Abstract
During plant growth and development, the phytohormone auxin induces a wide array of changes that include cell division, cell expansion, cell differentiation, and organ initiation. It has been suggested that the actin cytoskeleton plays an active role in the elaboration of these responses by directing specific changes in cell morphology and cytoarchitecture. Here we demonstrate that the promoter and the protein product of one of the Arabidopsis vegetative actin genes, ACT7, are rapidly and strongly induced in response to exogenous auxin in the cultured tissues of Arabidopsis. Homozygous act7-1 mutant plants were slow to produce callus tissue in response to hormones, and the mutant callus contained at least two to three times lower levels of ACT7 protein than did the wild-type callus. On the other hand, a null mutation in ACT2, another vegetative actin gene, did not significantly affect callus formation from leaf or root tissue. Complementation of the act7-1 mutants with the ACT7 genomic sequence restored their ability to produce callus at rates similar to those of wild-type plants, confirming that the ACT7 gene is required for callus formation. Immunolabeling of callus tissue with actin subclass-specific antibodies revealed that the predominant ACT7 is coexpressed with the other actin proteins. We suggest that the coexpression, and probably the copolymerization, of the abundant ACT7 with the other actin isovariants in cultured cells may facilitate isovariant dynamics well suited for cellular responses to external stimuli such as hormones.
INTRODUCTION
Phytohormones are believed to play a critical role in influencing virtually every aspect of plant growth and development (Davies, 1995). At the cellular level, the hormone auxin acts by altering the turgor, elongation, division, and differentiation of cells. Auxin also is known to induce the rapid synthesis of specific mRNAs and proteins suggested to be necessary to regulate these growth processes (Key, 1964; Theologis, 1986; Brummell and Hall, 1987; Hagen, 1989; Estelle, 1992; Takahashi et al., 1994; Abel and Theologis, 1996). Despite the wealth of information on the polar transport and physiological roles of auxin in plants (Davies, 1995; Muday, 2000), much remains to be learned regarding auxin's mode of action in regulating the dynamics and expression of cytoskeletal proteins, which elaborate the response to this hormone (Loof et al., 1996). Most attempts to examine the activity of plant hormones on the cytoskeleton have been directed toward analyzing changes in the pattern of organization of cytoskeletal networks within the cytoplasm (Thimann et al., 1992; Zandomeni and Schopfer, 1993; Shibaoka, 1994; Nick, 1999).
Exogenous application of hormones initiates a variety of biochemical events that culminate in processes directed by the cytoskeleton, such as the initiation of rapid cell proliferation, cell expansion, and differentiation. Therefore, understanding the role of hormones in the regulation of plant morphogenesis requires a thorough knowledge of the differential expression of the cytoskeletal genes in response to hormones. In the present study, we investigated the differential regulation of actin genes, which are fundamental to plant growth and morphogenesis, after application of the hormone auxin to cultured Arabidopsis tissues and organs.
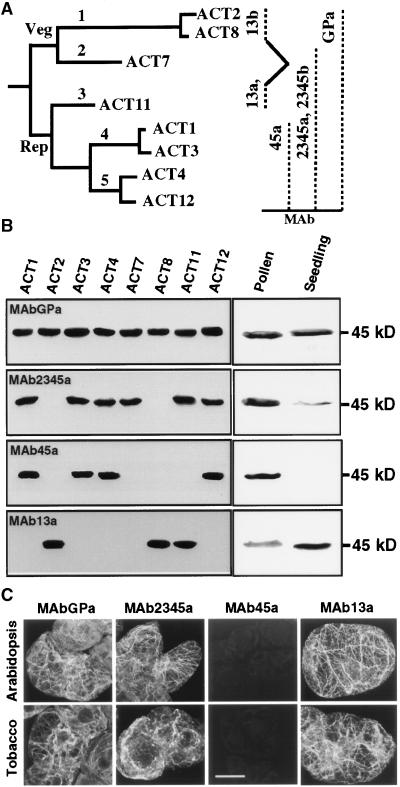
Higher plants contain actins encoded by a relatively ancient and highly divergent multigene family. Arabidopsis is an excellent model system for studying actin function and regulation because it has only eight functional actin genes, all of which have been well characterized. On the basis of their sequence and expression, these eight actin genes have been grouped into two major phylogenetic classes, reproductive and vegetative, and five subclasses (McDowell et al., 1996b; Meagher et al., 1999b), as shown in Figure 1A. These ancient actin genes encode proteins that are relatively divergent in their primary structures compared with proteins encoded by actin families in other kingdoms (Meagher et al., 1999a), and each of the genes is expressed in a distinct tissue-specific and temporal fashion (Meagher et al., 1999b). Moreover, there is a developmental switch in the regulation of actin isovariants during cell differentiation and maturation in plants. For example, during Arabidopsis and tobacco pollen development, there is a switch from completely vegetative to predominantly reproductive actin isovariants (Kandasamy et al., 1999; Meagher et al., 2000). Also, cellular responses rapidly evoked by external stimuli such as fungal infection (Jin et al., 1999) and hormones (Hightower and Meagher, 1985) can result in altered expression of specific actin mRNAs. These observations suggest that different cell types may differ in their preference for actin isovariants to fulfill their distinct cellular functions and that there are functional bases for actin isovariant multiplicity. A number of observations in animals strongly support this hypothesis, because their different actin isoforms have unique properties and they are not functionally equivalent (Rubenstein, 1990; Herman, 1993; Fyrberg et al., 1998). The functional significance of the actin isovariants in plants, however, has not been studied in detail.
Figure 1.
Reactivity of Anti-Actin Antibodies.
(A) Phylogenetic relationship of the eight expressed actins of Arabidopsis (left) and the specificity of monoclonal antibodies (right). Veg, vegetative; Rep, reproductive; MAb, monoclonal antibody.
(B) Protein gel blot analysis showing differential binding of the antibodies with the eight Arabidopsis recombinant actins (3 μg/lane) and actins in pollen and seedling extracts (25 μg total protein/lane).
(C) Immunofluorescence staining of actin filaments in Arabidopsis Fi-3 and tobacco BY-2 suspension cells. Note that all of the antibodies except MAb45a detected dense arrays of actin filaments in both cell types. Bar = 25 μm.
Using a battery of actin isovariant-specific antibodies and the act7-1 mutant allele (Gilliland et al., 1998), which shows a highly reduced level of expression of the ACT7 protein (L.U. Gilliland and R.B. Meagher, unpublished data) and poor induction of callus in response to auxin, we have demonstrated that the ACT7 isovariant is essential for normal phytohormone response during callus formation. We found that there is a significant increase in the expression of this protein in response to hormones in different organs of wild-type Arabidopsis seedlings. Moreover, the activity of the ACT7 promoter, which contains several predicted hormone-responsive elements (McDowell et al., 1996a), is enhanced rapidly during hormonally induced callus formation in the root tissue of transgenic Arabidopsis plants carrying ACT7-GUS fusion genes. By complementing act7-1 with ACT7 genomic sequence, we have shown that the hormone response and callus formation can be restored to normal levels. Together, our observations provide insights into the role of the ACT7 isovariant in hormone-induced cell proliferation and callus formation.
RESULTS
Distinguishing Different Actin Isovariants
Analysis of the differential regulation of the eight functional actins of Arabidopsis in response to exogenous hormones required isovariant-specific antibodies. Production of such antibodies depends only on a dozen or so nonconservative amino acid changes among plant actins (McDowell et al., 1996b). We recently produced MAb45a, a monoclonal antibody specific to late pollen-specific reproductive actin subclasses 4 and 5 (Kandasamy et al., 1999). To isolate additional actin subclass-specific antibodies, several mice were immunized with purified recombinant ACT2, ACT7, or ACT11 proteins. Hybridoma cell lines secreting monoclonal antibodies that react with only a subset of Arabidopsis actins in ELISA or on protein gel blots were isolated. The strategy used for the purification of recombinant actins and the isolation of the new antibodies was the same as that described previously (Kandasamy et al., 1999). Two of the antibodies produced from two independent mice had the same specificity and reacted with the actin subclasses 2, 3, 4, and 5 (Figures 1A and 1B), representing the entire reproductive class of actins (ACT1, ACT3, ACT4, ACT11, and ACT12) and one vegetative actin, ACT7. On the basis of the specificity of these two antibodies to actin subclasses 2, 3, 4, and 5, we named them MAb2345a and MAb2345b. These antibodies did not show any cross-reactivity with ACT2 and ACT8 (Figures 1A and 1B). Two additional antibodies reacted with actin subclasses 1 and 3, representing the two predominantly expressed vegetative actins, ACT2 and ACT8, and the reproductive actin ACT11 (Figure 1B). We named these monoclonal antibodies MAb13a and MAb13b. The general control antibody MAbGPa, on the other hand, detected uniformly all five subclasses of Arabidopsis actins, as shown in Figure 1B.
