Abstract
Herpes simplex virus (HSV) infections of humans are characterized by intermittent, lytic replication in epithelia. Circulating HSV-specific CD4 T cells express lower levels of preformed cutaneous lymphocyte-associated antigen (CLA), a skin-homing receptor, than do circulating HSV-specific CD8 T cells but, paradoxically, move into infected skin earlier than CD8 cells. Memory CD4 T cells develop strong and selective expression of CLA and E-selectin ligand while responding to HSV antigen in vitro. We now show that interleukin-12, type I interferon, and transforming growth factor beta are each involved in CLA expression by memory HSV type 2 (HSV-2)-specific CD4 T cells in peripheral blood mononuclear cells (PBMC). A reduction of the number of monocytes and dendritic cells from PBMC reduces CLA expression by HSV-2-responsive CD4 lymphoblasts, while their reintroduction restores this phenotype, identifying these cells as possible sources of CLA-promoting cytokines. Plasmacytoid dendritic cells are particularly potent inducers of CLA on HSV-reactive CD4 T cells. These observations are consistent with cooperation between innate and acquired immunity to promote a pattern of homing receptor expression that is physiologically appropriate for trafficking to infected tissues.
T-cell homing to skin is the subject of intense investigation. E-selectin expression by skin venular endothelial cells is increased during inflammation (49). A minority (5 to 10%) of circulating memory T cells express a ligand for E-selectin (ESL) (57). The E-selectin-ESL interaction promotes rolling adhesion of circulating lymphocytes to dermal endothelium. This is followed by tight adhesion and chemotaxis, processes involving integrins, chemokines, and their lymphocyte receptors (8). Cutaneous lymphocyte-associated antigen (CLA) is an antibody-defined, fucose-containing structure that is associated with, although not identical to, ESL (57). The principal scaffold for CLA on lymphocytes is P-selectin glycoprotein ligand 1 (16). Both CLA and ESL are controlled by fucosyltransferase VII (FTVII), which is regulated at the transcriptional level (21). Some innate cytokines (type I interferon, interleukin-12 [IL-12], and transforming growth factor beta [TGF-β]) can increase CLA levels, while IL-4 and all-trans retinoic acid can reduce CLA expression (34, 45). Effects on cell surface CLA levels have been linked by mechanistic studies to upstream intracellular signaling and FTVII promoter control events (7, 59, 61). These modulatory influences are detected in the presence of simultaneous mitogenic stimulation through a T-cell receptor (TCR) surrogate (anti-CD3) or phytohemagglutinin (PHA), leading to models in which T cells integrate signals from the TCR and other receptors to control FTVII and CLA expression.
Herpes simplex virus type 2 (HSV-2) is a prevalent human pathogen that typically infects keratinized epithelia such as the skin. HSV-2-specific CD4 T cells are enriched within herpetic skin lesions by a factor of 100 to 1,000, compared to blood, as early as day 2 of symptoms (22, 23). These CD4 cells execute a variety of antiviral functions (24). HSV-2-specific CD8 cytotoxic T lymphocytes are also highly enriched in lesions, infiltrating slightly after CD4 cells (25). We have previously shown that the same HSV-2-specific CD4 and CD8 T-cell clonotypes are present in both blood and HSV-2 skin lesions (6, 47), supporting their active movement from blood to infected tissues.
Tetramer-positive HSV-2-specific CD8 T cells in peripheral blood express high levels (50 to 70%) of preformed CLA and ESL (26, 27). This finding was observed in persons infected for more than 1 year when sampled between recurrences or when subjects with no history of symptomatic disease, a very common situation (60), were sampled at random times. HSV-2-specific CD8 memory cells therefore express CLA and ESL in the absence of proximal temporal or spatial reexposure to antigen. In contrast, tetramer-positive cytomegalovirus (CMV)- and Epstein-Barr virus-specific CD8 T cells express little CLA or ESL, correlating with a lack of skin tropism for these infectious agents (26, 27). Despite considerable experience with HLA class II tetramers to detect HSV-2-specific T cells (13, 32, 43), we have not been able to use them to study CLA/ESL expression by HSV-2-specific CD4 T cells in fresh blood. This is likely due to the low abundance of CD4 cells reactive with individual HSV-2 peptides (33). To compare CD4 and CD8 T cells, we have therefore begun in vitro restimulation studies of HSV-2-specific CD4 T cells from subjects with chronic (>1 year) HSV-2 infections who were sampled between recurrent lesion episodes (17).
The expression of CLA by circulating HSV-2-specific CD4 T cells, identified in short-term (6-hour) ex vivo assays, was quite modest (about 20%). Some specificity was noted, as CMV-reactive cells in the same assays had lower levels of CLA (17). Remarkably, exposure to whole HSV-2 antigen for several days in culture led to significant up-regulation of CLA and ESL on the resultant dividing HSV-2-specific cells. This was not simply due to stimulation through the TCR in our specific media, as recall stimulation with CMV or influenza virus antigens did not lead to CLA or ESL expression. We excluded the possibility that a subset of memory HSV-2-specific CD4 T cells with preformed CLA were preferentially restimulated by exposure to HSV-2 antigen in vitro by completely depleting CLA+ CD4+ cells from the initial responder population. After 5 days of culture with HSV-2 antigen and appropriate antigen-presenting cells (APC), the responding cells again expressed high levels of CLA and ESL (17).
In this report, we investigate the mechanism of CLA expression induced on HSV-2-reactive CD4 T cells by HSV-2 antigen in vitro. We first determined the requirements for CLA induction in response to HSV-2 and investigated the transferability of this phenomenon in antigen-mixing experiments. Using blocking antibodies, we then investigated the effects of the CLA-inducing cytokines, i.e., IL-12, type I interferon, and TGF-β, during short-term restimulation of memory HSV-2-specific CD4 T cells. Recent data indicate that plasmacytoid dendritic cells (pDC) and monocytes react to contact with HSV by secreting IL-12 and type I interferon (37, 53), possibly through Toll-like receptor-dependent mechanisms (18, 41). We therefore compared defined APC in an in vitro model of the recall phase of the CD4 response to HSV-2.
(This work was presented in part at the 29th International Herpesvirus Workshop, Reno, Nev., July 2004.)
MATERIALS AND METHODS
Subjects and specimens.
Informed consent was obtained from human immunodeficiency virus type 1-seronegative adults. Some samples were obtained 2 to 4 weeks after intramuscular influenza vaccination. Samples were obtained during periods of no clinically active HSV-2 infection from persons with symptomatic HSV-2 infections of >1 year's duration. HSV-2 shedding decreases after 1 year of infection for unknown reasons (28). We chose subjects with established infections to reduce possible heterogeneity due to differing rates of maturation of the acquired immune response. Serology for CMV, HSV-1, and HSV-2 was performed as described previously (17, 55). Anticoagulated blood (with acid citrate dextrose or sodium heparin) or leukapheresis products were processed by Ficoll-Hypaque centrifugation, and peripheral blood mononuclear cells (PBMC) were cryopreserved in liquid nitrogen. Fresh heparin-anticoagulated blood was processed within 4 hours for pDC and monocyte isolation.
