Abstract
Perforin-mediated cytotoxicity is a major effector function of virus-specific CD8 T cells. We have investigated the expression of perforin in the gut, an important site of simian immunodeficiency virus (SIV) pathogenesis, during experimental SIV infection of rhesus macaques. We observed significant increases in perforin protein and mRNA expression levels in the colons of SIV-infected macaques as early as 21 days after infection. However, during chronic infection, despite ongoing viral replication, perforin expression returned to levels similar to those detected in SIV-naïve animals. These findings demonstrate the presence of a robust perforin-positive response in gastrointestinal CD8 T cells during acute, but not chronic, SIV infection.
CD8 T cells play a major role in the control of human immunodeficiency virus type 1 (HIV-1) replication in humans (4, 9) and simian immunodeficiency virus (SIV) replication in macaques (7, 17). Perforin- and granzyme-mediated induction of apoptosis in target cells is a primary effector function of CD8 T cells (16). Perforin clearly has a nonredundant role in successful granule-mediated apoptosis (20), and studies of perforin knockout mice have shown that it is essential for the control of a number of viral infections (8).
Mucosal tissues contain the majority of lymphocytes and are an important site of HIV-1 and SIV replication and pathogenesis (22). Studies of SIV-infected macaques have shown a profound depletion of CD4 T cells in the gastrointestinal mucosa as early as 14 days postinfection, before such depletion can be observed in blood or peripheral lymphoid tissues (19, 21). A similar early and preferential loss of intestinal CD4 T cells in HIV-1-infected humans has recently been reported (3, 11). The goal of this study was to examine the kinetics of perforin expression in the gastrointestinal tract as an indicator of a cytotoxic T-cell response during the course of acute, early, and chronic SIV infection. To this end, we examined animals euthanized at sequential time points post-intravaginal inoculation with SIVmac251. This provided us with a model of mucosal infection, the most common route of HIV-1 infection globally and thus the most important in terms of understanding disease pathogenesis (12, 13). Using this model, we have recently demonstrated that systemic virus replication is detectable between 6 and 10 days postinoculation (13).
We previously observed that CD8 T cells of the rectal mucosa expressed little perforin both in healthy humans and in patients with chronic HIV-1 infection (18). CD8 T cells in blood samples from HIV-positive individuals expressed ample perforin, while CD8 T cells in the rectal mucosa did not. This finding led us to hypothesize that expression of perforin protein in the gastrointestinal mucosa may be tightly regulated. Damage to the integrity of the mucosal epithelial surface could compromise the natural barrier to commensal or pathogenic bacteria; therefore, stringent control of cytotoxicity might be important for the host. However, constitutively low levels of perforin in the gastrointestinal tract might also provide an inadvertent advantage to HIV-1 and other intracellular pathogens.
Given the low level of perforin expression in the gastrointestinal mucosa, we hypothesized that gut CD8 T cells might fail to mount an adequate cytotoxic response to acute HIV or SIV infection. To address this hypothesis, we examined the relationship between virus replication and perforin expression in the blood, spleen, and colon in 20 SIV-infected rhesus macaques that were euthanized at acute, early, or late time points postinfection as well as three SIV-naïve animals. Animal designations and necropsy times postinfection are given in Table 1.
TABLE 1.