We further characterized the reactivity of MAb2345a and MAb13a, which belong to the IgG1 class, by reacting them with immunoblots containing extracts from Arabidopsis pollen and young seedlings. MAb2345a recognized a strong 45-kD actin band in the pollen and a weak band of similar size in the seedling extract. In contrast, MAb13a reacted very strongly with actins expressed in the seedling and weakly with pollen actin (Figure 1B). An identical blot stained with MAbGPa revealed that both lanes contained equal amounts of total actins. Moreover, we tested the ability of the antibodies to bind to F-actin by reacting them with fixed Arabidopsis (Davis and Ausubel, 1989) and tobacco (Nagata et al., 1981) suspension cells, which contain vegetative actins (Figure 1C). Both MAb2345a and MAb13a stained dense arrays of cortical actin filaments in the interphase cells of both cell types. The pattern of labeling was very much comparable to that obtained with the general anti-actin antibody MAbGPa (Figure 1C). However, the reproductive actin-specific antibody MAb45a did not bind to any actin filaments in either cell type (Figure 1C). Identical results were obtained when we stained the embryonic cultures of a very distant dicot in the magnolia family, yellow poplar, and a monocot, rice (data not shown). Therefore, the actins expressed in these cultured cells must belong to subclasses 1 (ACT2 and ACT8), 2 (ACT7), and 3 (ACT11).
ACT7 Gene Expression Is Enhanced Rapidly by Plant Hormones
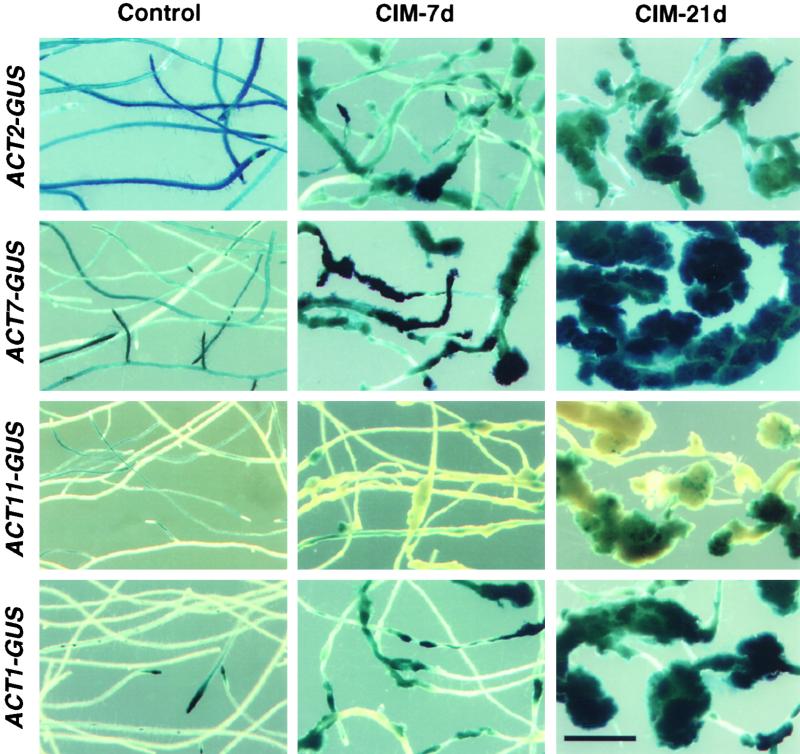
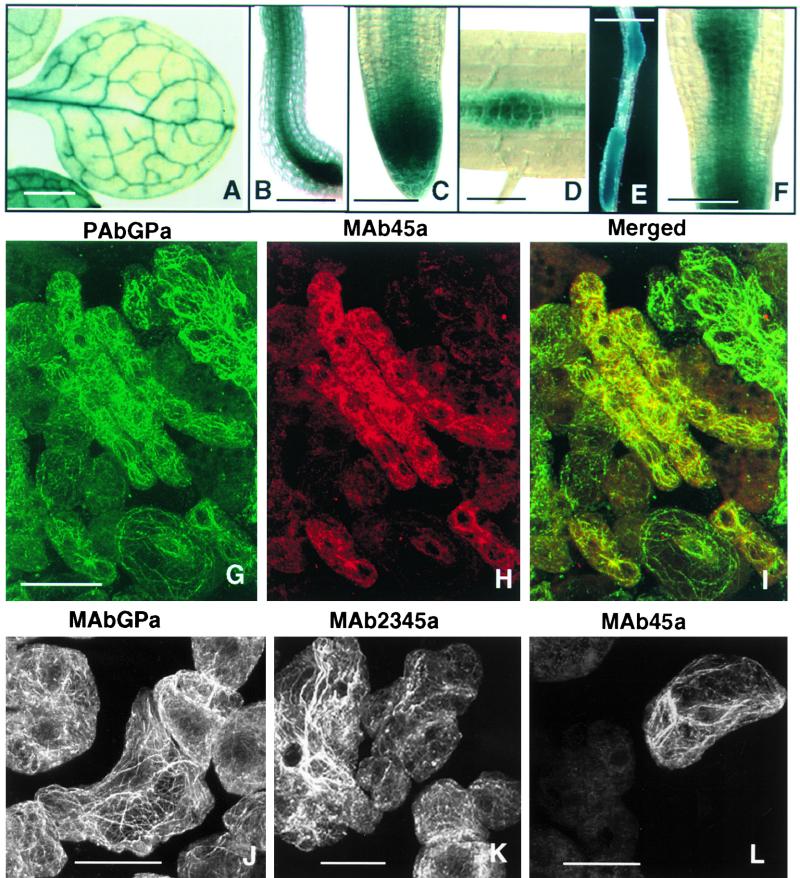
The promoter of ACT7 contains several putative hormone-responsive DNA sequence elements (McDowell et al., 1996a). Thus, we were interested to determine how this gene responds to the exogenous application of hormones during callus formation in Arabidopsis. We used roots from transgenic Arabidopsis plants carrying a translational fusion between the ACT7 5′ flanking region and the β-glucuronidase (GUS) reporter gene (McDowell et al., 1996a). Root explants were incubated on callus-inducing medium (CIM) containing 2,4-D or indoleacetic acid (IAA) and kinetin for induction of callus tissue. For comparison, we performed similar experiments with transgenic roots expressing the 5′ flanking region–GUS fusion from other actins (An et al., 1996a, 1996b; Huang et al., 1997). We then assayed GUS gene expression by histochemically staining the root explants at two different stages of callus formation, as shown in Figure 2. ACT7-GUS showed moderate staining in uninduced control roots but exhibited dark blue staining of all callus tissue 7 and 21 days after hormone treatment. ACT2-GUS, on the other hand, showed very strong staining before and moderate to low staining 7 and 21 days after hormone treatment. ACT1-GUS expression was observed in the lateral root primordia and root tips in untreated roots. In the hormone-induced samples, small segments of roots at the region of lateral root initiation (7 days) and portions of the callus tissue apparently derived from those regions (21 days) showed strong staining. Other fusion genes, such as ACT11-GUS, showed poor staining of roots before and after callus induction. Of all of the gene fusions, the ACT7-GUS fusion showed by far the strongest expression in the total callus tissue (Figure 2). Similar results were obtained in repetitions of this experiment with two other independent ACT7-GUS transgenic lines (data not shown).
Figure 2.
Histochemical GUS Staining of Control and Callus-Induced Roots.
Roots from Arabidopsis transgenic plants harboring four different actin gene promoter–GUS fusions were incubated on CIM containing 1 mg/L 2,4-D and 50 μg/L kinetin for 7 days (7d) or 21 days (21d) and then stained for GUS expression. Note that the strongest staining of root-derived callus tissue is from the ACT7-GUS transformant. Portions of callus from the ACT1-GUS transformant also show strong staining. Control roots were grown on germination medium without hormones. Bar = 2 mm.