PBMC culture.
PBMC were thawed, typically labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) (17), counted, and plated at 1 × 106/well in 48-well plates. T-cell medium (TCM) was 0.6 to 1.0 ml/well RPMI 1640 with 25 mM HEPES (Invitrogen, Carlsbad, CA), 1% penicillin-streptomycin, 2 mM l-glutamine, and 10% pooled heat-inactivated (56°C, 30 min) human serum from random healthy adult donors. In some experiments, CFSE-labeled PBMC were antigen loaded in 1 ml TCM in conical-bottomed polypropylene tubes for 2 h at 37°C in humidified 5% CO2, washed thrice, and plated (106/well) in 48-well plates. Stimuli included PHA-P (1.6 μg/ml; Remel, Lenexa, KS) and CMV viral lysate (diluted 1:1,000; ABI, Columbia, MD). Influenza virus protein antigen from the 2002 vaccine (dialyzed with phosphate-buffered saline; Aventis, Swiftwater, PA) was used at a dilution of 1:1,000. HSV-2 strain 333 was grown, titrated, and UV irradiated as described previously (23); mock virus samples were uninfected Vero cells. HSV stocks had titers of 3 × 108 (HSV-2) or 3 × 109 (HSV-1) PFU/ml prior to inactivation. Goat polyclonal anti-human IL-12 (AF-219-NA; R&D Systems, Minneapolis, MN), normal goat immunoglobulin G (IgG) (AB-108-C; R&D), murine anti-human alpha/beta interferon receptor (MMHAR-2; Calbiochem, San Diego, CA), control IgG2a (401123; Calbiochem), murine anti-TGF-β (MAb1835; R&D), control IgG1 (MAb002; R&D), or human IL-4 (R&D) was added at culture initiation. Some cultures received 100 nM all-trans retinoic acid (Sigma) or 0.06 to 0.2 μg/ml of recombinant vaccinia virus B18R protein (eBioscience, San Diego, CA). Cultures were typically held for 5 days. Some assays were scaled down to 96-well U-bottomed plates in duplicate or triplicate. PBMC or CD4-enriched, adherent cell-depleted cells (2 × 105/well) were incubated in 200 μl TCM with or without antigens, autologous cell fractions, and antibodies and analyzed by flow cytometry on day 5. Non-CFSE-pulsed replicates were pulsed with [3H]thymidine (see below).
Lymphoproliferation assays.
PBMC or fractionated, reconstituted cultures (see below) (1 × 105 or 2 × 105/well) were plated in triplicate 96-well U-bottomed plates in 200 μl TCM with a 1:1,000 dilution of UV-irradiated HSV-2 (corresponding to 3 × 105 PFU/ml prior to inactivation) or mock antigen. Antibodies were added to some wells on day 0, 1 μCi [3H]thymidine was added on day 5, and cells were harvested on day 6 (23). The change in counts per minute (cpm) was determined as mean cpm with antigen minus mean cpm with mock antigen, and the stimulation index was determined as mean cpm with antigen divided by mean cpm with mock antigen (23).
PBMC fractionation.
CD4+ cells were isolated by first depleting adherent and culture-labile cells during overnight culture at 107 PBMC/well in six-well dishes in 6 ml fetal calf serum (FCS)-based LCL medium (58). CD4+ cells were enriched from nonadherent cells with the portion of a CD4+ CD25+ kit (Miltenyi, Auburn, CA) designed for purification of all CD4+ cells. Monocytes were enriched from fresh PBMC with a negative selection monocyte isolation kit II (Miltenyi). pDC cells were isolated with a BDCA-4 positive-enrichment kit (Miltenyi). CD4+ T cells were defined as CD4high CD14neg, monocytes were defined as large and CD4intermediate CD14high, and pDC were defined as CD123high BDCA-2high CD4low (53) Comparing fresh PBMC to the final products for four subjects, CD4+ T cells were enriched from 37.0% ± 3.0% (mean ± standard deviation) to 94.4% ± 3.0%, monocytes were enriched from 15.4% ± 3.9% to 65.4% ± 17.8%, and pDC were enriched from 0.36% ± 0.31% to 73.7% ± 14.7%. In each case, cells in the combined lymphocyte and monocyte region (forward/side light scatter) were analyzed.
Flow cytometry.
Leukocytes were stained with anti-CD14-fluorescein isothiocyanate (Pharmingen, San Diego, CA), anti-CD4-phycoerythrin, and anti-CD8-phycoerythrin-Cy5 (both from Caltag, Burlingame, CA) or with anti-CD123-fluorescein isothiocyanate and either anti-BDCA-2-phycoerythrin or anti-BDCA-4-phycoerythrin (Miltenyi), as described previously (23). For measurement of CLA, cultured CFSE-labeled cells were stained for 30 min at 4°C with anti-CD4-phycoerythrin-Cy5 (Caltag) and biotinylated anti-CLA (Pharmingen), washed twice in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline, 1% FCS, 0.02% sodium azide), stained with streptavidin-phycoerythrin or -allophycocyanin (Pharmingen), washed, and fixed. Forward/side scatter data for mock and HSV-2 stimulation in each experiment were used to set regions for small resting lymphocytes and larger, granular cells (lymphoblasts). CLA expression analyses were typically gated on lymphoblasts that were CD4high CFSElow, consistent with prior in vitro proliferation. For mitogens, gates were set to include CD4+ blasts with two or more divisions, as judged by CFSE dilution. For recall antigens, CD4+ blasts usually partitioned into CFSEhigh cells and a group of CFSElow cells without discrete internal levels of CFSE intensity. Few CFSEintermediate events were observed, and the principle CFSElow cell populations were analyzed. The fluorescence cutoff for CLA positivity was set and held constant within each experiment. To set the cutoff for CLA positivity, PHA-stimulated, CD4+ CFSElow lymphoblasts were analyzed in a histogram. The cutoff was set at the inflection between the lower-intensity Poisson-shaped clump and the CLAhigh positive tail. Cutoff values ranged from fluorescence intensities of 101.0 to 102.0. To estimate proliferation by flow cytometry, total mononuclear cells, total lymphoblasts, and CD4+ CFSElow cells in both of these gates were enumerated among >105 total events/specimen. The percentages of lymphoblasts in the total mononuclear cells and the percentages of CD4+ CFSElow cells in mononuclear and lymphoblast gates were calculated. Analyses used FacScan or FacsCalibur (Becton Dickinson, Franklin Lakes, NJ) cytometers and WinMDI 2.8 (http://facs.scripps.edu/software.html).
Statistics.
Comparisons of CLA expression between paired cultures with and without anticytokine antibodies were done with a two-tailed Wilcoxon matched-pair signed-ranks test. Comparisons of grouped data were done with a two-tailed Mann-Whitney U test. Evaluation of the effect of increasing doses of IL-4 was done with the Kruskal-Wallis nonparametric analysis of variance test. Instat 3.05 (GraphPad, San Diego, CA) was used for analyses.
RESULTS
Expression of CLA by HSV-2-specific CD4 T cells can be dissociated from proliferation.
