A). These antibodies also reacted with secreted vCD200 protein that was FLAG tagged for immunoprecipitation. In this assay, supernatants from CHO cells transfected with FLAG-tagged vCD200 or vector only were first immunoprecipitated with an anti-FLAG antibody and then separated for Western blot analysis with the monoclonal antibody 11B8 for detection (Fig. 3B).
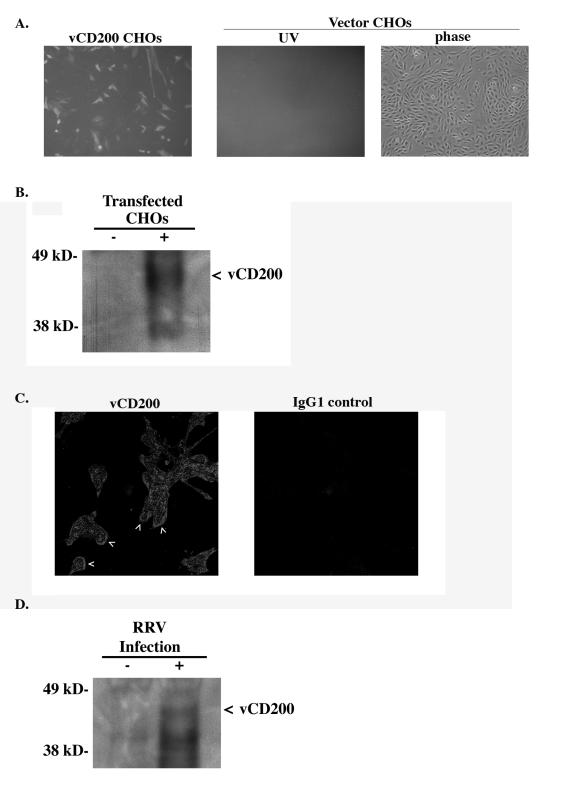
FIG. 3.
Expression analysis of vCD200. (A) vCD200 monoclonal antibodies (11B8) specifically detect the expression of vCD200 in CHO cells transfected with a full-length vCD200 expression vector and not in cells transfected with vector only. (B) Western blot analysis of vCD200 (arrowhead) from supernatants of CHO cells transfected with a FLAG-tagged vCD200 expression vector (+) or control vector (−), immunoprecipitated with an anti-FLAG antibody, and probed with antibody 11B8. (C) Specific staining of vCD200 was observed on the surfaces (arrowheads) of infected cells at 52 h postinfection. No specific staining was observed when infected cells were incubated with the isotype control antibody. Images represent an area of 281 μm by 281 μm. (D) Immunoprecipitation of vCD200 (arrowhead) from supernatants of RRV-infected fibroblasts at 72 h postinfection (+) or of uninfected cells (−).
Since we have previously demonstrated that R15, the ORF encoding vCD200, is transcribed as a late gene (20), RRV-infected cells (multiplicity of infection, 2.0) were fixed at 52 h postinfection with paraformaldehyde and stained with either monoclonal antibody 11B8 or an isotype control antibody (mouse IgG1 clone 11711; R&D Systems, Minneapolis, MN). Imaging of stained cells by confocal microscopy revealed specific vCD200 staining on the surfaces of infected cells and within the cytoplasm (Fig. 3C), which is consistent with our earlier report that vCD200 is expressed on the cell surface and present in vesicles destined for secretion or surface expression (20).
To demonstrate that vCD200 is secreted from infected cells, supernatants from infected fibroblasts were collected at 72 h postinfection, pelleted to remove cellular debris, filtered through a 0.22-μm filter, and concentrated for immunoprecipitation with monoclonal antibody 13D2. Immunoprecipitates from infected and noninfected cells were subsequently separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blot analysis with 11B8 antibody. As shown in Fig. 3D, supernatants from infected cells contained vCD200, supporting our earlier observation that the protein is secreted.