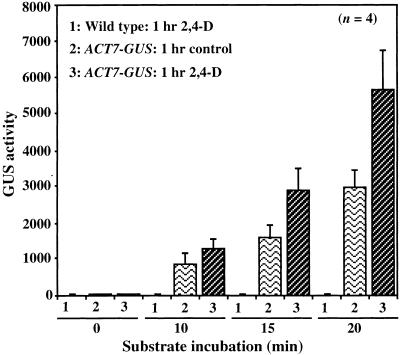
To examine how rapidly the ACT7 promoter responds to hormones, we performed quantitative fluorometric 4-methylumbelliferyl-β-d-glucuronide assays of roots from wild-type and ACT7-GUS transgenic plants at different times after auxin (2,4-D or IAA) treatment. As shown in Figure 3, the kinetics of GUS activity for several plant samples revealed that ACT7 promoter was induced even with a 1-hr auxin treatment. We observed a reproducible 30 to 100% increase in GUS activity in transgenic roots exposed to hormone compared with untreated controls. The wild-type plants showed no GUS activity before or after hormone treatment.
Figure 3.
Auxin Rapidly Accelerates ACT7-GUS Fusion Gene Expression in Transgenic Arabidopsis.
Wild-type and transgenic root samples were incubated on germination medium supplemented with or without (control) 2,4-D for 1 hr and then analyzed for GUS gene expression. Small root fragments were incubated in 4-methylumbelliferyl-β-d-glucuronide substrate, and GUS activity was assayed at different time intervals to validate the linearity of the response. GUS activity is measured in arbitrary fluorescence units (see Methods) per 2-mg sample. An average of four independent readings is presented with the standard error for each sample.
Callus Tissue Induced by Hormones Shows Enhanced ACT7 Isovariant Expression
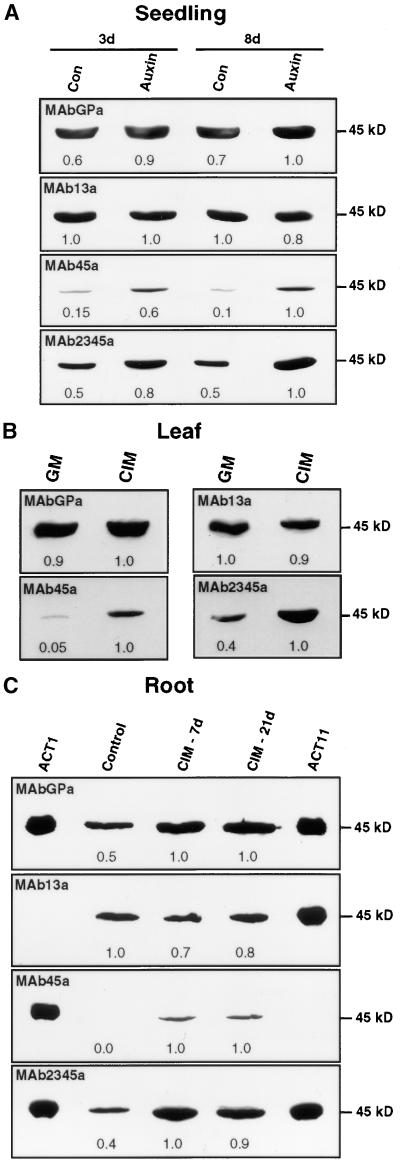
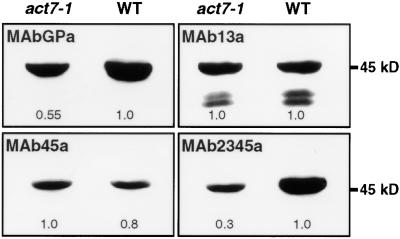
After observing an increase in the activity of ACT7 and ACT1 reporter fusions in response to hormones, we examined whether the expression of the actin isovariants changed in the hormone-induced callus tissue compared with that in the uninduced control. Qualitative and quantitative changes in the level of actin proteins in the hormone-treated tissue were examined by protein gel blot analysis using actin subclass-specific antibodies (Figure 4). Because there is no anti-actin antibody available with specificity for a single actin isovariant or subclass (Figures 1A and 1B), we compared different antibodies on identical blots to determine if particular actin isovariants were induced or suppressed upon hormone treatment.
Figure 4.
Differential Expression of Actin Isovariants during Hormone-Induced Callus Formation in Wild-Type Arabidopsis.
(A) Protein gel blot analysis of actin from seedlings grown in quarter-strength liquid germination medium with (3d and 8d) or without (Con) 2,4-D treatment. Identical blots containing 25 μg of total protein per lane were probed with the general antibody MAbGPa and the subclass-specific antibodies MAb13a, MAb45a, and MAb2345a. The numbers below the blots indicate the relative quantities of actin, with 1.0 being the highest amount in each blot. Similar results were obtained with IAA treatment (not shown).
(B) Protein gel blot analysis of actins in leaves after a 2-week incubation on solid medium with (CIM) and without (germination medium [GM]) hormones.
(C) Protein gel blot analysis of actins from root explants after 7 and 21 days of treatment (7d and 21d) on CIM. ACT1 and ACT11 recombinant proteins were used as controls to show the reactivity of the antibodies.
We probed blots containing protein samples from hormone-treated seedlings, leaves, and roots with different actin antibodies (Figure 4); we then quantified the intensity of the bands detected. MAbGPa revealed almost twice the amount of total actin per microgram of total protein present in the hormone-induced root callus tissues (7 and 21 days) as in the uninduced controls (Figure 4C), whereas the hormone-treated whole seedlings and leaves showed 40 to 50% and 10% increases in total actin, respectively (Figures 4A and 4B). MAb13a, which detects ACT2, ACT8 (subclass 1), and ACT11 (subclass 3), showed no change or only a slight reduction (10 to 20%) in the amounts of these actin isovariants in the hormone-induced leaves and seedlings compared with the control plants (Figures 4A and 4B). In root callus, there was 20 to 30% less of these actins than in control roots (Figure 4C). MAb45a, which detects the reproductive actins ACT1, ACT3, ACT4, and ACT12 (subclasses 4 and 5), did not detect any actin band in the control root extracts (Figure 4C) and detected only very faint bands in the extracts of seedlings (Figure 4A) and leaves (Figure 4B) incubated on germination medium without hormones. In the hormone-induced seedlings, leaves, and roots, there was a significant increase in the level of reproductive actins, but the amount of these actins was still low compared with the total actins present in these tissues (Figure 4).
A critical result was obtained with MAb2345a, which detects ACT7, ACT11, ACT1, ACT3, ACT4, and ACT12 of subclasses 2, 3, 4, and 5. This antibody showed a significant twofold to threefold increase in the amount of actin in the hormone-induced samples compared with the controls (Figure 4). The three subclass-specific antibodies, MAb2345a, MAb45a, and MAb13a, reacted with similar intensity to different recombinant Arabidopsis actins on protein gel blots, as shown in Figure 1B (see also ACT1 and ACT11 in Figure 4C). Therefore, a comparison of results obtained with these antibodies suggests that there are reductions in the levels of ACT2, ACT8, and/or ACT11 and increases in the levels of ACT1, ACT3, ACT4, ACT12, and/or ACT7 isovariants. Although MAb45a detected a significant increase in reproductive actins (ACT1, ACT3, ACT4, and ACT12), the level of their expression was 1 order of magnitude less compared with that of total actin or ACT7. Therefore, ACT7 made by far the greatest contribution to the increase in the total amount of actins in all of the hormonally induced samples.
Reproductive Actins Are Induced in a Subset of Cells during Callus Formation
Because protein gel blot analysis of the hormone-induced seedlings and organs revealed a minor induction of reproductive actins (Figure 4), we wanted to observe the spatial regulation of these actin genes before and after hormone treatment. Histochemical staining of transgenic Arabidopsis plants carrying the ACT1-GUS fusion gene demonstrated an extraordinarily high level of expression of the fusion gene product in pollen (An et al., 1996a). In addition, there was detectable staining in leaf veins (Figure 5A), the central cylinder of the hypocotyl (Figure 5B), the root apical meristem (Figure 5C), and lateral root primordia (Figure 5D). ACT3, another reproductive actin most closely related to ACT1 (Figure 1A), also showed a similar pattern of expression (not shown). Upon hormone treatment, the corresponding regions of leaf, hypocotyl, and roots that stained positively for ACT1 expression underwent rapid cell proliferation and produced callus. As shown for roots, in Figures 5E and 5F, the newly formed cells showed strong blue staining for ACT1-GUS expression.