Stimulation of PBMC by continuous exposure to HSV-2 antigen led to high expression of CLA by responding CD4+ lymphocytes (data for a representative subject are shown in Fig. 1A). To isolate dividing responder cells, we gated large, granular CD4+ CFSElow cells (by forward and side scatter). The small, less granular CD4+ cells were mostly CFSEhigh undivided cells, and these remained CLAlow (not shown). Among CD4+ CFSElow lymphoblasts, CLA expression was much lower after CMV or influenza virus stimulation (Fig. 1A).
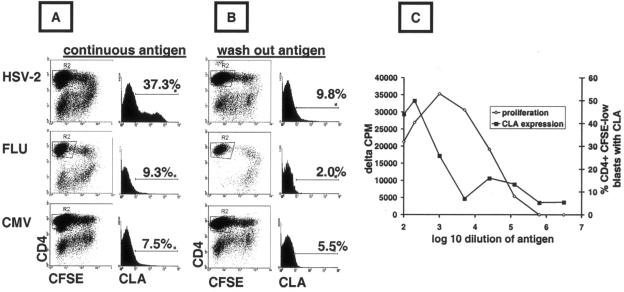
FIG. 1.
Dissociation of CLA induction and proliferation by HSV-2-reactive memory CD4 T cells. (A) PBMC were incubated continuously with antigen/mitogen and cultured for 5 days in 48-well plates. Large granular cells (gating not shown) that were CD4+ CFSElow (R2) were analyzed. Histograms and markers show the percent cells expressing CLA. (B) PBMC were pulsed with antigen/mitogen for 2 hours, washed, cultured, and analyzed as described above. The results shown in panels A and B are typical of five subjects. (C) PBMC from an HSV-1- and HSV-2-seropositive person were incubated with serial dilutions of whole HSV-1 or HSV-2 antigen. After 5 days, proliferation was measured in triplicate by [3H]thymidine incorporation. CLA expression was measured as described above. Results are typical of four subjects, and means are shown.
To investigate if stimulation through the TCR was both necessary and sufficient to induce CLA expression by HSV-2-specific memory CD4 T cells, PBMC were antigen pulsed for 2 hours, washed, and analyzed after 5 days in culture. Strong proliferative responses, as previously reported for pulsing with HSV-2 antigens (33), were detected by an accumulation of CFSElow CD4+ lymphoblasts (Fig. 1B). However, the CLA expression of cells responding to HSV-2 was of a low magnitude (9.8%), albeit somewhat higher than that for CMV (5.5%) or influenza virus (2.0%). Parallel experiments with the same responder cells, differing only in the fact that antigen was continuously present, led to strong, selective up-regulation of CLA in response to HSV-2 (Fig. 1A). Similar results were noted for five subjects. Titration of HSV-2 antigen could also dissociate proliferative responses from CLA expression. Continuous exposure of whole PBMC to highly diluted (1:25,000) HSV-1 or HSV-2 antigen resulted in proliferation, as detected by the accumulation of CFSElow CD4+ cells (not shown) and [3H]thymidine incorporation (Fig. 1C). However, the induction of CLA expression was observed only at higher concentrations of HSV-2 antigen.
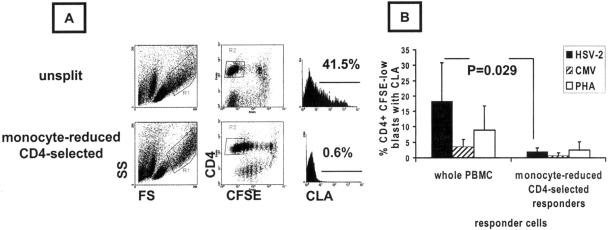
These experiments led to the hypothesis that accessory cells, possibly but not necessarily overlapping with functional APC, were involved in CLA expression by CD4+ T cells responding to HSV-2 antigen. We tested this hypothesis by reducing the number of non-CD4+ T cells prior to incubation with recall antigens. CD4+ T cells were enriched by overnight incubation of PBMC in FCS-based medium, allowing monocyte adherence, followed by a subtractive CD4+ T-cell selection protocol that included active removal of residual monocytes and dendritic cells. This treatment decreased large CD4intermediate CD14+ cells (monocytes) by 94%, from 24.8% ± 5.6% to 1.4% ± 0.9% (mean ± standard deviation; n = 4 subjects). pDC are known to be very labile to freeze-thaw processing of human PBMC and also to overnight culture unless IL-3 is supplied (29). We were unable to detect BDCA-2- or BDCA-4-positive cells by flow cytometry after the CD4 T-cell enrichment described above. Proliferation, presumably driven by residual APC, was noted in CD4-enriched cultures, as judged by an accumulation of large CFSElow CD4+ blasts (Fig. 2A). A reduction of the number of monocytes and other non-CD4 T cells led to a marked reduction in CLA expression by cells responding to HSV-2 compared to whole, nonadherent responders undergoing mock CD4 cell enrichment (Fig. 2B) (n = 4 subjects; P = 0.029 by a paired test). The differences for CMV and PHA stimulation were also significant, with P values of <0.05, but the absolute magnitudes of CLA expression among these responder cells were very low (Fig. 2B). These data show that a reduction of the number of APC to a residual level which is still adequate to stimulate proliferation also dissociates the stimulation of cell division and CLA expression.
FIG. 2.
CD4-enriched, monocyte-depleted PBMC cultures proliferating in response to HSV-2 antigen have low CLA expression. (A) Representative unsplit or monocyte-depleted, CD4-enriched PBMC from HSV-2/CMV dually seropositive donors were stimulated for 5 days with viral antigen or PHA and analyzed for size and granularity (left) or CFSE dilution and CD4 expression (middle). Large CD4+ CFSElow cells, gated as shown, were analyzed for CLA, and the percentages of positive cells are shown. (B) Means and standard deviations for four subjects with matched whole PBMC and CD4-enriched, monocyte-depleted cultures analyzed in parallel. Each responder cell population and antigen was tested in duplicate in a 96-well format, and the means for individual subjects were used to calculate means (bars) and standard deviations (error bars).
HSV-2 antigen influences bystander proliferating CD4 T cells to express CLA.