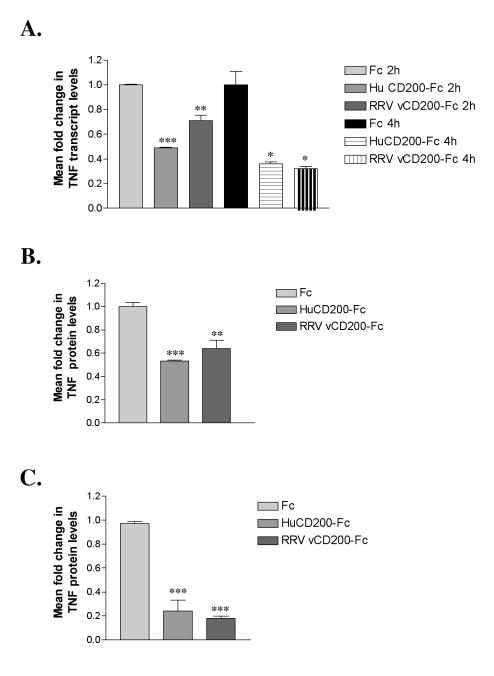
To examine the function of RRV vCD200-Fc, phorbol myristate acetate-treated (160 nM; Sigma) THP-1 monocyte-derived macrophages were incubated with a mixture of gamma interferon (100 U/ml; R&D Systems) and Fc proteins (90 pM) for various amounts of time. Specifically, cells were incubated with Fc alone (Chemicon, Temecula, CA), human CD200-Fc, or RRV vCD200-Fc. Two and 4 h following incubation, RNAs were harvested from cells (Tri reagent; Sigma) to detect changes in expression levels of transcripts encoding the TH1 cytokine TNF. Should RRV vCD200-Fc behave as a true homologue of CD200, levels of TNF message would be reduced compared to those in cells treated with Fc alone. Utilizing quantitative real-time reverse transcription-PCR (qRT-PCR) with cDNAs generated from THP-1 RNAs (Superscript II reverse transcriptase; Invitrogen Life Technologies, Carlsbad, CA), we observed 30% and 68% reductions in TNF transcripts from THP-1 cells that were incubated with RRV vCD200-Fc for 2 h and 4 h, respectively, compared with cells treated with Fc alone (Fig. 4A). Initially, the reduction in TNF message was highest for cells treated with human CD200-Fc (51% reduction); however, by 4 h, the levels of down regulation as a result of RRV vCD200-Fc treatment were similar to those resulting from human CD200-Fc treatment (64% reduction) (Fig. 4A). Next, we examined the effect of CD200 treatment on TNF protein production from THP-1 cells by using a TNF-specific enzyme-linked immunosorbent assay (ELISA; BD Pharmingen, San Diego, CA). Twenty-two hours following the addition of Fc proteins, THP-1 cells incubated with RRV vCD200-Fc displayed a 36% reduction in TNF levels, and cells incubated with human CD200-Fc displayed a 47% decrease in secreted TNF levels compared to Fc controls (Fig. 4B).
FIG. 4.
RRV vCD200-Fc down regulates TNF expression in monocyte-derived macrophages. (A) qRT-PCR to examine levels of TNF transcripts 2 and 4 h following incubation of gamma interferon-treated THP-1 cells with Fc proteins (Fc alone, HuCD200-Fc, or RRV vCD200-Fc). (B) ELISA to measure levels of secreted TNF in THP-1 cell supernatants 22 h following incubation with Fc proteins. Results shown are means normalized from three experiments (plus standard errors of the means). (C) TNF ELISA with primary rhesus monocyte-derived macrophage culture supernatants. Following a 4-hour incubation with Fc proteins, supernatants were assayed for TNF levels. Results shown are means normalized from four animals (plus standard errors of the means). *, significant difference compared with Fc treatment (P < 0.05); **, very significant difference compared with Fc treatment (P < 0.01); ***, extremely significant difference compared with Fc treatment (P < 0.001). No significant differences were detected between treatments with huCD200-Fc and RRV vCD200-Fc.
To examine the effect of RRV vCD200-Fc on a more native cell type, primary rhesus monocyte-derived macrophage cultures were prepared from peripheral blood mononuclear cells obtained from four specific-pathogen-free animals that were naïve for RRV infection (24). Primary rhesus monocyte-derived macrophage cultures incubated with RRV vCD200-Fc for 4 h secreted 12% of the TNF detected in the supernatants of cells treated with Fc alone (Fig. 4C). Supernatants from human CD200-Fc-treated cells contained 29% of the amount of TNF secreted from control cells (Fig. 4C).
Since RRV vCD200 shares an immunomodulatory function with HHV-8 vCD200, the role of this viral protein in promoting pathogenesis can be elucidated in a closely related animal model, the RRV/SIV-infected rhesus macaque, through the use of a virus lacking vCD200.
Acknowledgments
This work was supported by NIH grants CA75922 and RR00163.
We acknowledge D. Cawley for the production of vCD200 monoclonal antibodies. We are grateful for technical assistance with qRT-PCR experiments and data analysis from the laboratory of M. Iordanov. We also thank A. N. Barclay for a plasmid containing the human CD200 sequence and K. Langlais for technical assistance with confocal microscopy.