Figure 5.
ACT1 Is Expressed Only in a Subset of Vegetative Tissues.
(A) to (F) Histochemical GUS staining of a leaf (A), hypocotyl (B), and roots ([C] to [F]) of transgenic Arabidopsis plants containing ACT1-GUS fusions. (C) and (D) show a root tip (C) and a portion of root showing a lateral root primordium (D) before hormone treatment. (E) and (F) show roots incubated for 7 days on hormone-containing CIM.
(G) to (I) Confocal images of hormone-treated root callus tissue (21 days) double labeled with the general polyclonal anti-actin antibody PAbGPa and the reproductive actin-specific monoclonal antibody MAb45a. Actin filaments stained with PAbGPa are shown in green (G), and those stained with MAb45a are shown in red (H). The filaments appear yellow where the green and red signals overlap, as shown in (I).
(J) to (L) Immunofluorescence staining of 3-month-old root-derived callus tissue. Note that MAbGPa (J) and MAb2345a (K) stain actin filaments in all cells, whereas MAb45a (L) stains actin filaments only in a single cell in the field shown.
Bars in (A) and (E) = 500 μm; bar in (B) = 250 μm; bars in (C) and (F) = 100 μm; bar in (D) = 50 μm; bars in (G), (J), (K), and (L) = 25 μm.
To determine how reproductive actin expression was regulated at the cellular level, we double labeled the 21-day-old callus tissue with the general polyclonal antibody PAbGPa and the reproductive actin-specific antibody MAb45a. The latter stained only 30 to 40% of total cells (apparently the meristematic cells), whereas PAbGPa stained actin filaments in all cells (Figures 5G to 5I). In 3-month-old callus tissue, 15 to 20% of cells still stained positively with MAb45a for reproductive actins (Figure 5L). On the other hand, MAb2345a, which detected ACT7 along with all of the reproductive actins (subclasses 2, 3, 4, and 5), stained arrays of actin filaments uniformly in all cells (Figure 5K), very similar to the staining pattern of the general monoclonal antibody MAbGPa (Figure 5J). It is worth noting that long-established cell lines did not contain detectable levels of the reproductive actins as determined by protein gel blot analysis (data not shown) or immunofluorescence microscopy with MAb45a (Figure 1C). Moreover, MAb13a, which stained the two other vegetative actins (ACT2 and ACT8), detected dense arrays of actin filaments in these cell types (Figure 1C). Thus, the ACT7 protein in Arabidopsis and its homologous isovariant in tobacco cells were coexpressed along with the other vegetative actins, with ACT7 as the major actin constituent.
ACT7 Is Essential for Hormone-Stimulated Growth of Callus Tissue
Knowing that the ACT7 gene is induced rapidly in response to hormones and that the ACT7 isovariant constitutes the predominant actin protein in tissue culture cells, we assumed that this gene might have an essential role to play during hormone-induced callus formation. To test this hypothesis, we examined the ability of act7-1 plants (Gilliland et al., 1998), which exhibited significantly lower levels of ACT7 protein (data not shown), to regenerate callus in response to hormones. Although the mutant plants are morphologically very similar to the wild-type plants, they exhibit a deleterious phenotype (Gilliland et al., 1998). We incubated similarly sized cotyledons (Figures 6A and 6B), leaves, and roots from young seedlings of homozygous mutants and wild-type plants on hormone-containing medium to induce callus. There were no detectable differences between the mutant and wild-type samples after 7 days, as shown for cotyledons in Figures 6C and 6D, respectively. However, after 2 to 3 weeks of incubation on CIM, significant differences could be observed in the amount of callus regenerated from the mutant (Figure 6E, left) and wild-type (Figure 6E, right) samples. Enlarged images of callus produced from individual mutant and wild-type cotyledons and leaves after 21 days are shown in Figures 6F to 6I. Similarly, obvious differences in callus regeneration were observed between mutant (Figures 6J, left, and 6K) and wild-type (Figures 6J, right, and 6L) roots after 21 days. Overall, the act7-1 mutant was much slower in inducing callus tissue relative to the wild type in all of the organs tested.
Figure 6.
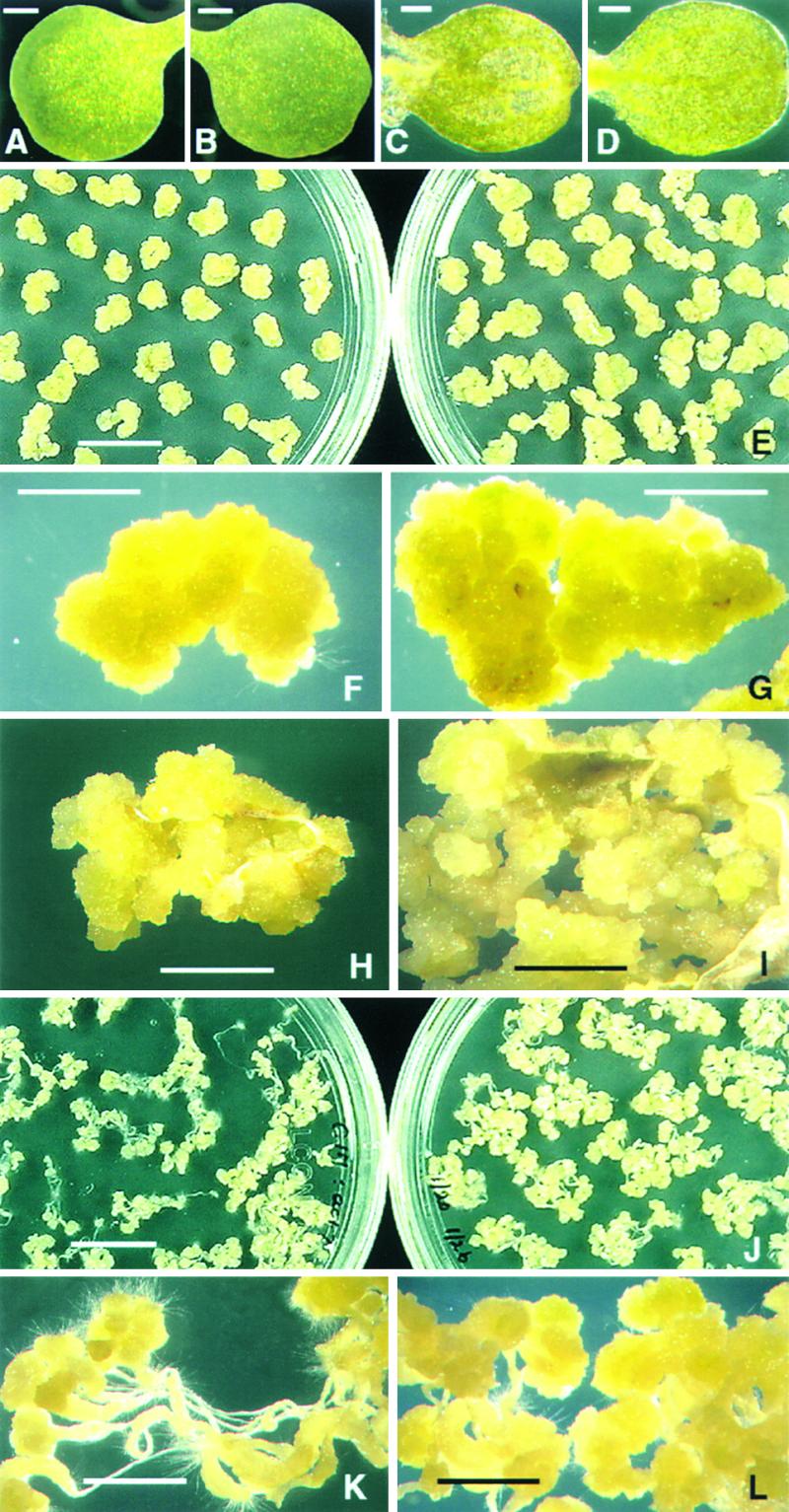
Effect of a Deleterious Mutation in the ACT7 Gene on Hormone-Induced Callus Formation.