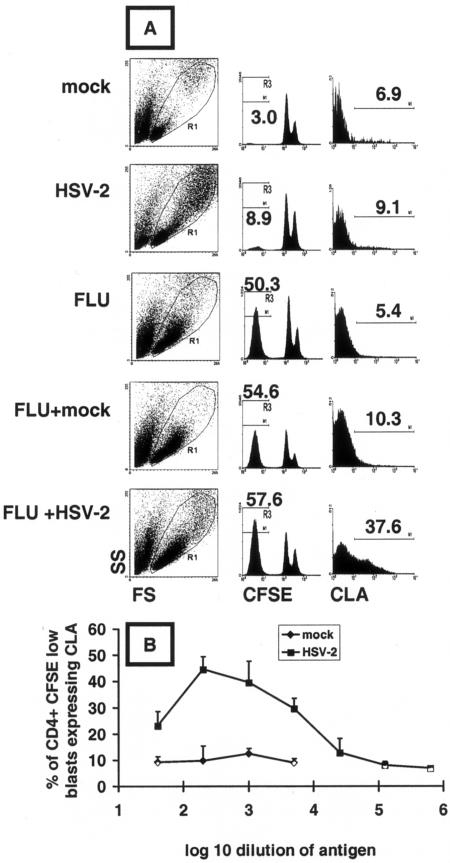
To investigate if the function provoked by HSV-2 antigen capable of stimulating CLA expression could act in trans, we performed antigen mixing using PBMC from an HSV-1/HSV-2 dually seronegative donor. UV-inactivated mock or HSV-2 antigen (diluted 1:1,000) alone caused an accumulation of 3.0% or 8.9%, respectively, of CFSElow cells among total (resting and lymphoblast) CD4+ lymphocytes (Fig. 3A). This background proliferation is commonly seen among HSV-seronegative persons and is of uncertain significance. In contrast, influenza virus antigen led to 50.4% CFSElow cells among the total CD4+ lymphocytes. These cells expressed low levels of CLA (5.4%). The addition of HSV-2 antigen diluted 1:1,000 to influenza virus protein antigen increased CLA expression among CFSElow CD4+ responder cells to 37.6%, while mock antigen had a much smaller effect. HSV-2 antigen cross-stimulation of CLA expression was dose dependent. The effect was maximal at a 1:200 dilution, with significant activity noted at 1:5,000 and a decline to baseline at 1:25,000 (Fig. 3B; see Materials and Methods for PFU equivalents). The differences were significant (P < 0.05 by a paired test) between the mock and HSV-2 antigens at each dilution tested, and similar results were noted for one other subject. At each dilution, little CLA expression was noted among bystander CFSEhigh CD4+ cells (not shown).
FIG. 3.
HSV-2 antigen induces CLA expression on bystander proliferating CD4+ memory T cells. (A) PBMC from an HSV-seronegative, influenza-vaccinated subject were incubated for 5 days with the antigen(s) indicated at left. Total lymphocytes (resting and blasts) in the indicated R1 forward/side scatter window were gated for CD4hi (not shown). Influenza virus antigen led to more CFSElow cells, indicated numerically above the histograms in the middle column, than did HSV-2. CLA expression on the gated (R3) CD4+ CFSElow large granular cells is shown in the right column, and positive cell percentages are indicated. (B) Dose dependence of bystander CLA expression induced by UV-inactivated HSV-2 or mock antigen. Data are means and standard deviations of triplicate results.
pDC restore CLA expression to CD4+ T cells responding to HSV-2 antigen.
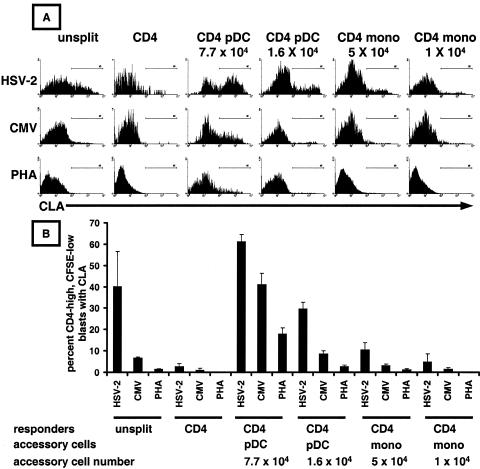
We showed above that depletion of DC and monocytes reduced CLA expression by HSV-2-reactive CD4+ lymphoblasts. Autologous pDC and monocytes were tested for the ability to restore CLA expression by purified CD4+ T cells responding to HSV-2 antigen. Triplicate assays were performed in a microtiter plate format, using graded numbers of accessory cells. Responding T cells were identified as large, granular CFSElow CD4high cells. The residual APC activity present in the purified CD4 cell preparation was enough to support proliferation, as detected by small numbers of large, granular CFSElow cells, but as reported above, CLA expression on these CD4+ responders was low. pDC preparations were potent inducers of CLA on T lymphocytes responding to HSV-2 antigen (Fig. 4A shows representative histograms; Fig. 4B shows means and standard deviations of triplicate assays for one subject). A dose-response relationship was present between the number of pDC added and CLA expression. Exogenous pDC, when added in sufficient number, also supported CLA expression by CD4 T cells responding to CMV antigen or PHA. However, the highest CLA expression level with either a low or high accessory cell number was noted for HSV-2 and added pDC. Monocytes were also able to induce CLA in response to HSV-2. Monocytes were less efficient for CLA induction on a per-cell basis and did not induce appreciable CLA expression on lymphoblasts when CMV antigen or PHA was used to stimulate purified CD4 T cells. Similar results for the restoration of CLA expression after reintroduction of pDC or monocytes were noted for three additional subjects.
FIG. 4.
Plasmacytoid dendritic cells induce CLA expression by memory CD4 T cells responding to HSV-2 antigen. (A) Representative histograms showing CLA expression after exposure of the indicated cell populations, gated for CD4+ CFSElow lymphoblasts, to viral antigens or PHA. Either the whole PBMC (left) or purified CD4+ T cells were labeled with CFSE. (B) Means and standard deviations of CLA expression levels from triplicate assays for a representative subject. Mono, monocytes.
CLA induction by HSV-2 is mediated by endogenous IL-12, type I interferon, and TGF-β.
The dissociation between HSV-2-driven proliferation and CLA expression and the results of the antigen- and cell-mixing experiments described above suggest that HSV-2 antigen acts on accessory cells to contribute to an environment supportive of CLA expression by proliferating cells. We investigated the two-hit model mentioned above, in which stimulation through the TCR plus cytokines promotes CLA and ESL expression. The roles of IL-12 and type I interferon were tested by adding blocking antibodies during culture setup, simultaneous with the addition of antigen. Initial experiments used antibodies at either 3 or 0.6 μg/ml and either a single addition at culture initiation or a repeat addition at 48 h. The effects were similar, and data for one addition of antibody are presented. Since there are many type I interferons, an antibody that blocks the receptor for both alpha and beta interferon was used.