Plasma, spleen, and colon viral loads of animals and times of necropsy postinfection with SIVmac251
| Animal | Time of necropsy (days postinfection) | No. of SIV vRNA copiesa in:
|
||
|---|---|---|---|---|
| Plasma | Spleen | Colon | ||
| 31426 | 1 | Neg | Neg | Neg |
| 31937 | 1 | Neg | Neg | ND |
| 30201 | 2 | Neg | Neg | 6.9 × 102 |
| 28420 | 3 | Neg | Neg | Neg |
| 31373 | 4 | Neg | Neg | Neg |
| 31385 | 4 | 1.5 × 103 | Neg | Neg |
| 30991 | 6 | 2.3 × 104 | 6.9 × 103 | 2.6 × 103 |
| 31523 | 6 | 7.3 × 104 | 4.6 × 104 | 3.8 × 104 |
| 26222 | 7 | 2.6 × 106 | ND | 1.3 × 105 |
| 27337 | 21 | 3.6 × 107 | 6.5 × 105 | 5.3 × 105 |
| 28240 | 21 | 4.0 × 106 | 2.5 × 105 | 1.8 × 105 |
| 31443 | 22 | 7.0 × 105 | 1.9 × 105 | 8.0 × 104 |
| 34474 | 29 | 1.0 × 105 | 3.9 × 104 | 9.2 × 102 |
| 34144 | 35 | 4.6 × 105 | 7.8 × 104 | 7.6 × 104 |
| 24181 | 47 | 6.9 × 107 | 6.6 × 105 | 1.6 × 106 |
| 29271 | 65 | 5.0 × 104 | 6.8 × 104 | 2.4 × 104 |
| 25537 | 180 | 7.1 × 105 | 7.2 × 105 | 1.0 × 105 |
| 31423 | 180 | 2.4 × 103 | 1.2 × 104 | 1.9 × 103 |
| 25402 | 180 | 6.6 × 102 | 2.8 × 102 | 6.2 × 102 |
| 31435 | 180 | 2.3 × 106 | 8.0 × 105 | 3.2 × 105 |
| 31931 | Naïve | ND | Neg | Neg |
| 34572 | Naïve | ND | Neg | Neg |
| 33630 | Naïve | ND | Neg | Neg |
Plasma viral load is reported as viral RNA (vRNA) copies per 1 ml of plasma, and tissue viral RNA levels are reported as viral RNA copies per 1 μg of total tissue RNA. All viral loads were measured by a quantitative branched-DNA assay (5). The detection limit of this assay is 125 copies/ml of plasma and 200 copies/μg of total tissue RNA (13). ND, not determined. Neg, no copies detected.
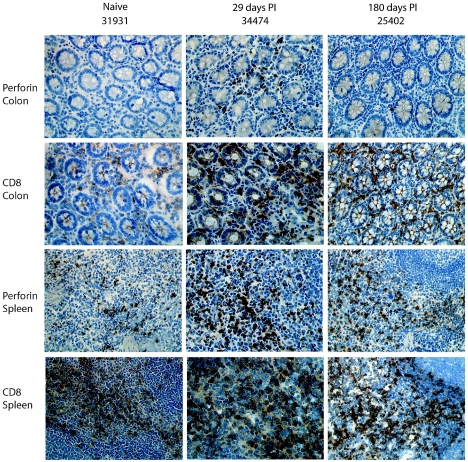
Figure 1 shows perforin expression in tissues as assessed by immunohistochemistry. This approach allowed us to detect the spatial localization of the protein and to compare sequential sections from the same tissue pieces. Spleen tissue provided a good positive control for perforin detection, since it contains many granular lymphocytes that have recently left the peripheral blood (10). Monoclonal mouse anti-human perforin immunoglobulin G (IgG) (clone PF344, which cross-reacts with macaque perforin) (14), biotinylated anti-mouse and the substrate 3′-diaminobenzidine tetrahydrochloride (both from Vector Laboratories, Burlington, CA), or goat anti-mouse Alexa-488 (Molecular Probes, Eugene, OR) were used to stain perforin (Fig. 1). Photomicrographs of tissue sections were captured using a Leica RXM light microscope and analyzed using computerized acquired-image analysis to measure the total perforin-positive area and express it as a percentage of the total cell area, as previously described (2). A minimum area of 0.4 mm2 within at least 10 random magnified fields was analyzed per tissue. For colon tissue, only the lamina propria was analyzed, and thus epithelial cells, enterocytes, and intraepithelial lymphocytes were excluded. For the spleen, only the red pulp was measured to avoid analyzing B-cell follicles, since they are typically low in T cells. CD8+ cells were stained with monoclonal rabbit anti-CD8 IgG from DBS (Pleasanton, CA) and biotinylated goat anti-rabbit IgG from Vector Laboratories or Alexa-568-conjugated goat anti-rabbit IgG (Molecular Probes). Intracellular perforin was assessed for CD3+ CD8+ peripheral blood mononuclear cells by flow cytometry using directly conjugated monoclonal antibodies, anti-CD3 Per-CP, anti-CD8 allophycocyanin-Cy7 (BD Pharmingen, California), and anti-perforin-fluorescein isothiocyanate. Regulation of perforin expression at the transcriptional level may be important for the safe and effective production and release of this cytotoxic protein (6). To investigate the relationship between perforin mRNA and protein levels in tissues, we quantified perforin mRNA in colon and spleen tissues using real-time PCR (1). Colon, spleen, and plasma viral loads were assessed at the time of necropsy by a quantitative branched-DNA assay (5), and the resulting measurements are presented in Table 1.
