Abstract
Rabies virus (RV) phosphoprotein P is an interferon (IFN) antagonist counteracting transcriptional activation of type I IFN (K. Brzózka, S. Finke, and K. K. Conzelmann, J. Virol 79:7673-7681, 2005). We here show that RV P in addition is responsible for preventing IFN-α/β- and IFN-γ-stimulated JAK-STAT signaling in RV-infected cells by the retention of activated STATs in the cytoplasm. Expression of IFN-stimulated response element- and gamma-activated sequence-controlled genes was severely impaired in cells infected with RV SAD L16 or in cells expressing RV P protein from transfected plasmids. In contrast, a recombinant RV expressing small amounts of P had lost the ability to interfere with JAK-STAT signaling. IFN-mediated tyrosine phosphorylation of STAT1 and STAT2 was not impaired in RV P-expressing cells; rather, a defect in STAT recycling was suggested by distinct accumulation of tyrosine-phosphorylated STATs in cell extracts. In the presence of P, activated STAT1 and STAT2 were unable to accumulate in the nucleus. Notably, STAT1 and STAT2 were coprecipitated with RV P only from extracts of cells previously stimulated with IFN-α or IFN-γ, whereas in nonstimulated cells no association of P with STATs was observed. This conditional, IFN activation-dependent binding of tyrosine-phosphorylated STATs by RV P is unique for a viral IFN antagonist. The 10 C-terminal residues of P are required for counteracting JAK-STAT signaling but not for inhibition of transcriptional activation of IFN-β, thus demonstrating two independent functions of RV P in counteracting the host's IFN response.
The interferon (IFN) systems represent powerful defense elements of higher organisms that integrate innate and adaptive immunity. Type I IFN (IFN-α/β) is produced in response to virus infection in most tell types, including neurons, and upon recognition of conserved exogenous pathogen-associated molecular patterns by several Toll-like receptors (2, 4, 14). Expression of IFN-γ is mostly confined to T cells and NK cells; however, some neurons can also produce IFN-γ (32).
IFN-α/β and IFN-γ act through binding to ubiquitous receptors, the IFN-α/β receptor (IFNAR) and the IFN-γ receptor (IFNGR), respectively, and activation of two variants of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway (44). IFN-α/β binding to IFNAR results in TYK2- and JAK1-mediated tyrosine phosphorylation of the latent transcription factors STAT1 and STAT2 and formation of a heteromeric complex (IFN-stimulated gene factor 3 [ISGF3]) containing STAT1, STAT2, and IFN regulatory factor 9 (IRF-9; p48). IFNGR signaling involves tyrosine phosphorylation of STAT1 by JAK1 and JAK2 and formation of STAT1 homodimers, known as gamma-activated factor. ISGF3 and gamma-activated factor drive the expression of two big sets of genes that are controlled by specific promoter sequences, the interferon stimulated response elements (ISRE) and the gamma-activated sequences (GAS), respectively (reviewed in references [1, 34, and 44]). Expression of interferon-stimulated genes (ISG) leads to establishment of a powerful antiviral status and supports the development of an adequate adaptive Th1-biased immune response.
IFN expression and IFN effector functions are therefore vital targets of viruses (14, 17, 20, 51). It turns out that even small viruses with a limited coding capacity, including nonsegmented negative-strand RNA viruses (order Mononegavirales), which comprise the important Paramyxoviridae and Rhabdoviridae families, have evolved multiple mechanisms to target different functions of the IFN networks (10, 13, 29). Members of the Paramyxoviridae family are well known for their effective “weapons of STAT destruction,” represented, for example, by the nonessential V protein, which lead to depletion of STATs from virus-infected cells and thereby demolish the IFN JAK-STAT signaling pathway (18, 52).
In contrast, interference with IFN signaling has not been shown so far for members of the Rhabdoviridae family including the prototypic neurotropic rabies virus (RV) of the Lyssavirus genus. RV encodes merely five viral proteins, all of which are essential for virus amplification, namely the nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and a large (L) RNA-dependent RNA polymerase (gene order: 3′-N-P-M-G-L-5′). We have previously identified the RV phosphoprotein P as an IFN-α/β antagonist preventing expression of IFN-β in RV-infected cells by interfering with the phosphorylation of the critical IFN transcription factor IRF-3 (5). Although RV P is essential for viral RNA synthesis, we could generate a recombinant IFN-β-inducing RV (SAD ΔPLP) by shifting the P gene to a promoter-distal position of the genome. The low levels of P expressed were sufficient to support viral RNA synthesis but not to block activation of IRF-3.