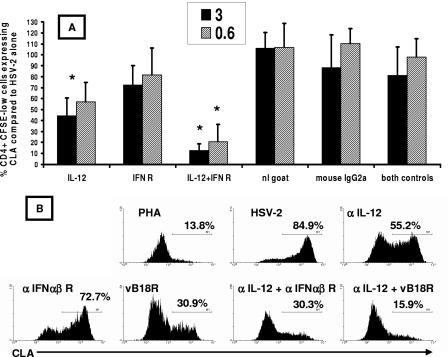
The blockade of IL-12 caused a significant reduction of CLA expression by herpesvirus-reactive CD4+ lymphocytes. Among five HSV-2-infected subjects (Fig. 5A), CLA expression decreased by 56% and 43% when anti-IL-12 antibody was added at 3 and 0.6 μg/ml, respectively. In absolute terms, this represented a decrease from 42.2% ± 15.7% (mean ± standard deviation) for no antibodies to 20.0% ± 14.3% for 3 μg/ml anti-IL-12 and 24.7% ± 15.8% for 0.6 μg/ml anti-IL-12. Anti-type I interferon receptor antibody alone had a smaller effect (mean 19% and 27% reductions at 0.6 and 3 μg/ml, respectively). However, the addition of anti-type I interferon receptor significantly increased the inhibition observed with anti-IL-12. CLA expression was reduced by 93% and 81% when both antibodies were added at 3 and 0.6 μg/ml, respectively. The means and standard deviations for CLA expression were 6.3% ± 3.5% at 3 μg/ml and 9.4% ± 3.9% at 0.6 μg/ml. When the values for CLA expression in wells treated with active antibodies were compared to those for CLA expression in the most relevant isotype control wells (single or double antibodies), the decrease in CLA expression was statistically significant for anti-IL-12 alone (P = 0.008 at 3 μg/ml) as well as anti-IL-12 plus anti-interferon receptor (P = 0.008 at both dose levels), while anti-interferon receptor alone did not appear to be active. The isotype control antibodies tested alone or in combination had no significant impact on CLA expression compared to no antibody (P > 0.05 at both 0.6 and 3 μg/ml for either or both antibodies). The effects of active and control antibodies on PBMC proliferative responses to HSV-2 antigen were studied in triplicate in four subjects. The active antibodies in combination or alone at concentrations up to 10 μg/ml had no significant impact on proliferation in triplicate PBMC proliferation assays (data not shown).
FIG. 5.
(A) Blockade of IL-12 acts alone and synergistically with blockade of class I interferon to prevent CLA expression by HSV-2-reactive CD4 T cells. PBMC from five HSV-2-infected donors were CFSE labeled, and antibodies were added on day 0 with HSV-2 antigen (1:1,000). The dose of antibody (3 or 0.6 μg/ml) is indicated in the legend. On day 5, CFSElow CD4+ blasts were gated and examined for CLA expression. CLA expression in the absence of antibodies was set to 100% for each patient. Bars and error bars are means and standard deviations, respectively, for five subjects for antibody at 0.6 μg/ml (hatched) or 3.0 μg/ml (solid). Asterisks refer to significant (P < 0.05) reductions of CLA expression compared to the wells containing the relevant control antibody or antibodies. Control antibodies (right) led to no significant differences (P > 0.05) from the no-antibody control. (B) Effect of neutralizing antibodies and soluble recombinant vaccinia virus B18R protein on CLA expression in a representative HSV-2-seropositive subject. PBMC were incubated with PHA or HSV-2 antigen (1:1,000) in the absence or presence of the indicated neutralizing antibodies (0.6 μg/ml) or B18R (0.2 μg/ml). Gated blasts that were CFSElow CD4+ were analyzed for CLA expression. Data are percentages of cells positive for CLA.
To further examine the effect of type I interferon, we tested the effect of adding recombinant vaccinia virus B18R protein at the time of PBMC stimulation with UV-inactivated HSV-2. This protein binds to and neutralizes all type I interferons (1). In isolation, a stronger inhibition of CLA expression was noted for B18R than for anti-interferon receptor (data for a representative subject are shown in Fig. 5B). A combination of anti-IL-12 and vaccinia virus B18R led to a profound inhibition of CLA expression by vaccinia virus-reactive CD4 T cells. Similar data were noted for two additional subjects.
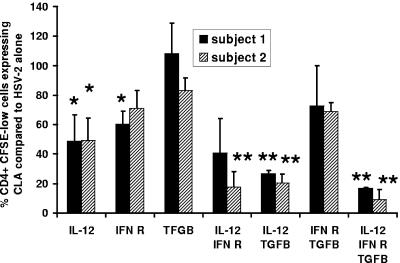
TGF-β has also been reported to increase CLA expression by lymphocytes responding to TCR stimulation and to be a component of the response to HSV (39). We tested the effect of a TGF-β blockade, using a microtiter plate format with duplicate cultures and antibodies at only the lower dose of 0.6 μg/ml. Anti-TGF-β alone had little effect on CLA expression (Fig. 6). Anti-IL-12 again had significant effects, as did anti-interferon receptor (for one subject). Anti-TGF-β synergized with anti-IL-12 in reducing CLA. Overall, among five persons studied, anti-IL-12 alone inhibited CLA by 50.1% ± 10.0%, while the combination of anti-IL-12 and anti-TGF-β led to 81.1% ± 7.8% inhibition of CLA (P = 0.02 compared to anti-IL-12 alone). Little additional inhibition was noted by combining all three antibodies.
FIG. 6.
Neutralization of TGF-β reduces CLA expression in combination with blockade of IL-12. PMBC from two HSV-2-infected donors (as indicated in the legend) were CFSE labeled, and antibodies (all used at 0.6 μg/ml) were added on day 0 in duplicate microtiter plate cultures. Cultures were analyzed on day 5, with CLA expression in the absence of antibodies set to 100%. Bars and error bars are means and standard deviations, respectively, of the percentages of CLA expression by CFSElow CD4+ blasts for two subjects relative to the no-antibody control. Single asterisks refer to significant (P < 0.05) reductions of CLA expression compared to the no-antibody control (100% on the y axis). Double asterisks refer to significant (P < 0.05) decreases of CLA expression compared to that with anti-IL-12 alone.
DISCUSSION
Our previous results showed that HSV-2-reactive CD4 T cells in seropositive subjects express CLA after proliferating in response to exposure to HSV-2 recall antigen in vitro. These data were consistent with the hypothesis that these cells are somehow programmed to express CLA upon cell division. The initial results in the current study showed that stimulation of cell division by HSV-2-specific memory CD4 T cells was not sufficient to cause CLA expression on resultant lymphoblasts. Reductions in the antigen dose or contact time dissociated cell division from CLA expression. In antigen-mixing experiments, CLA was induced on T cells proliferating in response to influenza virus antigen when HSV-2 antigen was also present, notably when the donor was seronegative for HSV-1 and HSV-2. The innate response to HSV-2 in these subjects influenced a shift in skin-homing receptor expression by “bystander” proliferating CD4 T cells. The reciprocal antigen-mixing experiment would add influenza virus protein antigen to PBMC from influenza virus-naïve, HSV-2-infected persons. Suitable PBMC are difficult to obtain, however, as almost all persons are immunologically experienced with influenza virus via infection and/or vaccination. It might be expected that whole influenza virus would stimulate CLA expression because influenza virions elicit type I interferon expression by pDC (12). However, the influenza virus antigens used in this study were purified surface proteins that do not contain appreciable viral nucleic acid, one likely immunostimulatory compound in the whole virus (35). Experiments studying the effects of whole influenza virus on CLA expression by memory CD4 T cells have not been described. The effects of innate responses to other viruses, such as CMV, on CLA expression by lymphoblasts may be studied by using PBMC from CMV-negative individuals and a second recall antigen to which the subject is sensitized. A series of such experiments will determine how specific the enhancement of CLA is to the innate response to HSV antigen.