Mutant samples are shown in the left panels and wild-type samples are shown in the right panels.
(A) to (G) Young cotyledons of almost similar size before ([A] and [B]) and after 7 days ([C] and [D]) and 21 days ([E] to [G]) of hormone treatment. (F) and (G) show enlarged cotyledon-derived calli from (E).
(H) and (I) Leaf explants after 21 days of incubation on CIM. Similar sized leaves were used for callus regeneration.
(J) to (L) Root explants after 21 days of incubation on CIM.
Bars in (A) to (D) = 1 mm; bars in (E) and (J) = 10 mm; bars in (F), (G), (H), (I), (K), and (L) = 5 mm.
To determine whether the callus phenotype observed in the mutant was attributable to defects in ACT7 gene expression, we performed protein gel blot analysis of protein extracts from the callus tissue produced from wild-type and act7-1 mutant leaves with the general and actin subclass-specific antibodies. The results are depicted in Figure 7. The general antibody MAbGPa revealed an approximately twofold reduction in the level of total actin in the act7-1 mutant compared with the wild-type callus. MAb45a showed a slight increase in the level of reproductive actin subclasses 4 and 5 in the mutant callus, whereas MAb13a showed no detectable changes in the levels of ACT2, ACT8 (subclass 1), and ACT11 (subclass 3) in the mutant and wild-type samples (Figures 1A and 7). MAb2345a detected approximately three times less actin in the mutant callus than in the wild-type control. Thus, the expression of ACT7 of subclass 2 was reduced greatly in the mutant callus tissue. The slow induction of callus in the act7-1 mutant, therefore, can be attributed to the significantly lower level of ACT7 protein expression.
Figure 7.
The ACT7 Protein Is Essential for Normal Callus Formation.
Protein gel blot analysis of leaf callus extracts from the act7-1 mutant and wild-type (WT) plants with the general antibody MAbGPa and the subclass-specific antibodies MAb13a, MAb45a, and MAb2345a.
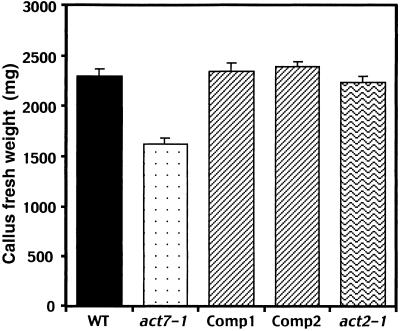
To support this hypothesis, we quantified the levels of callus formation from young cotyledons, leaves, and roots. The act7-1 mutant samples produced 30 to 50% less callus than did the corresponding wild-type controls (Figure 8). Furthermore, we examined the hormone-stimulated growth of callus tissue in act7-1 plants complemented with the ACT7 genomic transgene. We tested young cotyledons and leaves from two independent ACT7-complemented transgenic lines. Both lines showed hormone-induced callus formation restored to the normal level seen in the wild-type control (Figure 8). In addition, we assayed act2-1 plants (Gilliland et al., 1998) for hormonal response. They exhibited no significant defects in callus production, and the amounts of callus formed from leaves and cotyledons closely resembled those in the wild type (Figure 8). Moreover, act2-1 act7-1 double mutants, which looked morphologically stunted (Figure 9A) compared with wild-type plants (Figure 9B) at the seedling stage (L.U. Gilliland, unpublished data), showed poor induction of callus (Figures 9C to 9F). Callus formation from the cotyledons and roots of these double mutants was reduced by approximately 50% compared with that in the wild-type control.
Figure 8.
Complementation of act7-1 Plants with the ACT7 Gene Sequence Reverses the Slow Regeneration of Callus to the Normal Wild-Type (WT) Level.
Forty milligrams of young leaves was incubated on CIM, and after 21 days the fresh weight of the leaf explants was measured. Comp1 and Comp2 represent two independent complemented lines. Note that the callus regenerated from act7-1 mutant leaves had approximately 30% less weight than the wild-type leaf callus. Leaves from the complemented plants and act2-1 mutants produced callus almost equal to the wild-type leaves. The mean values of three different experiments with standard errors are shown.
Figure 9.
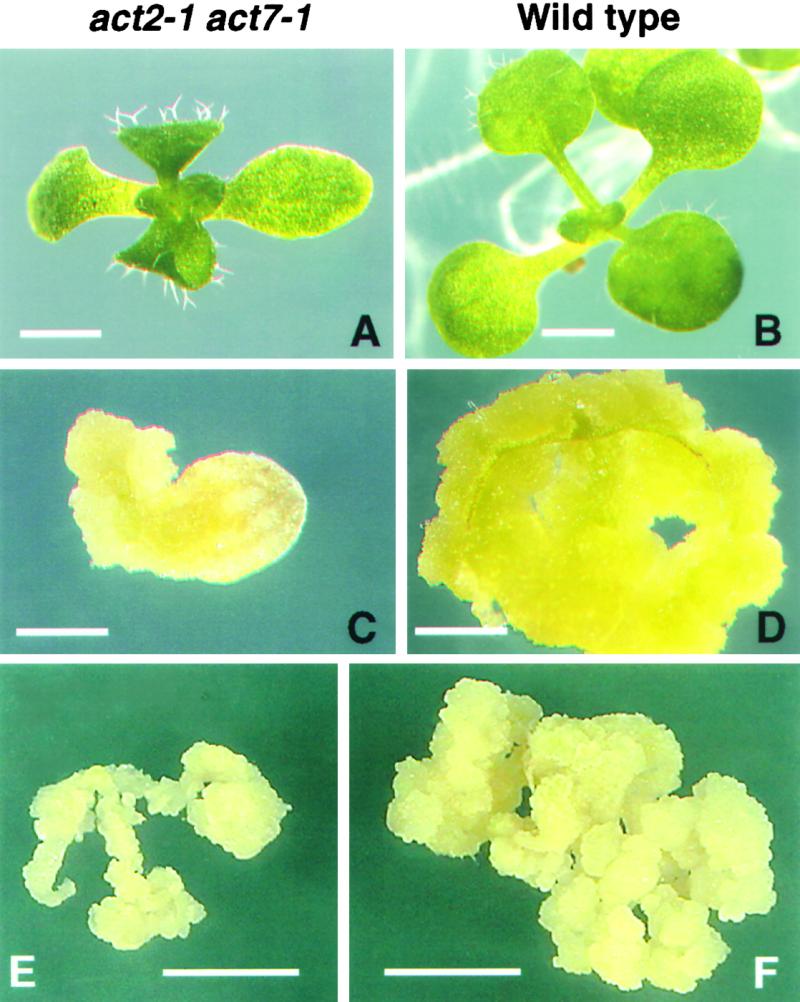
Callus Regeneration Is Severely Affected in the act2-1 act7-1 Double Mutant.
(A) Ten-day-old act2-1 act7-1 double mutant seedling grown on Murashige and Skoog (1962) (MS) medium containing 3% Suc.
(B) Ten-day-old wild-type seedling grown on similar medium.
(C) and (D) The double mutant (C) and wild-type (D) cotyledons after 18 days of incubation on CIM.
(E) and (F) Root explants of double mutant (E) and wild-type (F) plants after 25 days of incubation on CIM.
Bars in (A) to (D) = 1 mm; bars in (E) and (F) = 5 mm.
