Abstract
Herpes simplex virus type 1 (HSV-1) is a neurotropic virus that causes severe disease and death in newborn humans but, to date, it remains unclear how neonatal infection occurs. We show here that the vertical transmission of HSV-1 in mice is mainly hematogenous and involves the colonization of the neonate central nervous system (CNS). HSV-1 DNA was mainly detected in the blood and CNS of the offspring born to latently infected mothers; no significant differences were seen between the viral DNA concentrations in the blood of these mothers and their female progeny (either neonate or adult). The administration of acyclovir during gestation reduced or eliminated both the maternal and the neonatal viral DNA in the blood. Embryo transfer was performed to ensure (as far as possible) that only vertical hematogenous infection took place. Immunohistochemical analysis detected viral proteins in the encephalon of the offspring. Immunofluorescence studies provided immunoreactive evidence of HSV-1 proteins in the neurons of the hippocampus and showed that these viruses can molecularly reactivate after hyperthermia. Neonatal HSV-1 infection therefore appears to be mainly caused by hematogenous vertical transmission, and the viruses that colonize the offspring CNS are capable of molecular reactivation after a period of latency.
Neonatal infection with herpes simplex virus type 1 (HSV-1) or HSV-2 causes severe disease; the mortality and morbidity associated with such infections is high. The source of infection in newborns, however, is often obscure, although it is thought that neonatal HSV-2 infection occurs in the birth canal during delivery. Vertical transmission implies the transmission of the virus from the mother to the fetus, but this can occur via several pathophysiological mechanisms, e.g., true in utero transmission across the placenta, retrograde infection, infection during labor and delivery, or through breast feeding in the immediate postnatal period. HSV infection in neonates can have devastating consequences (14, 23, 43) and usually affects the skin, the eyes, the mucous membranes (SEM disease), or the central nervous system (CNS). In the United States, neonatal HSV-2 infection currently occurs around one in every 2,500 births, but rates are increasing (17, 39). HSV infection during pregnancy may result in miscarriage, the death of the fetus, congenital fetal infection, and malformations (16, 25, 27, 37, 38, 45).
The majority of studies related to vertical transmission of herpesviruses are restricted to HSV-2, and all are based on epidemiological observations that in no case analyze viral infection further than contamination in the birth canal or in neonates. It is accepted that HSV-2 can infect newborns, but no experimental work has ever been performed to show that HSV-1 infection actually occurs during birth. Most neonatal infections are due to HSV-2, although 30% are caused by HSV-1 (1). HSV-1 and HSV-2 infections behave differently even when the CNS is involved. Children with HSV-1 infections tend to suffer a milder, meningitis-like course of disease (46), whereas those infected with HSV-2 have a more serious clinical condition involving encephalitis and convulsions. However, HSV-1 has been specifically implicated in the pathogenesis of a number of neurological diseases, and there is an increasing body of evidence linking it to Alzheimer's disease (AD) (21). Thus, the colonization of a newborn's neural tissues may also be a risk factor for these HSV-1-associated diseases.
To date, neonatal HSV infection has been described as the result of contact between the newborn and HSV-1 or HSV-2 present in the birth canal of an asymptomatic mother during delivery (4, 30, 42). However, the possibility of vertical transmission has not been analyzed. In the present study, several approaches were attempted in order to detect and localize HSV-1 during several pre- and postnatal developmental stages in mice. Special interest was given to detecting HSV-1 in the CNS, both at the DNA and at the protein level. Viral DNA and protein detection among the offspring of latently infected mother mice was demonstrated in fetuses, neonates, and adults. It was noted that females were preferentially infected. The results indicate that viral DNA in the blood is more common than previously believed and that its presence is essential for neonatal infection to occur. Acyclovir treatment of infected mothers during pregnancy reduced or eliminated maternal viral DNA in the blood, as well as viral DNA concentrations in the offspring nervous system. The present study is the first to clearly demonstrate vertical transmission from maternal blood to the offspring CNS. HSV-1 was immunodetected in the hippocampal neurons; viral antigen signals increased after hyperthermic stress.
MATERIALS AND METHODS
Inoculation and dissection.
All experiments were performed in accordance with the guidelines of the European Community Animals Act (Scientific Procedures) of 1986. All animals underwent a period of quarantine. Strict precautions were taken to prevent contamination during inoculation and dissection.
The experimental animals were 478 wild-type C57BL/6 mice. HSV-1 was propagated and titrated by plaque assay in confluent monolayers of Vero cells (8). The HSV-1 KOS strain (kindly supplied by L. Carrasco) was used in all experiments. Fifty-nine female mice were intraperitoneally inoculated with 106 PFU of virus suspension as previously described (8) (Fig. 1). At 37 days postinfection (i.e., at latent infection), these mice were mated with mock-infected (uninfected) male mice. Mock-infected animals were used as controls (n = 10). All of the female mice used as mothers remained without clinical disease and survived until the end of the experiment. Their pups were sacrificed by decapitation at different times around delivery—as fetuses (−1 day), neonates (1 to 3 days), or adults (14 weeks)—and their organs were carefully removed and frozen at −70°C. The organs analyzed in the fetuses were the placentas, spinal cords, and encephalons. Whole blood, the spinal cord, and the encephalon were analyzed in neonates, and whole blood, the adrenal glands, the gonads, the spinal cord, the trigeminal ganglia, and the brain were studied in adults and mothers. For more precise analyses, the brain samples were subdivided into four regions: the midbrain (including the midbrain and nearby structures such as the pons, the medulla, and the superior and inferior colliculus), the ventricles (including the third and lateral ventricles, the thalamus, the hypothalamus, the preoptic area, and the striatum), the cerebral cortex (including the cortex, the temporal, frontal, parietal and occipital lobes, the hippocampus, the corpus callosum, and the olfactory bulbs), and the cerebellum.
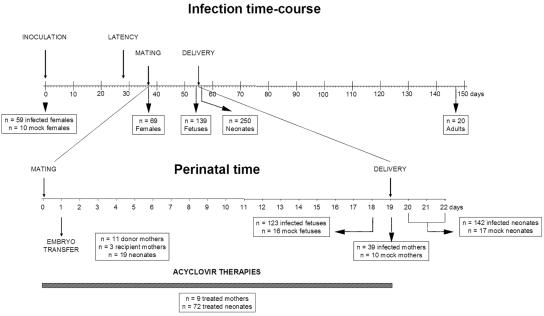
FIG. 1.
Infection and pregnancy, showing the main events in the life of the mouse, the temporal distribution of manipulations, and the number of animals used in each category. The infection time course is shown at the top. A total of 59 14-week-old wild-type C57BL/6 female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) (on day 0). After latency was established (from day 28 onward), these females were mated with mock-infected male mice (on day 37). Their pups were sacrificed and analyzed at different times around delivery, e.g., as fetuses (1 day predelivery or at 18 days of gestation), neonates (1 to 3 days after delivery), or adults (14 weeks postdelivery). The perinatal time is shown at the bottom, indicating the distribution of the experimentally used animals around delivery. At 1 day after mating, rederivation procedures were performed, transferring two-cell embryos from eleven donor C57BL/6 female mice to three recipient Swiss females. Nineteen neonates were obtained. The total numbers of mock-infected and infected mothers, fetuses, and neonates are given. Finally, for acyclovir therapies, nine infected mothers were treated with the antiviral agent, and their viral DNA concentrations were compared to those of nontreated infected mothers. Their offspring were also analyzed in the same way (72 treated compared to 53 nontreated infected neonates).