We next examined the possible roles of cytokines and accessory cells in the induction of CLA expression. Our data with blocking antibodies suggest that there are contributions from both IL-12 and type I interferon to the induction of CLA. Type I interferons may have a stronger effect, because the blockade of interferon only with vaccinia virus B18R protein led to a strong reduction of CLA expression. The blockade with an anti-interferon receptor monoclonal antibody may have been less complete, allowing residual stimulation that could synergize with IL-12 (Fig. 6). TGF-β seemed to have the weakest effect, as an isolated blockade led to no reduction in the proportion of responding CD4 cells with CLA expression (Fig. 6). In addition, TGF-β appeared to be synergistic with IL-12 but not with type I interferon. This finding is consistent with emerging findings concerning the regulation of expression of FTVII (21), a key enzyme involved in the creation of CLA (see below). Ideally, cytokines would have been measured in the supernatants of unfractionated, split, and add-back cultures and correlated with CLA expression. These studies are technically demanding due to cytokine lability and low concentrations and are currently under way.
Our data with blocking antibodies and the B18R protein suggest roles for type I interferon and IL-12 in regulating CLA expression by HSV-2-reactive CD4 T cells. IL-4 is known to down-regulate CLA expression by CD4 and CD8 T cells responding to stimulation through the TCR (42, 52). All-trans retinoic acid was recently shown to inhibit ESL expression on cells primed by Peyer's patch-derived DC (20). We extended these observations to memory CD4+ T cells responding to antigen in vitro. Exogenous IL-4 and all-trans retinoic acid were potent inhibitors of CLA expression by CD4+ lymphoblasts responding to HSV-2 antigen in vitro. Inhibition by IL-4 was dose dependent and active down to 100 picograms/ml (data not shown).
Our cell reconstitution experiments suggest that pDC are potent inducers of CLA in the setting of HSV-2 stimulation. HSV elicits a complex innate immune response from DC and monocytes/macrophages. Natural alpha interferon-producing cells among PBMC are characterized as pDC (53), recognize several different viruses, and produce a variety of interferon subtypes (9, 35). It is not known if pDC are present in HSV lesions, but pDC-like cells express CLA and localize to foci of melanomas and some inflammatory skin conditions (5, 48, 50, 63). The added pDC and monocytes were not labeled with CFSE and did express CLA and CD4. It is unlikely, however, that the CLAhigh CD4+ CFSElow cells detected after 5 days of antigen stimulation (Fig. 4) represented carryover of these input cells. The analyzed cells were CD4bright, while monocytes/macrophages and pDC are CD4intermediate (53). Back-gating of pDC that were positive for CD123 and BDCA-2 in PBMC showed that they have low side scatter (data not shown), while the cells that were analyzed for CLA expression were in the lymphoblast region with high forward scatter and high side scatter. In addition, the survival of pDC in culture at 72 to 96 h is <5% in the absence of exogenous IL-3 and/or FLT3 ligand (19, 29, 35), neither of which was supplied, and our analyses were carried out even later, at 120 h.
While pDC are well known as sources of type I interferon, their secretion of IL-12 is controversial (35). pDC purification based on BDCA-4 expression, as used in our work, can coenrich a small percentage of CD11c+ myeloid DC and other cells that can produce both interferon and IL-12 (35, 46). Ideally, we would also have studied myeloid DC in depletion and add-back experiments. These systems require several hundred milliliters of fresh blood (44) and are planned for future studies. Monocytes were also biologically active inducers of CLA in our system. While producing less HSV-stimulated alpha interferon on a per-cell basis than pDC (15, 46), monocytes are more abundant in vivo. Macrophages secrete IL-12 in response to HSV stimulation (37), and cells with monocyte/macrophage markers have been identified in HSV lesions (11). Whole PBMC produce large amounts of TGF-β in response to HSV, but which cell type(s) is active is not known (39). Ultimately, in vivo systems will likely be necessary to fully define the role(s) of cytokines and accessory cells in T-cell homing in response to HSV.
The HSV-2 structures and cellular receptors that elicit innate cytokine secretion are not fully characterized. Glycoprotein D (gD) of HSV-1 or HSV-2 is a potent trigger of alpha interferon from mixed PBMC, but the responder cell population is not known (3). DC express receptors for gD (51), and the use of purified gD in our system would clarify its possible role as a recall antigen or innate stimulus. Purified HSV DNA is also stimulatory to dendritic cells and contains sequences similar to agonistic CpG oligonucleotides. Recent studies have suggested roles for Toll-like receptors 2 and 9 in HSV pathogenesis, but it is not yet known if these receptors are involved in the promotion of CLA expression by responding lymphoblasts in our in vitro system (30, 31, 36).
It is likely that positive and negative signals, mediated by both innate and acquired responses to HSV-2, are integrated to control homing molecule expression by HSV-2-specific memory T cells. The expression of CLA and ESL is dependent on the expression of FTVII. FTVII appears to be essential for the expression of CLA and all selectin ligands (21, 38). FTVII expression is constitutive in myeloid cells and inducible in lymphoid cells (21). The control of FTVII enzyme activity appears to correspond with transcriptional regulation of mRNA levels. For example, FTVII mRNA levels are initially low and peak 1 to 2 days before CLA and ESL expression when PBMC are cultured under specific conditions known to stimulate CLA expression (57). The second-messenger pathways and promoter elements connecting cell surface signaling from IL-12 and interferon and FTVII expression are actively being dissected (21, 61). Recently, data consistent with both epigenetic regulation of genes controlling selectin ligand synthesis (56) and reprogramming of homing molecule expression by specific dendritic cells (40) have been presented. Consistent with plasticity within the memory T-cell compartment, we found that specific pDC could “overdrive” CMV-specific CD4+ T cells to express CLA, a nonphysiologic outcome for the host response to this virus that seldom infects the skin. HSV-reactive CD4 T cells were, however, differentially sensitive to the effects of exogenous pDC. This could be due to imprinting of the cells or a direct interaction with the HSV-2 antigen. Since HSV-2-specific memory CD8 T cells constitutively express CLA and ESL in the absence of antigen stimulation (26), it appears that FTVII expression can also be tonically up-regulated. This issue is currently under study, using tetramer-sorted HSV-2-specific CD8 T cells.
Several unresolved issues remain to be addressed. CLA expression by T cells in response to a dual TCR plus cytokine signal seems to require several cell divisions in vitro (10, 56), while CD4 migration to herpetic lesions is quite fast in vivo. Persons with deficiencies in IL-12 or IL-12 receptors are not known to have severe HSV infections (14), so IL-12 may not be necessary and other factors may provide a backup system. We have used in vitro culture conditions that are generally restrictive for CLA expression (4, 10) but cannot reproduce the complex in vivo milieu. The ratio of pDC or monocytes to responder CD4 T cells in our reconstitution experiments was higher than the expected ratio in PBMC, but high local concentrations of these cells are found in some disease states (5). It is possible that innate stimuli provided by non-HSV molecules, such as lipopolysaccharide from skin bacteria also present in herpetic lesions, could also be involved in up-regulating CLA in vivo (54). It is also possible that CLA expression by CD4 T cells with HSV-2-specific gamma interferon secretion or cytotoxicity but lacking proliferative potential in vitro could differ from CLA expression by the CD4 T cells that proliferated in vitro in this study, exhibiting diluted CFSE fluorescence. These areas are each amenable to additional research studies.