DISCUSSION
The data presented here provide strong evidence for the active involvement of the Arabidopsis ACT7 gene in the regulation of hormone-induced plant cell proliferation and callus formation. This conclusion is derived from the following six significant observations: (1) the plant hormones auxin and cytokinin or auxin alone preferentially and rapidly stimulated the activity of the ACT7-GUS reporter gene fusion over that of the other actin promoter–GUS fusion genes in transgenic Arabidopsis; (2) the hormones strongly enhanced the expression of the ACT7 protein during induction of callus tissue from different organs of wild-type Arabidopsis plants; (3) the act7-1 mutant showed slow formation of callus compared with the wild-type control; (4) complementation of the act7-1 mutants with the ACT7 gene sequence restored the hormone-induced callus formation to the normal wild-type level; (5) the callus produced from the mutant leaves contained at least two to three times less ACT7 protein compared with the wild-type callus, whereas the expression of all of the other major actin isovariants in leaves was basically unaffected; and (6) in the established culture cell lines, the ACT7 isovariant appeared to be the major actin constituent, whereas the other vegetative actins were present only at lower levels. These hormone-evoked responses of the ACT7 gene corroborated our earlier findings that the 5′ untranslated region of ACT7 contains an active core auxin-responsive sequence, TGTCTC (McDowell et al., 1996a). Direct or palindromic repeats of this element have been shown to be sufficient for auxin induction (Ulmasov et al., 1997, 1999) and form the basis of the DR5-GUS construct that is used by many auxin biologists (Sabatini et al., 1999). Moreover, the ACT7 promoter contains other hormone-responsive elements and responds to several external stimuli, including wounding and other hormones (McDowell et al., 1996a). Also, recently it was shown that an immediate evolutionary homolog of the Arabidopsis ACT7 gene in Malva pusilla is induced during a compatible plant–fungus pathogen interaction (Jin et al., 1999).
Analysis of mRNA steady state levels and the expression of actin-reporter fusions in transgenic plants have shown clearly that each of the eight functional actin genes of Arabidopsis exhibits a distinct pattern of tissue-specific and developmental regulation (Meagher et al., 1999b). Our next objective was to determine whether there is any differential use of actin isovariants during plant growth (cell division and elongation) and morphogenesis (cell differentiation and organ initiation). It is well known that the actin cytoskeleton is very dynamic during cell morphogenesis, forming a number of structurally distinct and functionally specialized arrays in dividing (Eleftheriou and Palevitz, 1992), elongating (Thimann et al., 1992; Thimann and Biradivolu, 1994; Miller et al., 1999), and differentiating (Jung and Wernicke, 1991; Seagull and Falconer, 1991) cells. Furthermore, actin undoubtedly plays a significant role in establishing cell polarity (Fowler and Quatrano, 1997). However, little information is available concerning the preferential deployment of different actin isovariants in any of the processes that contribute to plant development or in the responses of plants to external stimuli. A complete understanding of the functional importance of higher plant actin gene multiplicity requires a thorough knowledge of the spatiotemporal regulation of all of the subclasses of encoded actin protein isovariants. To address this problem, we produced monoclonal antibodies specific to different actin subclasses and used them to determine how the different actin isovariants respond to hormone-induced changes in the morphology and architecture of plant cells and organs. The subclass-specific antibodies were essential to identifying the induction of specific actin isovariants during auxin-induced meristem formation and cell proliferation.
Experiments have indicated that auxin, in concert with other plant growth regulators, profoundly affects cell elongation, cell division, and cell differentiation, thereby altering plant morphogenetic processes such as the development of lateral and adventitious roots and shoots, vascular tissues, trichomes, and tropic responses (Estelle and Klee, 1994; Davies, 1995). It appears that auxin regulates these processes by rapidly modulating the activity of specific auxin-responsive genes that are involved in the execution of vital cellular functions and developmental processes (Abel and Theologis, 1996). Experimental approaches designed to elucidate the molecular mechanism of auxin action have focused on auxin perception, genetic determination of the signaling apparatus, and specific gene activation (reviewed by Abel et al., 1996). In the present study, we investigated the auxin-induced responses of actin cytoskeletal genes, which also play important roles during plant growth and morphogenesis.
When we incubated Arabidopsis cotyledons, leaves, and roots, which showed primarily ACT2 and ACT8 expression, on auxin-containing CIM, initially there was a high degree of cell proliferation in the veins of the cotyledons and the leaves and initiation of organ primordia in the central stele region of the roots. Formation of an undifferentiated mass of callus tissue followed during later stages of subculturing. Protein gel blot analysis revealed an overall increase in the amount of actin present in the hormone-treated explants or the callus tissue derived from them compared with the untreated controls. A significant increase in the level of the ACT7 isovariant contributed primarily to the increase in total actin. This was determined by the comparative quantitative analysis of protein gel blots probed with four different anti-actin antibodies differing in their subclass specificity. A notable induction of reproductive actins (ACT1, ACT3, ACT4, and ACT12) also was detected in the callus tissue using MAb45a. On the other hand, there was little change or even a slight reduction in the levels of ACT2, ACT8, and ACT11 isovariants, as shown by MAb13a. The fine friable callus produced after several subsequent subcultures on CIM still contained increased levels of ACT7 and detectable amounts of reproductive actin isovariants. However, the lack of detectable amounts of reproductive actins in the Fi-3, BY-2, and other long-established suspension cell lines suggested that reproductive actins such as ACT1 and ACT3, which are expressed primarily in the pollen, ovule, and meristematic tissue (Meagher et al., 1999b), are lost during prolonged subculturing. Therefore, the ACT7 protein is present as the major actin component in the well-established tissue culture cells, along with the other vegetative actins ACT2 and ACT8. The high level of ACT7 protein may be due to the constant induction by the phytohormones required to maintain rapidly growing cell cultures.
Our immunocytochemical studies indicated that there is coexpression of the predominant ACT7 and other actin isovariants (e.g., ACT2 and ACT8) in the tissue culture cells and that the monomers of all of these isovariants may polymerize to form heteropolymers of F-actin (our unpublished observations). The copolymerization of different actin isovariants might facilitate more flexibility in the dynamic behavior of actin proteins by allowing them to interact specifically with different actin binding proteins (Meagher et al., 1999a). Because ACT7 is the major actin present in these cells, it is likely that the actin filaments are rich in ACT7 isovariant, and such filaments may be better suited for the dynamic response of the cells to hormones in CIM. For example, among several dynamic properties, ACT7 might polymerize and/or depolymerize more rapidly than other isovariants. Our results suggest that increased expression of the ACT7 gene may be required specifically for the growth and proliferation of tissues and organs in cultures, because this gene might be capable of responding rapidly to auxin and other growth hormones.
Moreover, the act7-1 mutant, which exhibits relatively normal plant morphology but is defective in ACT7 protein expression, was slow to produce callus in response to hormones. In addition, the complementation of the act7-1 mutation with the ACT7 gene sequence restored the ability of the mutant plants to produce callus to wild-type plant levels, confirming that the ACT7 gene is involved in normal callus formation. On the other hand, plants with a mutation in the ACT2 gene, which is more highly expressed in mature vegetative tissue than ACT7, were capable of inducing callus tissue at rates comparable to the wild-type controls. This suggests that ACT2 is not likely to play an active role in callus formation.
In conclusion, our study demonstrates that the Arabidopsis ACT7 gene appears to be essential for the normal growth of callus tissue. A mutant defective in the expression of this gene was unable to produce callus tissue at rates equivalent to the wild type, and complementation of this mutation with the ACT7 gene restored normal levels of callus production. Coexpression of the predominant ACT7 isovariant with the other actins might facilitate isovariant dynamics in a unique manner, a process that would enhance the responses of the actin cytoskeleton to external stimuli such as hormones, wounding, and pathogen attack. This study strongly suggests that the ACT7 protein and its homologs in other plants play an active role in cell proliferation and respond to external stimuli in higher plants.
METHODS
Plant Material and Hormone Application
Arabidopsis thaliana ecotype RLD or Wassilewskija seedlings were grown on plates containing germination medium (full-strength Murashige and Skoog [1962] [MS] salts, MS vitamins, 1% sucrose, and 0.8% phytagar, pH 5.7) at 22°C with a 16-hr photoperiod. Cotyledons and leaf and root tissue from 2- to 3-week-old seedlings were harvested and treated with hormones by incubating them on callus-inducing medium (CIM; MS salts, MS vitamins, 3% sucrose, 0.8% phytagar, 1 mg/L or 4.5 μM 2,4-D, and 50 μg/L or 25 nM kinetin, pH 5.7). For a control, samples were incubated on similar medium without hormones. At different times, proteins were extracted from the explants and analyzed by protein gel blotting for changes in actin isovariant expression. Portions of samples were maintained on CIM for up to several months by regularly subculturing onto new plates every 2 weeks. The callus tissue also was used at different stages for immunofluorescence microscopy. To understand the effect of auxin on actin gene regulation in whole seedlings, 2,4-D (1 mg/L or 4.5 μM) or indoleacetic acid (IAA; 1 mg/L or 5.7 μM) was added directly to 1-week-old seedlings grown in flasks containing quarter-strength liquid germination medium. The hormone-treated seedlings were harvested at different times for protein analysis. For quantitative analysis of callus production, equal amounts (∼40 mg) of young leaves or cotyledons from control and experimental plants were incubated on CIM, and after 21 days the fresh weight of explants was measured. Samples were transferred to new plates after 10 days of incubation, and the experiments were repeated at least three times. The means and standard errors were calculated, and the values were plotted using Excel (Microsoft, Redmond, WA).