HSV-1 DNA quantification in tissue homogenates.
DNA from homogenized samples was extracted by using conventional methods (the High Pure PCR template preparation kit [catalog no. 1 796 828]; Roche, Germany). The concentration of HSV-1 DNA in several organs was then quantified by using real-time quantitative PCR as previously described (9). An appropriate concentration range of purified HSV-1 DNA was used for the optimization of the standard curve, and the viral DNA concentration was expressed in terms of viral copy numbers. PCR calibration was performed by using the β-actin housekeeping gene (results were expressed as nanograms of host DNA). Cross-contamination of samples and false-positive PCR results were carefully avoided by frequent changes of gloves, the exclusive use of pipettes, and strict spatial separation of the three main PCR stages. Real-time PCR was performed by using a LightCycler rapid thermal cycler (Roche Diagnostics, Ltd., Lewes, United Kingdom) and a LightCycler FastStart DNA Master SYBR Green I kit (catalog no. 3 003 230; Roche, Germany). β-Actin primers (5′-AAC CCT AAG GCC AAC CGT GAA AAG ATG ACC-3′ and 5′-CCA GGG AGG AAG AGG ATG CGG C-3′) were used as a positive control for the reaction (379-bp PCR product). Specific primers for a sequence in the viral DNA polymerase (Pol) gene (5′-GGT GAA CGT CTT TTC GCA CT-3′ and 5′-GTG TTG TGC CGC GGT CTC AC-3′; 120-bp amplicon) and the thymidine kinase (TK) gene (5′-CG ACG ATC TGC GAC CT-3′ and 5′-TTG CCG TCA TAG CGC GG-3′; 110-bp product) were prepared. The reaction conditions were 95°C for 10 min, followed by 50 cycles at 95°C for 30 s, 55°C for 30 s (for β-actin) or 60°C for 5 s (for TK) or 60° for 30 s (for Pol), and finally 72°C for 40 s. Each experiment was performed in triplicate. Melting curve analyses, agarose and acrylamide gel electrophoresis, restriction analysis, and nested-PCR for TK fragment amplification (primers 5′-CCG ACG ATC TGC GAC CTG-3′ and 5′-ATA GCG CGG GTT CCT TCC-3′; reaction conditions, 95°C for 10 min, followed by 50 cycles of 95°C for 15 s, 60°C for 5 s, and 72°C for 10 s; product, 96 bp) confirmed the specificity of the products. The amplicons of the genes analyzed were cut with either the endonuclease AvaI (for amplicon TK, producing two fragments of 35 and 75 bp) or with Nla IV (producing 87- and 23-bp fragments). The β-actin amplicon was cut with RcaI (producing 236- and 143-bp fragments).
Rederivation procedures.
For rederivation experiments, 11 latently infected C57BL/6 females were used as oocyte donors for in vitro fertilization. After several washes with antibiotic, 45 two-cell-stage embryos from infected mothers were collected and transferred to three pseudopregnant Swiss female mice according to the protocol of Suzuki et al. (40). Briefly, 1-day-old embryos were collected from the oviducts of superovulated latently infected donor female mice kept with uninfected males to obtain fertilized embryos. The collected embryos were incubated in prewarmed medium until their transfer to pseudopregnant Swiss recipients (8 to 12 weeks old). These animals were anesthetized by an intraperitoneal injection with 15 ml of a solution containing 2% Rompun (Bayer, Germany) and 50 mg of ketolar (Parke-Davis, United States)/ml per kg, and the oviduct and top of each uterus were carefully exposed. Pools of embryos were then transferred into the oviduct of each recipient (seven to eight in each uterine horn) by using a mouth pipette with a hand-pulled transfer needle. After transfer, the body wall was sutured, and the skin was closed by using wound clips. The offspring (nine females and ten males in all) and recipient mothers were sacrificed at day 1 postdelivery, and their organs were analyzed for HSV-1 DNA by using real-time PCR as previously described.
Acyclovir therapy.
To determine the effect of acyclovir (Zovirax; Glaxo Wellcome, Spain) on the vertical transmission of HSV-1, 16 latently infected mothers (nine treated and seven nontreated mice) and their progenies were analyzed by scoring the mortality and viral DNA concentrations (expressed as viral copy numbers). Three groups of mothers were treated with acyclovir under different regimens from the day they presented the vaginal plug until the day of birth. These were compared to a control infected group that received no acyclovir. The acyclovir was administered by adding it to the drinking water (250 mg/ml provided ad libitum) or by administering a fixed subcutaneous or oral dose of 30 mg/kg/day administered three times per day. Comparisons were made with the usual doses used in the treatment of clinical neonatal HSV infection (41).
Histological procedures and immunodetection of viral antigens.
Immunohistochemical detection was used to assess HSV-1 infection in the offspring. Two antibodies were used to demonstrate the immunoreactivity of the virus: one that reacts with the tegument protein VP16 (a transcriptional activator of immediate-early gene products (31) and another that recognizes all of the major glycoproteins present in the viral envelope (known as HSV-1). Spinal cords and brains belonging to 15 fetuses and 30 neonates from latently infected mothers were examined for immunoreactive evidence of HSV-1. Fetuses were obtained from pregnant mice at 18 days of gestation; the neonates were obtained at 1 day postdelivery. The organs were washed with 10 ml of phosphate-buffered saline (PBS) and fixed in formaldehyde 10% in phosphate buffer, dehydrated, and embedded in paraffin wax. Sections (7 μm thick) were then processed by using the avidin-biotin-phosphatase complex (ABC-AP) method for VP16 and indirect immunofluorescence for the HSV-1 antigen. To retrieve the antigen, sections were deparaffinated, hydrated, and incubated with 0.1 M citrate buffer (pH 6) for 1 min in a conventional pressure cooker (28).
For the ABC-AP method, the sections were rinsed in Tris-buffered saline (TBS) and incubated with 3% normal donkey serum (NDS) in TBS containing 0.05% Triton X-100 for 30 min to prevent nonspecific binding of the primary antibody. The sections were then incubated overnight at 4°C with rabbit polyclonal anti-VP16 (BD Biosciences Clontech; 1:100 dilution) primary antibody diluted in TBS containing 3% NDS and 0.05% Triton X-100. The sections were then washed in TBS and incubated for 1 h with an anti-rabbit biotinylated immunoglobulin (Amersham Biosciences, United Kingdom), diluted 1:500 in TBS plus 0.3% NDS and 0.005% Triton X-100. After being washed in TBS, the sections were incubated with avidin-biotin-phosphatase complex (Dako, Barcelona, Spain) for 45 min and developed by using the AP-red substrate kit (Zymed Laboratories, Inc., San Francisco, CA). Counterstaining was performed with hematoxylin to emphasize the absence of nonspecific immunostaining. All sections were mounted in Acuatex (Merck, Darmstadt, Germany).