We show here, by analysis of SAD ΔPLP and wild-type (wt) RV and by expression of P from cDNA, that RV P is also effective in preventing IFN-α/β- and IFN-γ-mediated signaling and expression of ISGs. Inhibition of JAK-STAT signaling and IFN induction are two separate functions of RV P since a deletion mutant lacking the C-terminal 10 residues has lost the ability to counteract JAK-STAT signaling but retained activity in preventing IFN induction by TBK-1. The STAT inhibitory activity of RV involves a unique mechanism among viral IFN antagonists, in that it targets STAT1 and STAT2 exclusively after activation by IFN-α/β or IFN-γ. Such a purposive activity only on demand may stem from a limited coding capacity of the virus and the busy nature of P, allowing P to perform its many other functions in virus replication.
(This work represents part of the doctoral thesis of K. Brzózka in fulfillment of the requirements for a Ph.D. degree from L-M-University, Munich, Germany, 2006)
MATERIALS AND METHODS
Cells, viruses, and reagents.
HEp-2 cells (ATCC CCL-23) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum and antibiotics. HEK 293 and U3A cells (25) were propagated in Dulbecco's modified Eagle's medium with 10% fetal calf serum, l-glutamine, and antibiotics, and BSR-T7/5 cells (7) were propagated in minimal essential medium supplemented with 10% newborn calf serum.
Recombinant RV SAD L16 (42) comprising the consensus sequence of the attenuated vaccine strain SAD B19 (11) was used as wt RV. Cloning and recovery of SAD ΔPLP was described previously (5). Mouse monoclonal antibodies to RV N (W239) were kindly provided by J. Cox (Tübingen, Germany), and monoclonal antibodies and polyclonal anti-P serum (9, 37) were provided by D. Blondel (Gif-sur-Yvette, France). Anti-STAT1 p84/p91 (sc-392), anti-STAT2 (sc-476), anti-ISGF-γ p48 (IRF-9) (sc-496), and anti-NFκB p65 (sc-109) antibodies were obtained from Santa Cruz Biotechnology; anti-pY701-STAT1 and anti-pS727-STAT1 were obtained from Cell Signaling; anti-pY689-STAT2 was obtained from Upstate Biotechnology; and anti-actin was obtained from Sigma. Cytokines were purchased from Sigma (tumor necrosis factor alpha [TNF-α]) and PBC Biomedical Lab (IFN-α A/D and human IFN-γ).
Plasmids and transfection.
The plasmid encoding RV P (pCR3-RVP) was described previously (5). Similarly, the pCR3-RVPD288-297 expression vector was created by PCR using an alternative antisense primer, PΔ288-297NotI3′ (5′-ATGCGGCCGCTTACATGATTTTACTCAG-3′), leading to a deletion of the 10 C-terminal amino acids of P. The pCR3-Ig (where Ig is immunoglobulin) vector (6) was used to create an N-terminally Ig-tagged P fusion protein. PCR primers (5′-ATATGAATTCATGAGCAAGATCTTTGTCAATCC-3′ and 5′- ATATGCGGCCGCTTAGCAAGATGTATAGCGATTCAA-3′) were used for amplification of P cDNA and cloning into EcoRI/NotI restriction sites.
For reporter gene assays in virus-infected cells, 2 × 105 cells (HEp-2 or BSR-T7/5) were seeded in 24-well plates and infected at multiplicities of infection (MOIs) of 1 or 3. After 16 h, cells were transfected with 0.5 μg of pISRE-luc or 0.5 μg of pGAS-luc (Stratagene) per well using Lipofectamine 2000. In all experiments, 10 ng of pCMV-RL encoding Renilla luciferase were cotransfected as an internal control. Medium was changed 6 h later, and the cells were stimulated with either universal IFN type I (IFN-α A/D) or human IFN-γ. After an additional 24 h, cell extracts were prepared and subjected to the reporter gene assay using a Dual Luciferase Reporter system (Promega). Luciferase activity was measured in a Luminometer (Berthold) according to the supplier's instructions. For reporter gene assays using P cDNA transfection, 2 × 105 HEp-2 cells were seeded in 24-well plates, and after 16 h cells were transfected with 0.8 μg of DNA (0.4 μg of pISRE-luc or 0.4 μg of pGAS-luc cotransfected with 0.4 μg of empty vector or RV P expression construct) per well using Lipofectamine 2000. At 24 h posttransfection, the medium was changed, and cells were treated with the amounts of IFN indicated in the figures. At 24 h poststimulation, cell lysates were subjected to the ISRE and GAS reporter gene assays. The reporter gene assays for monitoring IFN-β promoter activity (p125luc) upon TBK-1 expression were performed as described previously (5).