Taken together, our data and reports in the literature permit the proposal of a working model for additional testing. The recurrence of HSV-2 may lead to the uptake of antigen by mobile, functional DC, as recently demonstrated with mice (2, 64), that present antigen to memory CD4 T cells in regional lymphoid tissue. Innate stimulation of either these functional APC or other cells may elicit secretion of IL-12, type I interferon, or other factors that influence the HSV-2-specific memory CD4 T cells to express CLA and ESL during their activation. These cells are then better equipped to traffic to infected tissues. In the next stages of T-cell trafficking, chemokines may trigger changes in integrins and/or chemotaxis towards areas of infection (8, 62). Ultimately, in vivo models will be required to determine the cells and molecules most involved in the trafficking of antigen-specific effector cells.
Acknowledgments
This work was supported by NIH grants AI30731 and AI50132. Michael Hensel was supported the Graduate School of the University of Washington.
We are grateful for the administrative, data management, and clinical staff of the Virology Research Clinic, Seattle, Wash., supervised by Anna Wald, who recruited the subjects and obtained the specimens.
REFERENCES
- 1.Alcami, A., J. A. Symons, and G. L. Smith. 2000. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 74:11230-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, R. S., C. M. Smith, G. T. Belz, A. L. van Lint, L. M. Wakim, W. R. Heath, and F. R. Carbone. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science 301:1925-1928. [DOI] [PubMed] [Google Scholar]
- 3.Ankel, H., D. F. Westra, S. Welling-Wester, and P. Lebon. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317-326. [DOI] [PubMed] [Google Scholar]
- 4.Armerding, D., and T. S. Kupper. 1999. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int. Arch. Allergy Immunol. 119:212-222. [DOI] [PubMed] [Google Scholar]
- 5.Bangert, C., J. Friedl, G. Stary, G. Stingl, and T. Kopp. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Investig. Dermatol. 121:1409-1418. [DOI] [PubMed] [Google Scholar]
- 6.Barcy, S., M. L. Huang, L. Corey, and D. M. Koelle. 2005. Longitudinal analysis of herpes simplex virus-specific CD4+ cell clonotypes in infected tissues and blood. J. Infect. Dis. 191:2012-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry, S. M., D. G. Zisoulis, J. W. Neal, N. A. Clipstone, and G. S. Kansas. 2003. Induction of FucT-VII by the Ras/MAP kinase cascade in Jurkat T cells. Blood 102:1771-1778. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, D. J., C. H. Kim, and E. C. Butcher. 2003. Chemokines in the systemic organization of immunity. Immunol. Rev. 195:58-71. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168:4796-4801. [DOI] [PubMed] [Google Scholar]
- 10.Colantonio, L., B. Rossi, G. Constantin, and D. D'Ambrosio. 2004. Integration and independent acquisition of specialized skin- versus gut-homing and Th1 versus Th2 cytokine synthesis phenotypes in human CD4(+) T cells. Eur. J. Immunol. 34:2419-2429. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, A. L., R. R. Turner, A. C. Miller, M. F. Para, and T. C. Merigan. 1985. Evolution of recurrent herpes simplex lesions: an immunohistologic study. J. Clin. Investig. 75:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai, J., N. J. Megjugorac, S. B. Amrute, and P. Fitzgerald-Bocarsly. 2004. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 173:1535-1548. [DOI] [PubMed] [Google Scholar]
- 13.Danke, N. A., D. M. Koelle, and W. W. Kwok. 2005. Persistence of HSV-2 VP16-specific CD4 T-cells. Hum. Immunol. 66:777-787. [DOI] [PubMed] [Google Scholar]
- 14.Fieschi, C., and J. L. Casanova. 2003. The role of interleukin-12 in human infectious diseases: only a faint signature. Eur. J. Immunol. 33:1461-1464. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald-Bocarsly, P. 2002. Natural interferon-alpha producing cells: the plasmacytoid dendritic cells. BioTechniques 2002(Suppl.):16-20, 22, 24-29. [PubMed] [Google Scholar]
- 16.Fuhlbrigge, R. C., J. D. Kieffer, D. Armerding, and T. S. Kupper. 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389:978-981. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, J. C., W. W. Kwok, A. Wald, C. L. McClurkan, and D. M. Koelle. 2005. Programmed expression of cutaneous lymphocyte-associated antigen amongst circulating memory T-cells specific for HSV-2. J. Infect. Dis. 191:243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, T., R. Amakawa, M. Inaba, S. Ikehara, K. Inaba, and S. Fukuhara. 2001. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 166:2961-2969. [DOI] [PubMed] [Google Scholar]
- 20.Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kate, and S.-Y. Song. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21:527-538. [DOI] [PubMed] [Google Scholar]
- 21.Kansas, G. S. 2004. Control of FucT-VII expression in CD4+ T cells. Ernst Schering Res. Found. Workshop 2004:95-107. [DOI] [PubMed] [Google Scholar]
- 22.Koelle, D. M., H. Abbo, A. Peck, K. Ziegweid, and L. Corey. 1994. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J. Infect. Dis. 169:956-961. [DOI] [PubMed] [Google Scholar]
- 23.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificities of human CD4+ T cell clones recovered from recurrent genital HSV-2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelle, D. M., Z. Liu, C. M. McClurkan, M. Topp, S. R. Riddell, E. G. Pamer, A. S. Johnson, A. Wald, and L. Corey. 2002. Expression of cutaneous lymphocyte-associated antigen by CD8+ T-cells specific for a skin-tropic virus. J. Clin. Investig. 110:537-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koelle, D. M., J. C. Gonzalez, and A. S. Johnson. 2005. Homing in on the cellular immune response to HSV-2 in humans. Am. J. Reprod. Immunol. 53:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelle, D. M., J. Benedetti, A. Langenberg, and L. Corey. 1992. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital infection. Ann. Intern. Med. 116:433-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250-3259. [PubMed] [Google Scholar]
- 30.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 31.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwok, W. W., J. A. Gebe, A. Liu, S. Agar, N. Ptacek, J. Hammer, D. M. Koelle, and G. T. Nepom. 2001. Rapid epitope identification from complex class II-restricted T-cell antigens. Trends Immunol. 22:583-588. [DOI] [PubMed] [Google Scholar]
- 33.Kwok, W. W., A. W. Liu, E. J. Novak, J. A. Gebe, S. N. Reymond, R. A. Ettinger, G. T. Nepom, and D. M. Koelle. 2000. HLA-DQ tetramers identify epitope-specific T-cells in peripheral blood of herpes simplex virus-2-infected individuals: direct detection of immunodominant antigen responsive cells. J. Immunol. 164:4244-4249. [DOI] [PubMed] [Google Scholar]