In indirect immunofluorescence assays to detect the HSV-1 antigen, the sections were rinsed in PBS and then incubated with 3% NDS in PBS containing 0.05% Tween 20 (PBST) for 30 min to prevent nonspecific binding of the primary antibody. This was followed by incubation for 1 h at 37°C with rabbit polyclonal anti-HSV-1 (Dako, Denmark) diluted 1:100 in PBST. The sections were then washed in PBST and incubated for 30 min at 37°C with Alexa 488 goat anti-rabbit immunoglobulin G (Invitrogen, California) diluted 1:50 in PBST. All sections were mounted in Vectashield HardSet mounting medium (Vector Laboratories, California); this is very appropriate for immunofluorescence techniques, given its antifade and antiphotobleaching properties.
For negative controls, contiguous sections of each sample were incubated as described above but without the primary or secondary antibodies or using the antibodies preabsorbed with an excess of purified antigens. The organs of offspring from mock-infected females were included in every experiment to monitor the specificity of viral localization. At least six sections per organ were examined to ensure sufficient observations were made.
Immunofluorescence analysis of hippocampal cells in culture.
Hippocampal cells were obtained from the fetuses of pregnant mice at 18 days of gestation as previously described (18). Cells were plated in Neurobasal medium (Gibco) containing 10% heat-inactivated horse serum and supplemented with N2 components (Gibco) and 2 mM glutamine. Experiments were performed after 1 day of growth in vitro. The following antibodies were used to detect HSV-1: rabbit polyclonal anti-HSV-1 antibodies (Dako, Denmark) (these react mainly with the major glycoproteins of the viral envelope); rabbit polyclonal anti-VP16 (BD Biosciences Clontech, United States), which recognizes the VP16 transcriptional activation domain; and mouse monoclonal anti-ICP4 immediate-early protein antibodies (Abcam, United Kingdom). Rabbit anti-cow glial fibrillary acidic protein (GFAP; Dako, Denmark) was used to identify glial cells. Neurons were stained by using the monoclonal anti-MAP2 antibody (Sigma); this antibody recognizes all forms of this major microtubule-associated protein of brain tissue. The secondary antibodies used for immunostaining were horseradish peroxidase-coupled goat anti-mouse antiserum (Vector, United States), fluorescein isothiocyanate-coupled goat anti-rabbit (BD Biosciences Clontech, United States), and Texas red-coupled goat anti-rabbit immunoglobulin G (Amersham Biosciences, United States). Cells were grown on coverslips, gradually fixed with increasing concentrations of paraformaldehyde (2, 3, and 4%), and incubated with antibodies. To visualize DNA, DAPI (4′,6′-diamidino-2-phenylindole) was added 10 min before the end of the procedure. Cells were examined with a Bio-Rad confocal microradiance microscope or Zeiss Axiovert 135 fluorescence microscope equipped with a ×100 oil immersion objective lens (Neofluor), and filters were optimized for triple-label experiments (fluorescein isothiocyanate, Texas red, and DAPI fluorescence). Pictures were taken with a digital camera Spot RT slider (Diagnostic) using Metamorph imaging software. The images obtained were processed with Adobe Photoshop 7.0.
Molecular reactivation of latent infection in offspring hippocampal neurons.
Hippocampal neuron cultures from fetuses of latently infected mothers were prepared as described above. Cultures were heated in a constant-temperature incubator capable of controlling the temperature to within ±0.1°C (model 311; Forma Scientific, United States; equipped with a digital readout). Cultures were incubated for 1 h or 3 h at 43°C in 5% CO2. At 24 h posthyperthermia, immunoreactive evidence of HSV-1 was detected by using the immunofluorescence protocol described above.
Statistical analysis.
The Fisher exact test was used to compare viral DNA concentrations. The Student two-tailed t test was used to compare litter survival and size. Significance was set at P < 0.05.
RESULTS
Survival of offspring born to latently infected mothers.
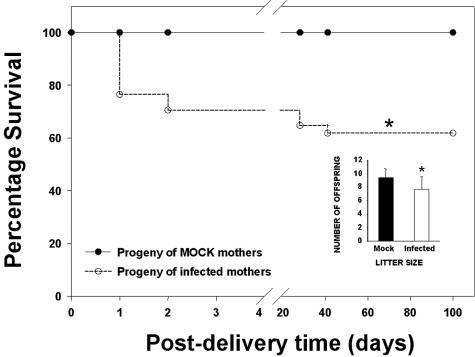
The offspring of mock (inoculated with saline solution) and latently infected mother mice were monitored daily from postdelivery day 1 to 100. All offspring of mock-infected mothers survived until the end of the experiments; those born to infected mothers, however, suffered significantly higher mortality (Fig. 2). The most critical moment was between days 1 and 2 postdelivery; during this period the mortality was close to 30%. The infected female progenitors, although always asymptomatic (especially in genital analyses), experienced mating problems. The fact that a vaginal plug forms in these mice does not necessarily indicate they are pregnant. In addition, those that do become pregnant may have a miscarriage or give birth to malformed offspring. The sex ratio in descendants was close to 1 (50.1% females to 49.9% males). Infected females experienced a sharp reduction in litter size, generating on average 7.7 ± 1.9 offspring per female compared to 9.4 ± 1.3 in their mock-infected counterparts (P < 0.01) (Fig. 2, inset).
FIG. 2.
Kaplan-Meier plot showing the percent survival postdelivery of mock-infected animal offspring (solid line) and of mothers latently infected with HSV-1 (dotted line). A group of female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice, and progeny survival was monitored until postdelivery day 100. Uninfected control mothers received a saline solution, and the survival of their progeny was recorded identically. (Inset) Infected females generated significantly fewer offspring than did mock-infected females. ✽, P < 0.01 (Student two-tailed t test).
Detection and quantification of HSV-1 DNA in the offspring at different developmental stages.
The detection and quantification of HSV-1 DNA was performed in fetuses, newborns, and adult offspring. A total of 110 of 116 fetuses (94.8%), 106 of 120 neonates (88.3%), and all of the 20 adults examined (100%) showed detectable levels of HSV-1 DNA. This indicates that the majority of offspring born to infected mothers were also infected, independent of their developmental stage. These results also suggest there may be an enrichment of HSV-1 DNA with age. The encephalon consistently showed the greatest infectivity, confirming the preferential tropism of HSV-1 toward the CNS of the offspring in this route of infection.
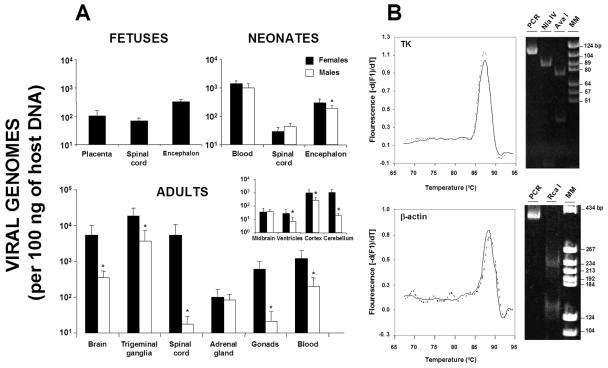
HSV-1 DNA was predominantly detected in the nervous system of the fetuses (Fig. 3A), with the encephalon showing the highest concentrations, followed by the spinal cord. In neonates, viral DNA was mainly located in the blood, followed by the encephalon and spinal cord. Interestingly, the HSV-1 DNA concentrations of the encephalon in fetuses and neonates were not significantly different (P > 0.05). In the adult offspring, HSV-1 DNA showed a preference for the nervous system (trigeminal ganglia, brain, and spinal cord), followed by the blood. In the brain, HSV-1 DNA was mainly detected in the cortex and cerebellum, followed by the midbrain and ventricles. Every PCR product generated prominent bands of the expected sizes in gel electrophoresis and restriction analyses (Fig. 3B). Moreover, the amplicons showed the same specific melting temperatures as the standards used.