Monoclonal Antibody Production
To produce actin subclass-specific antibodies, Arabidopsis ACT2, ACT7, and ACT11 proteins were expressed in Escherichia coli using the pET15b expression system (Novagen, Madison, WI). The recombinant actins were isolated from inclusion bodies, purified to near homogeneity by preparative gel electrophoresis, and partially renatured as described previously for other plant actins (Kandasamy et al., 1999). Several mice were immunized with the purified recombinant actins, and two or three mice showing high antibody levels were identified for each protein by ELISA of serum from a tail bleed. Splenocytes isolated from these mice were fused with myeloma cells (SP2/O) to produce the hybridomas. Monoclonal cell lines producing anti-actin antibodies were identified by ELISA, and the specificity of the antibodies was determined by protein gel blot analysis of hybridoma supernatants with all of the expressed Arabidopsis actins, as described previously (Kandasamy et al., 1999). The cell lines of interest were expanded to produce large quantities of hybridoma supernatant. The antibodies in the supernatant were isolated by ammonium sulfate precipitation and then purified using the Affi-Gel Protein A MAPS II Kit (Bio-Rad). The antibodies were isotyped using Clonotyping System AP (Fisher Biotech, Pittsburgh, PA). The purified antibodies were then used for immunofluorescence microscopy or immunoblot analysis of plant extracts. We used the following antibodies as controls in the present study: MAbGPa, a monoclonal antibody with general plant actin specificity (Kandasamy et al., 1999); PAbGPa, a polyclonal antibody with general plant actin specificity (Kandasamy and Meagher, 1999); and MAb45a, a monoclonal antibody that reacted with actin subclasses 4 and 5 representing four of the five reproductive actins (Kandasamy et al., 1999).
Analysis of GUS Activity of Hormone-Induced Transgenic Roots
Transgenic plants carrying different actin promoter–β-glucuronidase (GUS) fusion genes and showing high levels of expression (ACT1-GUS, ACT3-GUS, ACT2-GUS, and ACT8-GUS [An et al., 1996a, 1996b]; ACT4-GUS and ACT12-GUS [Huang et al., 1996]; ACT11-GUS [Huang et al., 1997]; and ACT7-GUS [McDowell et al., 1996a]) were grown on germination medium. Root segments from these plants were induced with hormones by incubating them on CIM for histochemical analysis or on germination medium supplemented with 4.5 μM 2,4-D or 5.7 μM IAA for fluorometric assay, as described above. Histochemical GUS staining of the root samples before and after 7 or 21 days of hormone treatment was performed as reported previously (An et al., 1996a). For fluorometric quantitation of promoter-GUS expression, 2 mg of both control and hormone-treated (1-hr) root segments was incubated in 4-methylumbelliferyl-β-d-glucuronide substrate (Jefferson et al., 1987) placed in 96-well microtiter plates. After different times (0, 10, 15, and 20 min), the reaction was stopped with 2 M Na2CO3 and GUS activity was measured in fluorescence units using the Biolumin 960 fluorescence microtiter plate reader (Molecular Dynamics, Sunnyvale, CA) at 360/450 nm (excitation/emission). The experiments were repeated two times with duplicate samples for each treatment.
Protein Gel Blot Analysis
The methods for preparing protein samples from plant tissues or from E. coli expressing recombinant actins for protein gel blot analysis have been described (Kandasamy et al., 1999). The proteins were separated on 10% SDS-PAGE gels and blotted onto Immobilon membranes (Millipore, Bedford, MA) by semidry blotting (Hofer, San Francisco, CA). The membranes were then blocked in TBST (10 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween 20) containing 10% goat serum and 5% nonfat dry milk and probed with the anti-actin primary antibodies (0.25 or 0.5 μg/mL) in the blocking solution for 1 hr at room temperature. After washing in TBST (3 × 10 min), the blots were incubated with horseradish peroxidase–conjugated anti-mouse secondary antibody at 1:2000 dilution in the blocking solution for 30 min. The blots were washed again in TBST (3 × 10 min) and treated with enhanced chemiluminescence detection solution (Amersham) for approximately 2 min and then exposed to the Hyperfilm enhanced chemiluminescence system (Amersham). The actin bands were quantified by scanning the film in a densitometer loaded with Image Quant software (Molecular Dynamics). Protein gel blot analysis was repeated at least twice for each treatment and plant organ.
Immunofluorescence Labeling
The hormone-induced callus tissue from roots or Fi-3 (Davis and Ausubel, 1989) and BY-2 (Nagata et al., 1981) suspension cells were used for immunofluorescence microscopy. The samples were fixed in 4% paraformaldehyde in PME (50 mM 1,4-piperazinediethanesulfonic acid, pH 7.0, 5 mM EGTA, 1 mM MgSO4, and 0.5% casein) containing a protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany) for 1 hr at room temperature. Fixation was done either with or without maleimidobenzoyl-N-hydroxysuccinimide ester pretreatment (Sonobe and Shibaoka, 1989). After fixation, the samples were washed in PME (3 × 5 min) and permeabilized by treating with 1% Cellulysin (Calbiochem, San Diego, CA) and 0.1% Pectolyase (Sigma, St. Louis, MO) in PME containing the protease inhibitors for 40 to 60 min. After washing for 5 min in PME and twice for 10 min in PBS, the cells were immobilized onto chromium potassium sulfate– and gelatin-coated slides as described previously (Liu and Palevitz, 1992). The cells on slides were further permeabilized in 0.5% Triton X-100 for 30 min and −20°C methanol for 10 min. After 20 min of treatment with 0.1 M glycine and washing in PBS, the cells were blocked for 1 hr in TBST-BSA-GS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20, 5% BSA, and 10% goat serum).
The slides then were incubated in the primary anti-actin antibody (MAbGPa, MAb2345a, MAb45a, or MAb13a) at 2 to 5 μg/mL in the blocking solution overnight. After rinsing three times for 10 min in PBS, the slides were incubated in fluorescein isothiocyanate–conjugated anti-mouse secondary antibody (Sigma) at 1:100 dilution for 4 hr. Slides were double labeled by incubating in a mixture of PAbGPa and MAb45a overnight and then with fluorescein isothiocyanate–conjugated anti-rabbit (Sigma) and Texas Red–conjugated anti-mouse (Amersham) antisera for 4 hr as described above. After secondary antibody labeling, the slides were washed in PBS (3 × 10 min) and mounted with 80% glycerol in PBS containing 1 mg/mL p-phenylenediamine (Sigma). The actin microfilaments in the labeled cells were visualized with a Bio-Rad MRC-600 confocal laser scanning microscope using suitable filters. The images were then transferred to a PowerMac/7100 computer and further processed using Adobe (Mountain View, CA) PhotoShop software.
Complementation of act7-1 Mutant Plants with the ACT7 Gene Sequence
To verify that the mutant callus phenotype was attributable directly to the disruption of the ACT7 gene, a 4-kb genomic clone containing the ACT7 gene was transformed into Arabidopsis cv Wassilewskija plants by Agrobacterium tumefaciens–mediated transformation. Kanamycin-resistant transformants containing the ACT7 transgene were selected and crossed with act7-1 act7-1 plants. After a generation of selfing, numerous progeny plants were screened by polymerase chain reaction for the presence of the complementing genomic allele and the absence of the native ACT7 gene. Therefore, the resulting complemented plants were homozygous for act7-1, with at least one copy of the ACT7 transgene. Two independent mutant lines containing the transgene were used in the callus regeneration analysis.
Acknowledgments
We thank Dr. Marcus Fechheimer and Gay Gregson for critically reviewing the manuscript and Yolanda Lay and Elizabeth Lytle for providing technical assistance during antibody screening. The monoclonal antibodies were raised at the University of Georgia (UGA) Monoclonal Facility. The confocal microscopy work was performed at the UGA Center for Advanced Ultrastructural Research. This work was supported by funds from the National Institutes of Health (Grant GM 36397-12) and the UGA Research Foundation.