- 34.Leung, D. Y., M. Gately, A. Trumble, B. Ferguson-Darnell, P. M. Schlievert, and L. J. Picker. 1995. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J. Exp. Med. 181:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, Y. J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275-306. [DOI] [PubMed] [Google Scholar]
- 36.Lund, J., A. Sato, S. Akira, R. Medzhitov, and H. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malmgaard, L., S. R. Paludan, S. C. Mogensen, and S. Ellerman-Eriksen. 2000. Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-kappaB. J. Gen. Virol. 81:3011-3020. [DOI] [PubMed] [Google Scholar]
- 38.Maly, P., A. Thall, B. Petryniak, C. E. Rogers, P. L. Smith, R. M. Marks, R. J. Kelly, K. M. Gersten, G. Cheng, T. L. Saunders, S. A. Camper, R. T. Camphausen, F. X. Sullivan, Y. Isogai, O. Hindsgaul, U. H. von Andrian, and J. B. Lowe. 1996. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86:643-653. [DOI] [PubMed] [Google Scholar]
- 39.Mendez-Samperio, P., M. Hernandez, and H. E. Ayala. 2000. Induction of transforming growth factor-beta 1 production in human cells by herpes simplex virus. J. Interferon Cytokine Res. 20:273-280. [DOI] [PubMed] [Google Scholar]
- 40.Mora, J. R., G. Cheng, D. Picarella, M. Briskin, N. Buchanan, and U. H. von Andrian. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison, L. A. 2004. The Toll of herpes simplex virus infection. Trends Microbiol. 12:353-356. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama, F., Y. Teraki, T. Kudo, A. Togayachi, H. Iwasaki, T. Tamatani, S. Nishihara, T. Mizukawa, T. Shiohara, and H. Narimatsu. 2000. Expression of cutaneous lymphocyte-associated antigen regulated by a set of glycosyltransferases in human T cells: involvement of alpha1,3-fucosyl transferase VII and beta1,4-galactosyltransferase I. J. Investig. Dermatol. 115:299-306. [DOI] [PubMed] [Google Scholar]
- 43.Novak, E. J., A. W. Liu, J. A. Gebe, B. A. Falk, G. T. Nepom, D. M. Koelle, and W. W. Kwok. 2001. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J. Immunol. 166:6665-6670. [DOI] [PubMed] [Google Scholar]
- 44.Otero, M., G. Nunnari, D. Leto, J. Sullivan, F. X. Wang, I. Frank, Y. Xu, C. Patel, G. Dornadula, J. Kulkosky, and R. J. Pomerantz. 2003. Peripheral blood dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART. AIDS Res. Hum. Retrovir. 19:1097-1103. [DOI] [PubMed] [Google Scholar]
- 45.Picker, L. J., J. R. Treer, B. Ferguson-Darnell, P. A. Collins, P. R. Bergstresser, and L. W. M. M. Terstappen. 1993. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J. Immunol. 150:1122-1136. [PubMed] [Google Scholar]
- 46.Pollara, G., M. Jones, M. E. Handley, M. Rajpopat, A. Kwan, R. S. Coffin, G. Foster, B. Chain, and D. R. Katz. 2004. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J. Immunol. 173:4108-4119. [DOI] [PubMed] [Google Scholar]
- 47.Posavad, C. M., S. Barcy, M. L. Huang, D. M. Koelle, S. J. Polyak, and L. Corey. 2000. Long-term persistence of herpes simplex virus-specific CD8+ CTL clones derived from genital lesions. J. Immunol. 165:1146-1152. [DOI] [PubMed] [Google Scholar]
- 48.Salio, M., M. Cella, W. Vermi, F. Facchetti, M. J. Palmowski, C. L. Smith, D. Shepherd, M. Colonna, and V. Cerundolo. 2003. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur. J. Immunol. 33:1052-1062. [DOI] [PubMed] [Google Scholar]
- 49.Schaerli, P., L. Ebert, K. Willimann, A. Blaser, R. S. Roos, P. Loetscher, and B. Moser. 2004. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 199:1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt, C., H. Fohrer, S. Beaudet, P. Palmer, M. J. Alpha, B. Canque, J. C. Gluckman, and A. H. Dalloul. 2000. Identification of mature and immature human thymic dendritic cells that differentially express HLA-DR and interleukin-3 receptor in vivo. J. Leukoc. Biol. 68:836-844. [PubMed] [Google Scholar]
- 51.Schneider, K., K. G. Potter, and C. F. Ware. 2004. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 202:49-66. [DOI] [PubMed] [Google Scholar]
- 52.Seneviratne, S. L., L. Jones, A. S. Bailey, R. V. Samuel, A. P. Black, and G. S. Ogg. 2005. Interleukin-4 induced down-regulation of skin homing receptor expression by human viral-specific CD8+ T cells may contribute to atopic risk of cutaneous infection. Clin. Exp. Immunol. 141:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.-J. Liu. 1999. The nature of the principle type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 54.Sigmundsdottir, H., J. E. Gudjonsson, and H. Valdimarsson. 2003. Interleukin-12 alone cannot enhance the expression of the cutaneous lymphocyte associated antigen (CLA) by superantigen-stimulated T lymphocytes. Clin. Exp. Immunol. 132:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swetter, S. M., E. L. Hill, E. L. Kern, D. M. Koelle, C. M. Posavad, and S. Safrin. 1998. Chronic vulvar ulceration in an immunocompetent woman due to acyclovir-resistant, thymidine-kinase-deficient herpes simplex virus. J. Infect. Dis. 177:543-550. [DOI] [PubMed] [Google Scholar]
- 56.Syrbe, U., S. Jennrich, A. Schottelius, A. Richter, A. Radbruch, and A. Hamann. 2004. Differential regulation of P-selectin ligand expression in naive versus memory CD4+ T cells: evidence for epigenetic regulation of involved glycosyltransferase genes. Blood 104:3243-3248. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi, R., Y. Mizukawa, Y. Yamazaki, K. Hayakawa, J. Hayakawa, A. Kudo, and T. Shiohara. 2003. In vitro differentiation from naive to mature E-selectin binding CD4 T cells: acquisition of skin-homing properties occurs independently of cutaneous lymphocyte antigen expression. J. Immunol. 171:5769-5777. [DOI] [PubMed] [Google Scholar]
- 58.Tigges, M. A., D. M. Koelle, K. Hartog, R. E. Sekulovich, L. Corey, and R. L. Burke. 1992. Human CD8+ herpes simplex virus-specific cytotoxic T lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wagers, A. J., and G. S. Kansas. 2000. Potent induction of alpha(1,3)-fucosyltransferase VII in activated CD4+ T cells by TGF-beta 1 through a p38 mitogen-activated protein kinase-dependent pathway. J. Immunol. 165:5011-5016. [DOI] [PubMed] [Google Scholar]
- 60.Wald, A., J. Zeh, S. Selke, T. Warren, A. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]
- 61.White, S. J., G. H. Underhill, M. H. Kaplan, and G. S. Kansas. 2001. Cutting edge: differential requirements for stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J. Immunol. 167:628-631. [DOI] [PubMed] [Google Scholar]
- 62.Wickham, S., B. Lu, J. Ash, and D. J. Carr. 2005. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J. Neuroimmunol. 162:51-59. [DOI] [PubMed] [Google Scholar]
- 63.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Investig. Dermatol. 119:1096-1102. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, X., E. Deak, K. Soderberg, M. Linehan, D. Spezzano, J. Zhu, D. M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]