FIG. 3.
(A) Quantification of HSV-1 DNA concentrations in fetuses, neonates, and adults by gender. A group of female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice, and several organs from the descendants (116 fetuses [−1 day], 120 neonates [1 to 3 days postdelivery], and 20 adults [14 weeks of age]) were analyzed. The bar graph represents the viral copy number detected in each organ expressed on a logarithmic scale. The inset shows dissected areas of the brain in adults analyzed by gender. Values are the means ± the standard errors of the mean (SEM) of the quantity of viral DNA (expressed as viral genomes and normalized with respect to the quantity of mouse genomes in 100 ng of host DNA by amplifying the β-actin housekeeping gene). The Fisher exact test was used to compare the values for the two genders (✽, P < 0.01). (B) Confirmation of PCR amplification specificities of TK and β-actin. Melting peaks were examined for the TK and β-actin with quantitative standard samples (solid lines) and a total DNA sample (dashed line). Gel electrophoresis of the PCR products was performed on an 8% acrylamide gel. The melting temperatures and sizes were, respectively, 87.2 ± 0.2°C and 110 bp for the TK amplicon and 88.7 ± 0.2 and 379 bp for the β-actin amplicon. The identity of the amplified products was further confirmed by restriction analysis (TK was cut with the NlaIV endonuclease [producing two fragments of 87 and 23 bp] or with AvaI [fragments of 35 and 75 bp]). The β-actin amplicon was cut with RcaI, producing two fragments of 236 and 143 bp (MM; DNA Molecular Weight Marker V; Roche, Germany).
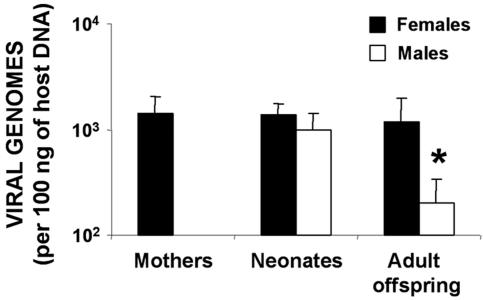
To examine the influence of gender on vertical HSV-1 transmission, the concentrations of viral DNA of the two sexes were studied in neonates and adults (Fig. 3A). No differences were seen between the sexes in the blood and spinal cord viral DNA concentrations of newborns, while the sex ratio was close to 1 (48.1% male and 51.9% female mice). However, the brains of female neonates showed significantly higher viral DNA concentrations than did those of male mice (P < 0.01). In adults, gender-based differences were more evident due to the enrichment of virus levels with aging; significantly higher viral DNA levels were found in the blood, gonads, and nervous systems of females than males (P < 0.01). The adrenal glands of both sexes showed similar viral DNA concentrations (P > 0.05). In the dissected brain, the order of viral DNA concentrations in female mice was cerebellum, cortex, midbrain, and ventricles; significantly greater viral DNA levels were seen in the females in every region, except in the midbrain. With respect to viral copy numbers in blood in the offspring, higher HSV-1 DNA concentrations were consistently related to female gender; the levels of viral DNA of the mothers, female newborns, and female adult offspring were not significantly different (Fig. 4).
FIG. 4.
Determination of HSV-1 DNA concentration in the blood of several groups of animals by gender. Fifty-nine female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice, and the viral copy numbers in the blood of the mothers and neonates were determined at 1 day postdelivery. The viral copy numbers in the blood of 20 adult descendants (14 weeks of age) were also analyzed. The bar graph represents the viral copy number expressed on a logarithmic scale. Values are the means ± the SEM of the quantity of viral DNA (expressed as viral genomes and normalized with respect to the quantity of mouse genomes in 100 ng of host DNA by amplifying the β-actin housekeeping gene). The Fisher exact test was used to compare the values for the two genders (✽, P < 0.001).
Viral screening in embryo transfer experiments.
Two-cell-stage embryos from latently infected females were transferred to recipient mothers, and the offspring were analyzed at 1 day postdelivery. Viral DNA was screened and quantified in the donors, recipient females, and offspring. All donor females were found to harbor HSV-1 DNA and showed organ viral DNA concentrations similar to those of the latently infected females (J. S. Burgos, C. Ramirez, A. Brachet, J. M. Alfaro, I. Sastre, and F. Valdivieso, unpublished data). No viral DNA was detected in the recipients or offspring. The apparent lack of the virus in the recipient mothers and their offspring shows that viral transmission does not occur via the oocyte and that no viral contamination of the recipient mothers occurred through experimental manipulation.
Effect of maternal acyclovir treatment on vertical transmission.
To determine the effects of prenatal acyclovir therapy on the offspring of latently infected mothers, the mortality and viral DNA concentrations of these progeny were determined in four groups of differently treated mother mice: (i) controls (no acyclovir treatment), (ii) mice that received acyclovir in their drinking water (250 mg/ml ad libitum), (iii) mice that received the agent via a subcutaneous injection (30 mg/kg/day), and (iv) mice that received acyclovir orally (30 mg/kg/day). A total of 125 newborns from the four categories were examined. The sex ratio was close to 1 (50.8% male and 49.2% female mice).
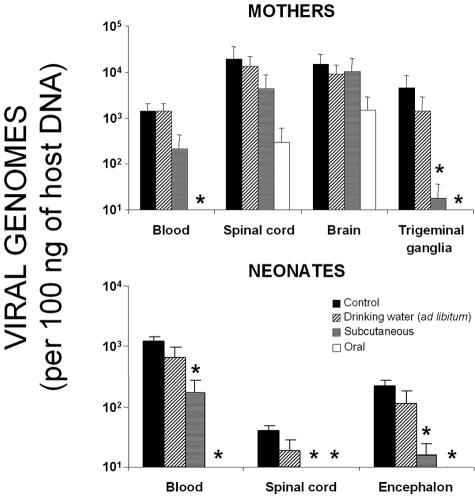
Analysis of the mothers consistently showed the most effective treatment to be oral administration, followed by subcutaneous injection and finally administration via the drinking water (Fig. 5). With the drinking water protocol, no significant differences in viral DNA concentration were seen for any tested organ with respect to the control group. Subcutaneous therapy reduced the HSV-1 DNA concentration in every organ assessed, although this was only significant in the trigeminal ganglia (P < 0.001). The oral treatment significantly reduced virus levels in the trigeminal ganglia and blood (P < 0.001). However, no significant reductions were seen in the brain or spinal cord compared to the controls.
FIG. 5.