REFERENCES
- 1.Barclay, A. N. 1981. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology 44:727-736. [PMC free article] [PubMed] [Google Scholar]
- 2.Beral, V., T. A. Peterman, R. L. Berkelman, and H. W. Jaffe. 1990. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 335:123-128. [DOI] [PubMed] [Google Scholar]
- 3.Bergquam, E. P., N. Avery, S. M. Shiigi, M. K. Axthelm, and S. W. Wong. 1999. Rhesus rhadinovirus establishes a latent infection in B lymphocytes in vivo. J. Virol. 73:7874-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukovsky, A., J. Presl, J. Zidovsky, and P. Mancal. 1983. The localization of Thy-1.1, MRC OX 2 and Ia antigens in the rat ovary and fallopian tube. Immunology 48:587-596. [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron, C. M., J. W. Barrett, L. Liu, A. R. Lucas, and G. McFadden. 2005. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J. Virol. 79:6052-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone, A., U. Tirelli, A. Gloghini, C. Pastore, E. Vaccher, and G. Gaidano. 1996. Herpesvirus-like DNA sequences selectively cluster with body cavity-based lymphomas throughout the spectrum of AIDS-related lymphomatous effusions. Eur. J. Cancer 32A:555-556. [DOI] [PubMed] [Google Scholar]
- 7.Chung, Y. H., R. E. Means, J. K. Choi, B. S. Lee, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J. Virol. 76:4688-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damania, B., M. DeMaria, J. U. Jung, and R. C. Desrosiers. 2000. Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus. J. Virol. 74:2721-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damania, B., M. Li, J. K. Choi, L. Alexander, J. U. Jung, and R. C. Desrosiers. 1999. Identification of the R1 oncogene and its protein product from the rhadinovirus of rhesus monkeys. J. Virol. 73:5123-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estep, R. D., M. K. Axthelm, and S. W. Wong. 2003. A G protein-coupled receptor encoded by rhesus rhadinovirus is similar to ORF74 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 77:1738-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster-Cuevas, M., G. J. Wright, M. J. Puklavec, M. H. Brown, and A. N. Barclay. 2004. Human herpesvirus 8 K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J. Virol. 78:7667-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaidano, G., C. Pastore, A. Gloghini, M. Cusini, J. Nomdedeu, G. Volpe, D. Capello, E. Vaccher, R. Bordes, U. Tirelli, G. Saglio, and A. Carbone. 1996. Distribution of human herpesvirus-8 sequences throughout the spectrum of AIDS-related neoplasia. AIDS 10:941-949. [DOI] [PubMed] [Google Scholar]
- 13.Gessain, A., J. Briere, C. Angelin-Duclos, F. Valensi, H. M. Beral, F. Davi, M. A. Nicola, A. Sudaka, N. Fouchard, J. Gabarre, X. Troussard, E. Dulmet, J. Audouin, J. Diebold, and G. de The. 1997. Human herpes virus 8 (Kaposi's sarcoma herpes virus) and malignant lymphoproliferations in France: a molecular study of 250 cases including two AIDS-associated body cavity based lymphomas. Leukemia 11:266-272. [DOI] [PubMed] [Google Scholar]
- 14.Hoek, R. M., S. R. Ruuls, C. A. Murphy, G. J. Wright, R. Goddard, S. M. Zurawski, B. Blom, M. E. Homola, W. J. Streit, M. H. Brown, A. N. Barclay, and J. D. Sedgwick. 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290:1768-1771. [DOI] [PubMed] [Google Scholar]
- 15.Kaleeba, J. A., E. P. Bergquam, and S. W. Wong. 1999. A rhesus macaque rhadinovirus related to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J. Virol. 73:6177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karcher, D. S., and S. Alkan. 1997. Human herpesvirus-8-associated body cavity-based lymphoma in human immunodeficiency virus-infected patients: a unique B-cell neoplasm. Hum. Pathol. 28:801-808. [DOI] [PubMed] [Google Scholar]
- 17.Nador, R. G., E. Cesarman, A. Chadburn, D. B. Dawson, M. Q. Ansari, J. Sald, and D. M. Knowles. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645-656. [PubMed] [Google Scholar]
- 18.Oksenhendler, E., M. Duarte, J. Soulier, P. Cacoub, Y. Welker, J. Cadranel, D. Cazals-Hatem, B. Autran, J. P. Clauvel, and M. Raphael. 1996. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS 10:61-67. [PubMed] [Google Scholar]
- 19.Otsuki, T., S. Kumar, B. Ensoli, D. W. Kingma, T. Yano, M. Stetler-Stevenson, E. S. Jaffe, and M. Raffeld. 1996. Detection of HHV-8/KSHV DNA sequences in AIDS-associated extranodal lymphoid malignancies. Leukemia 10:1358-1362. [PubMed] [Google Scholar]
- 20.Pratt, C. L., R. D. Estep, and S. W. Wong. 2005. Splicing of rhesus rhadinovirus R15 and ORF74 bicistronic transcripts during lytic infection and analysis of effects on production of vCD200 and vGPCR. J. Virol. 79:3878-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston, S., G. J. Wright, K. Starr, A. N. Barclay, and M. H. Brown. 1997. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur. J. Immunol. 27:1911-1918. [DOI] [PubMed] [Google Scholar]
- 22.Rabkin, C. S., S. Janz, A. Lash, A. E. Coleman, E. Musaba, L. Liotta, R. J. Biggar, and Z. Zhuang. 1997. Monoclonal origin of multicentric Kaposi's sarcoma lesions. N. Engl. J. Med. 336:988-993. [DOI] [PubMed] [Google Scholar]
- 23.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Investig. 100:3154-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 26.Webb, M., and A. N. Barclay. 1984. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J. Neurochem. 43:1061-1067. [DOI] [PubMed] [Google Scholar]
- 27.Wong, S. W., E. P. Bergquam, R. M. Swanson, F. W. Lee, S. M. Shiigi, N. A. Avery, J. W. Fanton, and M. K. Axthelm. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright, G. J., M. J. Puklavec, A. C. Willis, R. M. Hoek, J. D. Sedgwick, M. H. Brown, and A. N. Barclay. 2000. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13:233-242. [DOI] [PubMed] [Google Scholar]