FIG. 1.
Perforin expression in colon and spleen in SIV-positive and -naïve macaques. Immunohistochemical staining of colon and spleen tissues from macaques indicates various levels of perforin expression between SIV-naïve animals and SIV-positive animals at 29 and 180 days postinfection (PI). The frequencies of CD8+ cells can be observed in sequential sections. Perforin and CD8 are stained brown by diaminobenzidine, and cell nuclei are stained blue by hematoxylin.
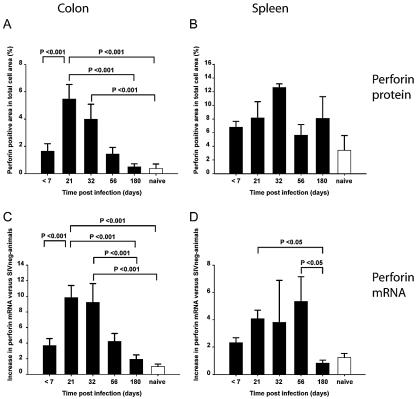
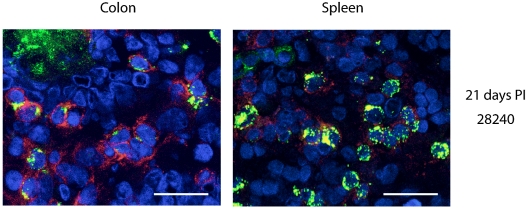
Within the first 7 days postinfection, we detected low levels of perforin protein and perforin mRNA in the colons of SIV-infected animals, similar to that in the colons of SIV-naïve animals. By day 21, however, there was a significant increase in the expression levels of perforin mRNA and protein in the colons of SIV-infected animals (P < 0.001, one-way analysis of variance and Holm-Sidak test) (Fig. 2). This increase occurred in parallel with an increase in the percentage of CD8+ cells in the lamina propria, as observed in situ by immunohistochemistry (Fig. 1 and data not shown). Analysis by confocal microscopy of double-stained cells with anti-CD8 and anti-perforin monoclonal antibodies indicated that perforin expression was predominantly occurring in CD8+ cells (Fig. 3). Perforin protein expression at baseline was higher in spleen than in colon, and while there was a trend towards increased expression in both spleen (Fig. 2) and peripheral blood mononuclear cells (data not shown) throughout infection, this trend did not reach statistical significance.
FIG. 2.
Quantitative assessment of perforin protein and perforin mRNA in colon and spleen tissues from SIVmac251-infected macaques. For analysis of perforin expression in tissues by immunohistochemical staining, each total perforin-positive area was measured and expressed as a percentage of the total cell area (A and B). Changes in perforin mRNA are reported as increases (n-fold) of perforin mRNA levels in SIV-infected colon (C) and spleen (D), compared to that in SIV-negative (SIVneg) animals (n = 8) (1). If statistical differences existed between groups as determined by a one-way analysis of variance, then a Holm-Sidak test was used to determine significant differences between pairs of groups, and all P values are indicated in the figure. Necropsy times shown in the figure are averages for each group of animals; for actual necropsy times for individual animals, see Table 1.
FIG. 3.
Immunofluorescent staining and confocal microscopy of colon and spleen tissues reveal expression of perforin in CD8+ cells. On day 21 postinfection (PI), animal 28240 shows perforin (green) expressed in CD8+ cells (red). Cell nuclei are stained with 4′,6′-diamidino-2-phenylindole, which appears blue, and colocalization of perforin with membrane CD8 appears yellow. Images were obtained using a Leica TCS-SP2 confocal microscope. Bars, 20 μm.
However, in the colons of animals that had been infected for 180 days, we observed much lower levels of perforin protein and mRNA expression than at early time points, and there was no significant difference compared to the results for SIV-naïve animals (Fig. 2). This finding was especially striking since the number of CD8+ cells as a percentage of all lamina propria cells was not significantly lower than that at earlier time points (Fig. 1 and data not shown). This result was irrespective of viral load, since the group with chronic infection included animals with both high and low tissue virus titers (Table 1). Although the dynamics of perforin protein expression and mRNA expression were similar, we did not observe a direct correlation between the two data sets at any stage of infection (data not shown).