Quantification of HSV-1 DNA concentrations in infected mothers and newborns treated under different acyclovir regimens. A group of 16 female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice, and the viral copy numbers of several organs from infected mothers and neonates were determined at 1 day postdelivery. Mothers were separated into four acyclovir treatments groups: control (saline solution), drinking water administration (acyclovir diluted 250 mg/ml in the drinking water; supplied ad libitum), subcutaneous injection (30 mg/kg/day in three injections), and oral administration (30 mg/kg/day in three oral administrations per day). Therapy started on the first day that the mothers showed the vaginal plug and lasted until the day of birth. The results were compared to those obtained in a control infected group that received no acyclovir. The viral copy numbers of the organs of 125 newborns (total) from the four categories were determined. The bar graph shows the viral copy number expressed on a logarithmic scale. Values are the means ± the SEM of the quantity of viral DNA (expressed as viral genomes and normalized with respect to the quantity of mouse genomes in 100 ng of host DNA by amplifying the β-actin housekeeping gene). The Fisher exact test was used to compare the results obtained with the different treatments (✽, P < 0.001).
The percent mortality of the offspring at day 1 postdelivery depended on the treatment received by the mother. The drinking water regimen led to lethality levels not significantly different from those observed when no treatment was received at all (23.3% compared to 23.5%; P > 0.5). The subcutaneous and oral administration routes were more effective at preventing mortality: the subcutaneous regimen led to an offspring mortality rate of ca. 4.0% (P < 0.05), and no newborns died when their mothers received acyclovir orally.
With respect to offspring HSV-1 DNA concentrations, the most effective treatment was again oral administration; with this regimen, no virus was detected in any of the tested organs (blood, spinal cord, and brain) (P < 0.001). The ad libitum regimen led to virus levels not significantly different to those seen in control mice. The subcutaneous injection of acyclovir led to a significant reduction in HSV-1 DNA concentrations in all of the studied organs but was less effective than the oral protocol. Interestingly, the viral DNA levels detected in offspring encephalons were directly proportional to the viral DNA concentrations in blood in their mothers after acyclovir treatment. This confirms the hematogenous connection between HSV-1 infection in mothers and their newborns.
Immunohistochemical detection of HSV-1 proteins in the nervous systems of fetuses and neonates.
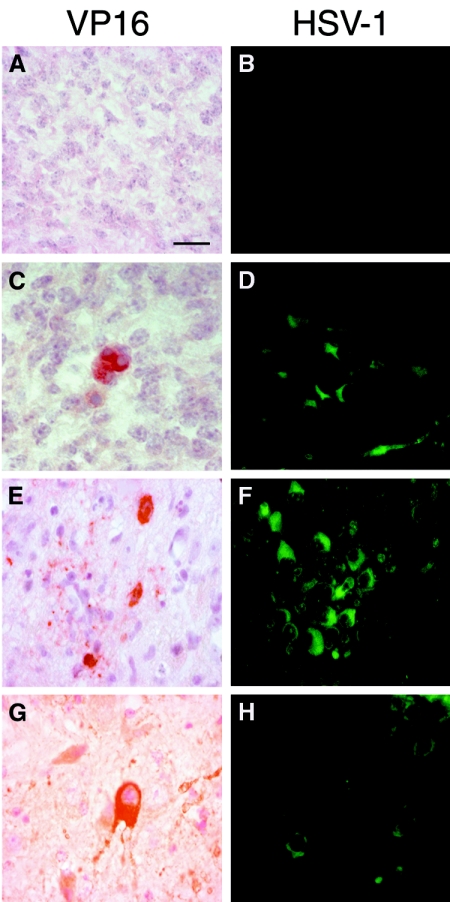
To rule out nonspecific staining, several negative controls were performed. First, the fetal encephalon samples from mock-infected mothers showed no positive VP16 or HSV-1 signal (Fig. 6A and B). Neither were any such signals observed in neonate brains from mock mothers (data not shown). Further, no reaction was observed in negative controls incubated with preimmune serum samples or when we used preabsorbed antibodies with an excess of purified antigens in samples of fetal and neonatal brains from infected female mice (data not shown).
FIG. 6.
Immunohistochemical detection of HSV-1 antigens in the encephalon of fetuses and neonates. A group of seven female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice. From these, 15 fetuses were obtained for analysis on day 18 of gestation, plus another 30 neonates which were analyzed at 1 day postdelivery. Their organs were embedded in paraffin wax and serially sectioned. Immunodetection was performed by using two specific antibodies: anti-VP16 (against the tegument viral protein, revealed by a red chromogenic substrate) and anti-HSV-1 (which recognizes viral glycoproteins, detected by indirect immunofluorescence) as described in Materials and Methods. To rule out nonspecific viral staining, several controls were performed. The sections of fetal encephalon obtained from mock-infected female mothers showed no reaction to VP16 (A) or HSV-1 (B) antigens. (C) VP16 specific staining was observed, however, in some neurons of the cortex of fetal encephalons obtained from infected female mice. (D) Similarly, HSV-1 glycoprotein signals were detected by immunofluorescence in several neurons of the fetal encephalon from infected mothers at a similar location as described above, although more infected cells were visible. (E and F) Coronal sections of female neonate brains from infected mothers showed specific VP16 staining in a few neurons of the midbrain region (E), while HSV-1 glycoprotein signals were detected in a higher number of neurons (F). (G and H) Sections of male neonate encephalons from infected mothers showed specific immunoreactions with VP16 in the cytoplasm of neurons from the midbrain region (G), while HSV-1 immunoexpression was detected more strongly in mesencephalic neurons (more weakly in male mice) (H). Bar, 20 μm.
VP16 and HSV-1 were detected, however, in the encephalons of fetuses and in the CNS of neonates from infected mothers. In fetuses, viral antigens were seen in the cortical neurons, where they showed a cytoplasmic staining pattern (Fig. 6C and D). In neonates, VP16 and HSV-1 were immunodetected in neurons of the midbrain in both females (Fig. 6E and F) and males (Fig. 6G and H). Antigens were also revealed in the gray matter of the spinal cord in both sexes (data not shown).
Anti-HSV-1 signals were detected in more neurons than were anti-VP16 signals. However, independent of the antibody used, the staining intensity and the number of infected neurons was greater in female brains than in male neonate brains (compare Fig. 6E and F to Fig. 6G and H). The location of the viral antigens was always cytoplasmic.
HSV-1 immunodetection in fetal hippocampal primary cultures.
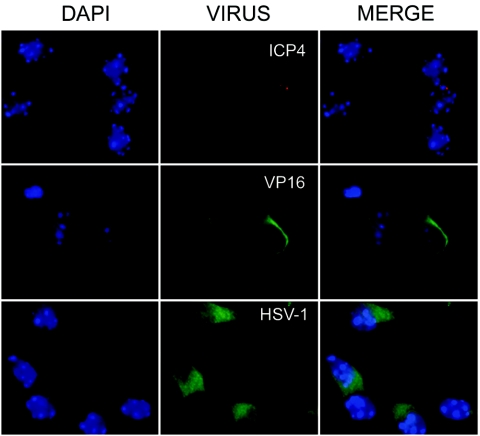
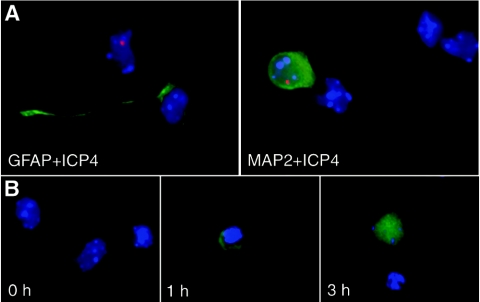
The detection of viral antigens in primary hippocampal neuron cultures showed HSV-1 antigens to be present in fetal cells. Staining for ICP4 revealed single or multiple discrete dots within cell nuclei (Fig. 7). VP16 showed perinuclear signals while immunodetection with the anti-HSV-1 antibody was restricted to the cytoplasm. Cell identification was based on morphology and on differential immunostainings for neurons (anti-MAP2 detection) and glial cells (anti-GFAP staining). HSV-1 antigens were detected only in neurons (positive for MAP2 and negative for GFAP); glial cells were consistently negative with every viral antibody tested (Fig. 8A). These results show that HSV-1 is located in the hippocampal neurons of fetuses but not in glial cells.