Precipitation assays.
HEK 293 cells were transfected using a calcium phosphate mammalian transfection kit (Stratagene), and U3A cells were transfected using Lipofectamine 2000 (Invitrogen). Precipitation was performed 48 h posttransfection. Briefly, cells were lysed in lysing buffer (50 mM NaCl, 150 mM Tris, 1 mM Na-vanadate, 1 mM EDTA, and protease inhibitor cocktail [Roche]), and after centrifugation (14,000 rpm for 10 min) protein A Sepharose (Amersham Biosciences) was used to pull down Ig-tagged complexes from the supernatant (2h at 4°C). After the incubation and washing steps, beads were resuspended in lysis buffer (62.5 mM Tris, 2% sodium dodecyl sulfate [SDS], 10% glycerol, 6 M urea, 5% β-mercaptoethanol, 0.01% bromophenol blue, 0.01% phenol red) and incubated at 95°C for 10 min in order to destroy bead-bound complexes.
Western blotting.
Cell extracts were prepared by treatment with cell lysis buffer (62.5 mM Tris, 2% SDS, 10% glycerol, 6 M urea, 5% β-mercaptoethanol, 0.01% bromophenol blue, 0.01% phenol red). Proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Schleicher and Schuell) using a semidry blotter (Peq-Lab). Membranes were incubated overnight at 4°C with primary antibodies. Protein signals were visualized with horseradish peroxidase-conjugated secondary antibodies and an ECL system (Perkin Elmer).
Immunofluorescence microscopy.
HEp-2 cells were seeded on glass coverslips and were infected with RV at an MOI of 1 or were transfected with plasmid cDNA using Lipofectamine 2000. The cells were fixed using 3% paraformaldehyde for 20 min at room temperature and were permeabilized in 0.5% Triton X-100 in phosphate-buffered saline (PBS). After incubation with primary antibodies (1:100 in PBS for 45 min at 37°C), the specimens were incubated with fluorescence-labeled secondary antibodies at a dilution of 1:200 in PBS for 1 h at 37°C (goat anti-rabbit Alexa Fluor 488 and anti-mouse tetramethylrhodamine, both from Molecular Probes). Nuclear chromatin was stained by adding TO-PRO-3-iodide (Molecular Probes) to the secondary antibodies (final concentration, 0.5 μM TO-PRO-3-iodide).
Confocal laser scanning microscopy was performed with a Zeiss LSM510 Meta laser system using a Zeiss Axiovert 200 microscope. Excitation of Alexa Fluor 488, tetramethylrhodamine, and TO-PRO-3-iodide occurred at wavelengths of 488 nm, 543 nm, and 633 nm, respectively. To avoid cross talk, the individual channels were scanned sequentially.
RESULTS
RV infection inhibits production of ISGs.
RV is not able to replicate in cells activated by IFN-α/β or IFN-γ, i.e., in cells in which an antiviral state has been established (data not shown). We noticed, however, that IFN had no obvious effect on viral replication and gene expression when cells were treated after RV infection. This suggested that RV encodes proteins to interfere with the establishment of an antiviral state by IFN or to counteract the antiviral activity of ISGs.
To explore whether RV is able to interfere with the production of ISGs, HEp2 cells were infected at a MOI of 1 with RV SAD L16 for 18 h and were then transfected with plasmids encoding firefly luciferase under the control of ISRE or GAS sequences (pISRE-luc or pGAS-luc). At 6 h posttransfection, cells were treated with IFN-α or IFN-γ. Cell lysates were prepared 24 h after IFN stimulation and processed for luciferase assays and for Western blotting.