References
- Abel, S., and Theologis, A. (1996). Early gene and auxin action. Plant Physiol. 111, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel, S., Ballas, N., Wong, L.M., and Theologis, A. (1996). DNA elements responsive to auxin. Bioessays 18, 647–654. [DOI] [PubMed] [Google Scholar]
- An, Y.-Q., Huang, S., McDowell, J.M., McKinney, E.C., and Meagher, R.B. (1996. a). Conserved expression of the Arabidopsis ACT1 and ACT3 actin subclass in organ primordia and mature pollen. Plant Cell 8, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Y.-Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996. b). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10, 107–121. [DOI] [PubMed] [Google Scholar]
- Brummell, D.A., and Hall, J.L. (1987). Rapid cellular responses to auxin and the regulation of growth. Plant Cell Environ. 10, 523–543. [Google Scholar]
- Davies, P.J. (1995). Plant Hormones: Physiology, Biochemistry and Molecular Biology (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Davis, K.R., and Ausubel, F.M. (1989). Characterization of elicitor-induced defense responses in suspension-cultured cells of Arabidopsis. Mol. Plant-Microbe Interact. 2, 363–368. [Google Scholar]
- Eleftheriou, E.P., and Palevitz, B.A. (1992). The effect of cytochalasin D on preprophase band organization in root tip cells of Allium. J. Cell Sci. 103, 989–998. [Google Scholar]
- Estelle, M. (1992). The plant hormone auxin: Insight in sight. BioEssays 14, 439–444. [DOI] [PubMed] [Google Scholar]
- Estelle, M., and Klee, H.J. (1994). Auxin and cytokinin in Arabidopsis. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring, NY: Cold Spring Harbor Laboratory Press), pp. 555–578.
- Fowler, J.E., and Quatrano, R.S. (1997). Plant cell morphogenesis: Plasma membrane interactions with the cytoskeleton and cell wall. Annu. Rev. Cell Dev. Biol. 13, 697–743. [DOI] [PubMed] [Google Scholar]
- Fyrberg, E.A., Fyrberg, C.C., Biggs, J.R., Saville, D., Beall, C.J., and Ketchum, A. (1998). Functional nonequivalence of Drosophila actin isoforms. Biochem. Genet. 36, 271–287. [DOI] [PubMed] [Google Scholar]
- Gilliland, L.U., Asmussen, M.A., McKinney, E.C., and Meagher, R.B. (1998). Detection of deleterious genotypes in multi-generational studies. I. Disruptions in individual Arabidopsis actin genes. Genetics 149, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen, G. (1989). Molecular approaches to understanding auxin action. New Biol. 1, 19–23. [PubMed] [Google Scholar]
- Herman, I.M. (1993). Actin isoforms. Curr. Opin. Cell Biol. 5, 48–55. [DOI] [PubMed] [Google Scholar]
- Hightower, R.C., and Meagher, R.B. (1985). Divergence and differential expression of soybean actin genes. EMBO J. 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., An, Y.-Q., McDowell, J.M., McKinney, E.C., and Meagher, R.B. (1996). The Arabidopsis ACT4/ACT12 actin gene subclass is strongly expressed in post-mitotic pollen. Plant J. 10, 189–202. [DOI] [PubMed] [Google Scholar]
- Huang, S., An, Y.-Q., McDowell, J.M., McKinney, E.C., and Meagher, R.B. (1997). The Arabidopsis ACT11 actin gene is strongly expressed in tissues of the emerging inflorescence, pollen and developing ovules. Plant Mol. Biol. 33, 125–139. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, S., Xu, R., Wei, Y., and Goodwin, P.H. (1999). Increased expression of a plant actin gene during a biotrophic interaction between round-leaved mallow, Malva pusilla, and Colletotrichum gloeosporioides f. sp. malvae. Planta 209, 487–494. [DOI] [PubMed] [Google Scholar]
- Jung, G., and Wernicke, W. (1991). Patterns of actin filaments during cell shaping in developing mesophylls of wheat (Triticum aestivum L.). Eur. J. Cell Biol. 56, 139–146. [PubMed] [Google Scholar]
- Kandasamy, M.K., and Meagher, R.B. (1999). Actin-organelle interactions: Association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil. Cytoskeleton 44, 110–118. [DOI] [PubMed] [Google Scholar]
- Kandasamy, M.K., McKinney, E., and Meagher, R.B. (1999). The late pollen specific actins in angiosperms. Plant J. 18, 681–691. [DOI] [PubMed] [Google Scholar]
- Key, J.L. (1964). Ribonucleic acid and protein synthesis as essential processes for cell elongation. Plant Physiol. 39, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B., and Palevitz, B.A. (1992). Organization of cortical microfilaments in dividing root cells. Cell Motil. Cytoskeleton 23, 252–264. [Google Scholar]
- Loof, A.D., Broeck, J.V., and Janssen, I. (1996). Hormones and the cytoskeleton of animals and plants. Int. Rev. Cytol. 166, 1–58. [DOI] [PubMed] [Google Scholar]
- McDowell, J.M., An, Y.-Q., McKinney, E.C., Huang, S., and Meagher, R.B. (1996. a). The Arabidopsis ACT7 actin gene is expressed in rapidly developing tissues and responds to several external stimuli. Plant Physiol. 111, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, J.M., Huang, S., McKinney, E.C., An, Y.-Q., and Meagher, R.B. (1996. b). Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics 142, 587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher, R.B., McKinney, E.C., and Kandasamy, M.K. (1999. a). Isovariant dynamics expands and buffers the responses of complex systems: The diverse plant actin gene family. Plant Cell 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher, R.B., Vitale, A., and McKinney, E.C. (1999. b). The evolution of new structures: Clues from plant cytoskeletal genes. Trends Genet. 15, 278–284. [DOI] [PubMed] [Google Scholar]
- Meagher, R.B., McKinney, E.C., and Kandasamy, M.K. (2000). The significance of diversity in the plant actin gene family: Studies in Arabidopsis. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 3–27.
- Miller, D.D., de Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). The role of actin in root hair morphogenesis: Studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17, 141–154. [Google Scholar]
- Muday, G.K. (2000). Interactions between the actin cytoskeleton and an auxin transport protein. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 541–556.
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nagata, T., Okada, K., Takebe, I., and Matsui, C. (1981). Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol. Gen. Genet. 184, 161–165. [Google Scholar]
- Nick, P. (1999). Signals, motors, morphogenesis: The cytoskeleton in plant development. Plant Biol. 1, 169–179. [Google Scholar]
- Rubenstein, P.A. (1990). The functional importance of multiple actin isoforms. BioEssays 12, 309–315. [DOI] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, P., Leyser, O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Seagull, R.W., and Falconer, M.M. (1991). In vitro xylogenesis. In The Cytoskeletal Basis of Plant Growth and Form, C.W. Lloyd, ed (London: Academic Press), pp. 183–194.
- Shibaoka, H. (1994). Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the cross-linking between microtubules and the plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 527–544. [Google Scholar]
- Sonobe, S., and Shibaoka, H. (1989). Cortical fine actin filaments in higher plant cells visualized by rhodamine-phalloidin after pretreatment with m-maleimidobenzoyl N-hydroxysuccinimide ester. Protoplasma 148, 80–86. [Google Scholar]
- Takahashi, Y., Ishida, S., and Nagata, T. (1994). Function and modulation of expression of auxin-regulated genes. Int. Rev. Cytol. 152, 109–144. [DOI] [PubMed] [Google Scholar]
- Theologis, A. (1986). Rapid gene regulation by auxin. Annu. Rev. Plant Physiol. 37, 407–438. [Google Scholar]
- Thimann, K.V., Reese, K., and Nachmais, V.T. (1992). Actin and the elongation of plant cells. Protoplasma 171, 153–166. [Google Scholar]
- Thimann, K.V., and Biradivolu, R. (1994). Actin and the elongation of plant cells. 2. The role of divalent ions. Protoplasma 183, 5–9. [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov, T., Hagen, G., and Guilfoyle, T.J. (1999). Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 96, 5844–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni, K., and Schopfer, P. (1993). Reorientation of microtubules at the outer epidermal wall of maize coleoptiles by phytochrome, blue-light photoreceptor and auxin. Protoplasma 173, 103–112. [Google Scholar]