FIG. 7.
HSV-1 immunolocation in mouse fetal hippocampal cells from latently infected mothers. Female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice. Hippocampal cells were then obtained from fetuses at 18 days of gestation, plated, and after 1 day in vitro were analyzed by using several antibodies. Immunofluorescence analysis shows discrete dots marked with the anti-ICP4 antibody (middle top; red) and perinuclear signals stained with anti-VP16 (middle center; green). Cytoplasmic immunodetection was demonstrated by using an anti-HSV-1 marker (middle bottom; green). DAPI images of chromatin staining are shown on the left, and merge images are shown on the right.
FIG. 8.
Characterization and molecular reactivation of HSV-1 in mouse fetal hippocampal cells from latently infected mothers. Female mice were infected intraperitoneally with 106 PFU of HSV-1 (KOS strain) and mated at 37 days postinfection with mock-infected male mice. Hippocampal cells were obtained from fetuses at 18 days of gestation and plated and analyzed after 1 day in vitro. (A) Culture characterized to identify the cell type where the viral signal was detected. For this purpose, immunofluorescence analysis was performed by using anti-ICP4 (red) viral and anti-GFAP (top left; green) glial or anti-MAP2 neuronal (top right; green) antibodies. The viral antigens were found only in the hippocampal neurons and not the glia. (B) Immunofluorescent image showing molecular reactivation after hyperthermia. After plating, cultures were subjected to 0, 1, or 3 h at 43°C in 5% CO2; after 24 h, VP16 (green) viral protein was detected with a clear increase in signal strength over treatment time. DAPI staining was used to mark the nuclei of the cells.
Increase in immunofluorescence over time after hyperthermic treatment of fetal hippocampal primary cultures.
To molecularly reactivate HSV-1 harbored in offspring neurons, hippocampal primary cultures were exposed to conditions of hyperthermia for 0, 1, and 3 h, and observed 24 h later. VP16 immunofluorescence analysis was used to test for viral amplification. The hyperthermia treatment increased the VP16 signals. Immunostaining was strong in the perinuclear region after 1 h of exposure, but at 3 h the entire cytoplasm was filled with viral antigens. The main neuron prolongations also showed signals after 3 h of treatment (Fig. 8B). These results indicate that the molecular reactivation of the HSV-1 in the hippocampus of the offspring can occur under stress conditions.
DISCUSSION
HSV-2 is one of the most commonly encountered perinatal pathogens; infection can cause considerable sequelae including long-term neurodevelopmental damage. Even infection acquired near the time of labor can cause neonatal herpes and perinatal morbidity (14, 23, 43). To date, the contraction of neonatal herpes has only been described in studies based on genital contamination, and the information available is restricted to HSV-2. The present results show that intrauterine infection with HSV-1 is likely to be more common than previously thought. However, it may often go unnoticed because of the low viral loads in the blood and CNS of offspring. The results also suggest, for the first time, that a truly hematogenous route of in utero infection is possible and may even be common when HSV-1 is latent in the mother. Once the fetus is infected, the viruses colonize the brain in general and the hippocampus in particular, from where they might be reactivated by a number of stimuli, including delivery. Since viral infection cannot be readily studied in humans, animal models are required, although they can be extremely hard to evaluate. An animal model that can monitor the vertical transmission of HSV-1 from the early stages of infection is highly desirable, although clearly there are many differences between the course of HSV-1 infection in humans and rodents. The present results suggest, however, that mice provide an appropriate model for the study of the hematogenous vertical route of infection and for work on treatment protocols, antiviral agents, and vaccines.
HSV-1 has been related to several neurological diseases, especially AD (20, 21, 26); indeed, a physical association between HSV-1 antigens and the amyloid precursor protein has been recently reported (2, 33). The present results show that enrichment of the virus occurs with aging and that infection is more strongly associated with female gender—also a risk factor for AD (15). Daw et al. (11) indicate that between 30 and 70% of the total variance in AD might be explained by the influence of an unidentified single gene (which should be fairly easy to identify after the human genome has been entirely deciphered) (11). Moreover, these authors suggest that “transmissible environmental effects could potentially mimic genes”; the results presented here indicate that HSV-1 may act in this way. We have also reported that HSV-1 infection may be a risk factor for AD, acting with apolipoprotein E (ApoE) and dependent upon ApoE dosage (8), ApoE4 isoform efficiency (7), the brain region involved, and female gender (6). Moreover, the APOE genotype of the latently infected mother affects the vertical transmission of HSV-1, where the encephalons of the offspring of APOE4 mothers present significantly more viral load than those of the APOE3 mothers (manuscript in preparation).
The present results show that HSV-1 can be detected at the DNA and protein level in the majority of fetuses and neonates (ca. 90%) born to latently infected mothers and that viral shedding increases with development and aging; all adults originally born to such females had detectable HSV-1 DNA. The presence of HSV-1 in fetuses (detected by real-time PCR and immunodetection), especially in the CNS, indicates that mother-to-infant transmission does not occur solely in the birth canal. The percentage of infected fetuses suggests that the risk of acquisition is relatively uniform throughout pregnancy and that at delivery HSV-1 is already lodged in the offspring CNS. The offspring encephalon was consistently the organ with the greatest viral copy number, indicating the neurotropic character of the virus in this infection pathway. The mortality levels detected here are in agreement with the fetal mortality reported in clinical studies (5, 10). The authors of the latter study report that HSV infection of the newborn occurs within a short time after birth, suggesting the possibility of intrauterine infection. In the present study we detected perinatal mortality among the offspring born to virus carrying females but which showed no sign of disease. Transmitting mothers shed virus at delivery but have subclinical HSV-1 infection (4); when combined with the sometimes subtle symptoms of infection in the infant, a diagnosis may not be made until the development of serious disease.