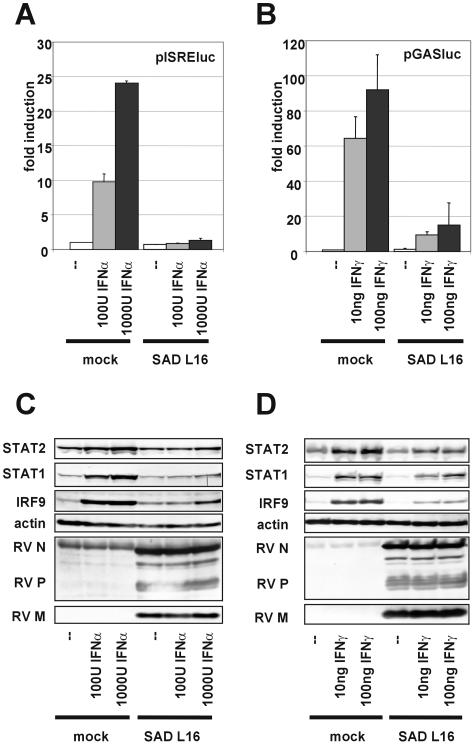
In mock-infected cells, stimulation with 1,000 U of IFN-α resulted in a more than 20-fold induction of luciferase activity from the ISRE plasmid, whereas in SAD L16-infected cells induction of luciferase activity was almost completely prevented. Indeed, luciferase activity was comparable to mock-infected nontreated cells (Fig. 1A). Similarly, IFN-γ stimulation of luciferase from the GAS-controlled plasmid was greatly impaired in RV-infected cells (Fig. 1B).
FIG. 1.
RV inhibits induction of ISGs by IFN-α and IFN-γ. (A and B) HEp-2 cells were infected with RV SAD L16 (MOI of 1), transfected with pISRE-luc (A) or pGAS-luc (B) at 18 h postinfection, and IFN-α or IFN-γ was added at 6 h posttransfection. Twenty-four hours later firefly luciferase activities were measured and corrected to the Renilla luciferase activity from a cotransfected control plasmid to normalize for variations in the transfection efficiency. Transfection experiments were repeated at least three times and averages and error bars are shown. (C and D) Expression of individual ISGs STAT1, STAT2, and IRF9 was analyzed by Western blot analysis of extracts from HEp-2 cells infected at an MOI of 1 for 24 h and a subsequent 24-h stimulation with IFN-α (C) or IFN-γ (D). In contrast to mock-infected cells, RV-infected cells do not upregulate ISRE- or GAS-controlled luciferase or STAT1, STAT2, and IRF9 upon IFN stimulation.
To check the expression of some individual ISGs upon IFN treatment, cell extracts were also analyzed by Western blotting with antibodies to IRF9, STAT1, and STAT2. In mock-infected cells, a conspicuous accumulation of these proteins, in particular, STAT2 and IRF9, was noticed already upon stimulation with 100 U of IFN-α (Fig. 1C) or 10 ng/ml IFN-γ (Fig. 1D). In contrast, in RV-infected cells the upregulation of STAT1, STAT2, and IRF9 was evidently impaired, confirming the luciferase reporter gene assays. Only at a high dose of 1,000 U of IFN-α was some increase of protein levels apparent, though levels remained below those of mock-infected IFN-stimulated cells. Notably, the basic levels of STAT1 and STAT2 were not reduced in RV-infected cells. This indicated that the observed inhibition of ISG expression by RV was not due to depletion of STATs.
STAT tyrosine phosphorylation is not affected.
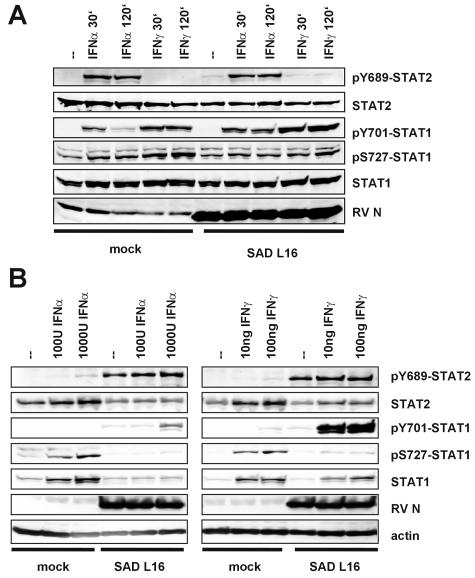
STATs are activated by Janus tyrosine kinases associated with the cytoplasmic domains of IFNAR and IFNGR. To analyze the phosphorylation status of STAT1 and STAT2 in RV-infected cells, Western blot experiments with phospho-specific STAT antibodies were performed. HEp-2 cells infected for 24 h with SAD L16 were treated with IFN, and cell extracts were prepared 30 min and 120 min after the IFN stimulation (Fig. 2A). As observed previously, the total levels of STAT1 and STAT2 were similar in mock- and virus-infected cells. As in mock-infected cells, tyrosine-phosphorylated STAT2 (pY689-STAT2) and STAT1 (pY701-STAT1) were readily detected in RV-infected cells at 30 min poststimulation with IFN-α. Similarly, IFN-γ stimulation resulted in comparable amounts of pY701-STAT1 in mock-infected and RV-infected cells at 30 min poststimulation (Fig. 2A). Thus, activation of STAT1 and STAT2 by tyrosine phosphorylation is not impaired in RV-infected cells.