T lymphocytes in the gastrointestinal mucosa of healthy humans and macaques express little perforin (18; this study). However, the results presented here demonstrate that perforin is robustly expressed in gut CD8 T cells by day 21 of SIV infection. This is consistent with the appearance of SIV-specific tetramer-positive CD8 T cells in the colon at day 21 postinfection (15). A study of CD8 T-cell effector function during early SIV infection has suggested that the magnitude of antiviral cytokine production is too low in the gastrointestinal tract and that such production occurs too late after peak viremia to have any significant influence on overall viral load reduction (15). The significant expression of perforin in the colon during early SIV infection may indeed lead to the elimination of many SIV-infected cells. However, this response does not prevent the establishment of SIV infection in the gastrointestinal mucosa, and a simmering viremia persists as in the periphery. It remains to be determined whether the lack of perforin expression by gut CD8+ cells during chronic infection is a consequence of tight regulatory control of cytotoxicity within the mucosa or if it reflects an aspect of SIV-induced immunopathogenesis.
Acknowledgments
We thank Katy Lantz, Tracy Rourke, and Zhong-Min Ma for help with tissues and microscopy, J. William Critchfield and Delandy H. Young for assistance with flow cytometry, Anette Hoffman for help with confocal microscopy, and the technicians of the Molecular Immunology Unit of the CNPRC Immunology Core.
This work was supported by the Sven Gard Foundation; the Swedish Foundation for Strategic Research, the Swedish International Development Agency, the Swedish Science Council, Karolinska Institutet; U.S Public Health Service Grant V51-00169; and NIH grants R01-A1-51239, R01-AI-48484, and R01-AI-57020.
REFERENCES
- 1.Abel, K., L. La Franco-Scheuch, T. Rourke, Z.-M. Ma, V. de Silva, B. Fallert, L. Beckett, T. A. Reinhart, and C. J. Miller. 2004. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J. Virol. 78:841-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjork, L., T. E. Fehniger, U. Andersson, and J. Andersson. 1996. Computerized assessment of production of multiple human cytokines at the single-cell level using image analysis. J. Leukoc. Biol. 59:287-295. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodie, S. J., D. A. Lewinsohn, B. K. Patterson, D. Jiyamapa, J. Krieger, L. Corey, P. D. Greenberg, and S. R. Riddell. 1999. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat. Med. 5:34-41. [DOI] [PubMed] [Google Scholar]
- 5.Dailey, P. J., M. Zamround, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantification of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. Presented at the 13th Annual Symposium on Nonhuman Primate Models for AIDS, Monterey, Calif.
- 6.Glimcher, L. H., M. J. Townsend, B. M. Sullivan, and G. M. Lord. 2004. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat. Rev. Immunol. 4:900-911. [DOI] [PubMed] [Google Scholar]
- 7.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 9.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mebius, R. E., and G. Kraal. 2005. Structure and function of the spleen. Nat. Rev. Immunol. 5:606-616. [DOI] [PubMed] [Google Scholar]
- 11.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, C., and M. B. Gardner. 1991. AIDS and mucosal immunity: usefulness of the SIV macaque model of genital mucosal transmission. J. Acquir. Immune Defic. Syndr. 4:1169-1172. [PubMed] [Google Scholar]
- 13.Miller, C. J., Q. Li, K. Abel, E.-Y. Kim, Z.-M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79:9217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley, M. F., J. W. Critchfield, B. Zuber, K. Abel, C. J. Miller, J. K. Sandberg, and B. L. Shacklett. 2005. New perforin detection reagents: an improved tool for analysis of SIV specific cellular immune responses. Presented at the 23rd Annual Symposium on Nonhuman Primate Models for AIDS, Portland, Oreg.
- 15.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z.-M. Ma, L. Compton, G. Napoé, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 79:9228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20:323-370. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 18.Shacklett, B. L., C. A. Cox, M. F. Quigley, C. Kreis, N. H. Stollman, M. A. Jacobson, J. Andersson, J. K. Sandberg, and D. F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173:641-648. [DOI] [PubMed] [Google Scholar]
- 19.Smit-McBride, Z., J. J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 21.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 22.Veazey, R. S., P. A. Marx, and A. A Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633. [DOI] [PubMed] [Google Scholar]