In the present study, several direct and indirect pieces of evidence demonstrate the hematogenous vertical transmission of HSV-1: (i) in the fetuses the placenta and the nervous system consistently show detectable viral DNA levels; (ii) the blood was the organ with the greatest viral DNA concentration in neonates, followed by the brain; (iii) the blood and nervous system were the organs with the highest viral DNA level in adult offspring; (iv) the number of viral genomes in the blood of the mothers, newborns, and adults were not significantly different; (v) reducing the maternal viral DNA concentration in the bloodstream with acyclovir caused newborn HSV-1 DNA levels to become reduced or even disappear; and (vi) the embryo transfer experiments clearly demonstrate that virus is not harbored in the oocyte; this confirms our previous finding that HSV-1 cannot be immunodetected in oocytes (6). Further, PCR techniques have confirmed that viremia is common in neonatal HSV infection (12, 22, 24). These results indicate that the virus passes through the placenta, with viral shedding occurring from the maternal bloodstream and infecting the fetus. The detection of HSV-1 DNA in the bloodstream during latency does not indicate that there is latent virus in the blood. The virus is present in blood only when the trigeminal ganglia are infected, suggesting that the latter play an important role as a viral reservoir in latently infected mice, supplying significant quantities of virus to the blood (6). This is the first report of a natural, hematogenous mechanism of HSV-1 infection leading to viral colonization of fetal nervous tissue. The results also explain the viremia commonly detected in neonates.
Gender-dependent infection was observed both in neonates and adults, with females showing significantly higher levels of viral DNA and proteins in the encephalon. This confirms our previous findings (6). The immunohistochemical tests detected HSV-1 antigens in the encephalic cortical neurons of fetuses and in the mesencephalic region of neonate brains, with more foci in female than male neonates. The immunofluorescence assays using primary cultures detected HSV-1 antigens in fetal neurons, where the viruses were molecularly reactivated after hyperthermia. It has long been known that artificial pyrexia is a very efficient inducer of HSV reactivation in humans (3, 32) and in cultures (34). Detectable VP16 signals increased after hyperthermia, with intense immunofluorescence 3 h into the treatment. This is a new approach for inducing HSV-1 molecular reactivation, which is notoriously difficult to achieve in experimental animals (19, 29, 36), especially for the KOS strain (34); the present study describes the earliest molecular reactivation ever recorded in the developing nervous system. It also provides the first definitive demonstration in a purely in vivo model that neurons are the site of HSV-1 molecular reactivation; this could be important with respect to the onset of neurological disease since reactivating neurons show degenerative changes eventually leading to their death (34).
Nucleoside analogues such as acyclovir that specifically inhibit herpesvirus DNA synthesis and replication (13, 35) can significantly shorten and ameliorate the severity of HSV infections (35, 44). This therapy has been of particular use in the treatment of neonatal infection (41). In the present study, the treatment of the mothers with this antiviral agent provided direct protection against the development of neonatal herpes in their offspring, with the oral administration route being the most effective.
The present results suggest that efforts to reduce the morbidity associated with neonatal HSV-1 infection should concentrate on preventing maternal acquisition of HSV-1 infection during pregnancy and attempting to block the vertical transmission of the virus to infants from seropositive mothers. The latter will commonly be asymptomatic but should have clinical histories showing incidences of herpes-related disease.
To our knowledge, this is the first report to demonstrate hematogenous vertical transmission of HSV-1. The results show that infection of the offspring occurs via the bloodstream, with the invading virus colonizing the fetal CNS. They also suggest that disseminated disease and mortality in neonatal HSV-1 infection is caused by viremia. HSV-1 DNA is present in the blood and CNS of fetuses, neonates, and adults born to infected mothers, indicating that infection occurs before birth. HSV-1 DNA concentrations then increase with age. In addition, HSV-1 was located in the brain of the offspring at both the DNA and the protein levels, especially in the hippocampal neurons where the virus can increase after hyperthermia.
Prenatal antiviral therapy was shown to be of benefit in preventing the vertical transmission of HSV-1. The present study provides insight into the pathobiology of HSV-1 and the treatment of its associated diseases. It may also throw light on the use of vectors based on herpesviruses to locate genetic constructs in the nervous system without the need for genetic, cellular, or surgical interventions.
Acknowledgments
This study was supported by a grant from the Obra Social Caja Madrid to the Asociación de Familiares de Enfermos de Alzheimer and by an institutional grant by the Fundación Areces to the Centro de Biología Molecular Severo Ochoa. J.S.B. and C.R. were, respectively, supported by a contract with and a grant from the Universidad Autónoma de Madrid.
We thank F. Mayor for his continuous encouragement and help and L. Carrasco for providing the HSV-1 KOS strain. We are grateful to J. Ripoll for excellent technical assistance.
REFERENCES
- 1.Annunziato, P. W., and A. Gershon. 1996. Herpes simplex virus infections. Pediatr. Rev. 17:415-424. [PubMed] [Google Scholar]
- 2.Bearer, E. L. 2004. Perspectives on herpes-APP interactions. Aging Cell 3:81-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boak, R., C. M. Carpenter, and S. L. Warren. 1934. Symptomatic herpetic manifestations following artificially induced fevers. J. Bacteriol. 27:83-87. [Google Scholar]
- 4.Brown, Z. A., J. Benedetti, R. Ashley, S. Burchett, S. Selke, S. Berry, L. A. Vontver, and L. Corey. 1991. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N. Engl. J. Med. 324:1247-1252. [DOI] [PubMed] [Google Scholar]
- 5.Brown, Z. A., S. Selke, J. Zeh, J. Kopelman, A. Maslow, R. L. Ashley, D. H. Watts, S. Berry, M. Herd, and L. Corey. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509-515. [DOI] [PubMed] [Google Scholar]
- 6.Burgos, J. S., C. Ramirez, I. Sastre, J. M. Alfaro, and F. Valdivieso. 2005. Herpes simplex virus type 1 infection via the bloodstream with apolipoprotein E dependence in the gonads is influenced by gender. J. Virol. 79:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgos, J. S., C. Ramirez, I. Sastre, M. J. Bullido, and F. Valdivieso. 2003. ApoE4 is more efficient than E3 in brain access by herpes simplex virus type 1. Neuroreport 14:1825-1827. [DOI] [PubMed] [Google Scholar]
- 8.Burgos, J. S., C. Ramirez, I. Sastre, M. J. Bullido, and F. Valdivieso. 2002. Involvement of apolipoprotein E in the hematogenous route of herpes simplex virus type 1 to the central nervous system. J. Virol. 76:12394-12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgos, J. S., C. Ramirez, R. Tenorio, I. Sastre, and M. J. Bullido. 2002. Influence of reagents formulation on real-time PCR parameters. Mol. Cell Probes 16:257-260. [DOI] [PubMed] [Google Scholar]
- 10.Corey, L., R. J. Whitley, E. F. Stone, and K. Mohan. 1988. Difference between herpes simplex virus type 1 and type 2 neonatal encephalitis in neurological outcome. Lancet i:1-4. [DOI] [PubMed] [Google Scholar]
- 11.Daw, E., H. Payami, E. Nemens, D. Nochlin, T. Bird, G. Schellenberg, and E. Wijsman. 2000. The number of trait loci in late-onset Alzheimer disease. Am. J. Hum. Genet. 66:196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond, C., K. Mohan, A. Hobson, L. Frenkel, and L. Corey. 1999. Viremia in neonatal herpes simplex virus infections. Pediatr. Infect. Dis. J. 18:487-489. [DOI] [PubMed] [Google Scholar]
- 13.Elion, G. B., P. A. Furman, J. A. Fyfe, P. de Miranda, L. Beauchamp, and H. J. Schaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 74:5716-5720.202961 [Google Scholar]
- 14.Enright, A. M., and C. G. Prober. 2002. Neonatal herpes infection: diagnosis, treatment, and prevention. Semin. Neonatol. 7:283-291. [DOI] [PubMed] [Google Scholar]
- 15.Evans, D., M. Ganguli, T. Harris, C. Kawas, and E. B. Larson. 1999. Women and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 13:187-189. [DOI] [PubMed] [Google Scholar]
- 16.Florman, A. L., A. A. Gershon, P. R. Blackett, and A. J. Nahmias. 1973. Intrauterine infection with herpes simplex virus. Resultant congenital malformations. JAMA 225:129-132. [PubMed] [Google Scholar]
- 17.Forsgren, M., E. Skoog, S. Jeansson, S. Olofsson, and J. Giesecke. 1994. Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983, and 1989: implications for STD epidemiology. Int. J. STD AIDS 5:113-116. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Billault, C., J. Avila, and A. Caceres. 2001. Evidence for the role of MAP1B in axon formation. Mol. Biol. Cell 12:2087-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbour, D. A., T. J. Hill, and W. A. Blyth. 1983. Recurrent herpes simplex in the mouse: inflammation in the skin and activation of virus in the ganglia following peripheral stimulation. J. Gen. Virol. 64(Pt. 7):1491-1498. [DOI] [PubMed] [Google Scholar]
- 20.Itzhaki, R. F., C. B. Dobson, and M. A. Wozniak. 2004. Herpes simplex virus type 1 and Alzheimer's disease. Ann. Neurol. 55:299-301. [DOI] [PubMed] [Google Scholar]
- 21.Itzhaki, R. F., W. R. Lin, D. Shang, G. K. Wilcock, B. Faragher, and G. A. Jamieson. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241-244. [DOI] [PubMed] [Google Scholar]
- 22.Kimura, H., M. Futamura, H. Kito, T. Ando, M. Goto, K. Kuzushima, M. Shibata, and T. Morishima. 1991. Detection of viral DNA in neonatal herpes simplex virus infections: frequent and prolonged presence in serum and cerebrospinal fluid. J. Infect. Dis. 164:289-293. [DOI] [PubMed] [Google Scholar]
- 23.Kohl, S. 1997. Neonatal herpes simplex virus infection. Clin. Perinatol. 24:129-150. [PubMed] [Google Scholar]
- 24.Malm, G., and M. Forsgren. 1999. Neonatal herpes simplex virus infections: HSV DNA in cerebrospinal fluid and serum. Arch. Dis. Child Fetal Neonatal 81:F24-F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell, J. E., and F. C. McCall. 1963. Transplacental infection by herpes simplex virus. Am. J. Dis. Child. 106:207-209. [DOI] [PubMed] [Google Scholar]
- 26.Mori, I., Y. Kimura, H. Naiki, R. Matsubara, T. Takeuchi, T. Yokochi, and Y. Nishiyama. 2004. Reactivation of HSV-1 in the brain of patients with familial Alzheimer's disease. J. Med. Virol. 73:605-611. [DOI] [PubMed] [Google Scholar]
- 27.Naib, Z. M., A. J. Nahmias, W. E. Josey, and J. H. Wheeler. 1970. Association of maternal genital herpetic infection with spontaneous abortion. Obstet. Gynecol. 35:260-263. [PubMed] [Google Scholar]
- 28.Norton, A. J., S. Jordan, and P. Yeomans. 1994. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J. Pathol. 173:371-379. [DOI] [PubMed] [Google Scholar]
- 29.Openshaw, H., L. V. Asher, C. Wohlenberg, T. Sekizawa, and A. L. Notkins. 1979. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J. Gen. Virol. 44:205-215. [DOI] [PubMed] [Google Scholar]
- 30.Prober, C. G., P. A. Hensleigh, F. D. Boucher, L. L. Yasukawa, D. S. Au, and A. M. Arvin. 1988. Use of routine viral cultures at delivery to identify neonates exposed to herpes simplex virus. N. Engl. J. Med. 318:887-891. [DOI] [PubMed] [Google Scholar]
- 31.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 32.Roizman, B., and A. E. Sears. 1990. Herpes simplex viruses and their replication, p. 1795-1841. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 33.Satpute-Krishnan, P., J. A. De Giorgis, and E. L. Bearer. 2003. Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of Alzheimer's disease. Aging Cell 2:305-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaeffer, H. J., L. Beauchamp, P. de Miranda, G. B. Elion, D. J. Bauer, and P. Collins. 1978. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272:583-585. [DOI] [PubMed] [Google Scholar]
- 36.Shimeld, C., T. J. Hill, W. A. Blyth, and D. L. Easty. 1990. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J. Gen. Virol. 71(Pt. 2):397-404. [DOI] [PubMed] [Google Scholar]
- 37.Sieber, O. F., Jr., V. A. Fulginiti, J. Brazie, and H. J. Umlauf, Jr. 1966. In utero infection of the fetus by herpes simplex virus. J. Pediatr. 69:30-34. [DOI] [PubMed] [Google Scholar]
- 38.South, M. A., W. A. Tompkins, C. R. Morris, and W. E. Rawls. 1969. Congenital malformation of the central nervous system associated with genital type (type 2) herpesvirus. J. Pediatr. 75:13-18. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan-Bolyai, J., H. F. Hull, C. Wilson, and L. Corey. 1983. Neonatal herpes simplex virus infection in King County, Washington: increasing incidence and epidemiologic correlates. JAMA 250:3059-3062. [PubMed] [Google Scholar]
- 40.Suzuki, H., K. Yorozu, T. Watanabe, M. Nakura, and J. Adachi. 1996. Rederivation of mice by means of in vitro fertilization and embryo transfer. Exp. Anim. 45:33-38. [DOI] [PubMed] [Google Scholar]
- 41.Whitley, R., A. Arvin, C. Prober, S. Burchett, L. Corey, D. Powell, S. Plotkin, S. Starr, C. Alford, J. Connor, et al. 1991. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. N. Engl. J. Med. 324:444-449. [DOI] [PubMed] [Google Scholar]
- 42.Whitley, R., A. Arvin, C. Prober, L. Corey, S. Burchett, S. Plotkin, S. Starr, R. Jacobs, D. Powell, A. Nahmias, et al. 1991. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. N. Engl. J. Med. 324:450-454. [DOI] [PubMed] [Google Scholar]
- 43.Whitley, R. J. 2002. Herpes simplex virus infection. Semin. Pediatr. Infect. Dis. 13:6-11. [DOI] [PubMed] [Google Scholar]
- 44.Whitley, R. J., and J. W. Gnann, Jr. 1992. Acyclovir: a decade later. N. Engl. J. Med. 327:782-789. [DOI] [PubMed] [Google Scholar]
- 45.Witzleben, C. L., and S. G. Driscoll. 1965. Possible transplacental transmission of herpes simplex infection. Pediatrics 36:192-199. [PubMed] [Google Scholar]
- 46.Yamamoto, L. J., D. G. Tedder, R. Ashley, and M. J. Levin. 1991. Herpes simplex virus type 1 DNA in cerebrospinal fluid of a patient with Mollaret's meningitis. N. Engl. J. Med. 325:1082-1085. [DOI] [PubMed] [Google Scholar]