FIG. 2.
RV infection does not prevent tyrosine phosphorylation of STAT1 and STAT2 (A) but leads to accumulation of tyrosine-phosphorylated STAT1 and STAT2 over time (B). Mock-infected and RV-infected HEp-2 cells (MOI of 1) were stimulated at 24 h postinfection with IFN-α or IFN-γ. After 30 or 120 min (A) cell extracts were processed for Western blotting and probed with phospho-specific antibodies as indicated. Tyrosine phosphorylation of STAT2 (pY689-STAT2) and STAT1 (pY701-STAT1) was similar in mock- and RV-infected IFN-stimulated cells, whereas serine phosphorylation of STAT1 (pS727-STAT1) by IFN was more effective in mock-infected cells. Tyrosine-phosphorylated STAT1 and STAT2 accumulate. As shown in panel B, at later stages after IFN stimulation (24 h poststimulation), abundant amounts of tyrosine-phosphorylated STATs are still detectable only in RV-infected cells but not in mock-infected cells, suggesting a defect in STAT recycling.
To follow the long-term fate of phosphorylated STATs, cells were allowed to grow for 24 h following IFN treatment (Fig. 2B). At this late time point, only trace amounts of pY701-STAT1 and pY689-STAT2 were left in mock-infected cells treated with IFN-α, while total levels of STAT1 and STAT2 were increased as a result of IFN stimulation. In striking contrast, abundant amounts of pY689-STAT2 were present in RV-infected cells at this late time point, as well as increased levels of pY701-STAT1 (Fig. 2B, left panel). In line with these findings, IFN-γ treatment resulted in the most pronounced accumulation of pY701-STAT1 and a less prominent accumulation of pY689-STAT2 in RV-infected cells during the 24-h period following IFN treatment. These results further confirmed that the initial tyrosine phosphorylation of STATs is unimpaired and pointed toward a defect in recycling of correctly activated STATs.
In contrast to tyrosine phosphorylation of STAT1, serine phosphorylation, which is not required for the transcriptional activity of STATs, appeared to be somewhat less efficient in RV-infected cells. Lower levels of pS727-STAT1 were observed both at early (Fig. 2A) and late time points after IFN stimulation (Fig. 2B).
RV prevents nuclear import of activated STAT1 and STAT2.
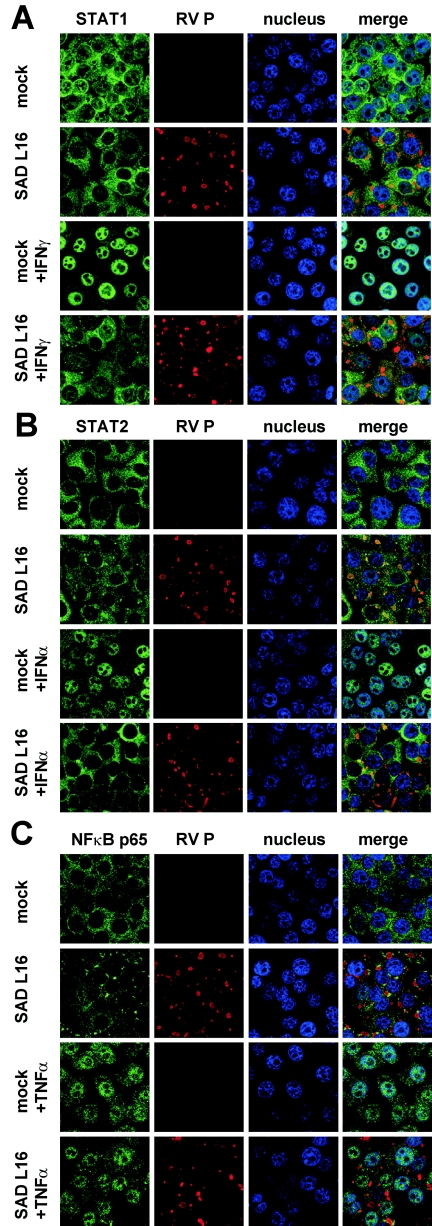
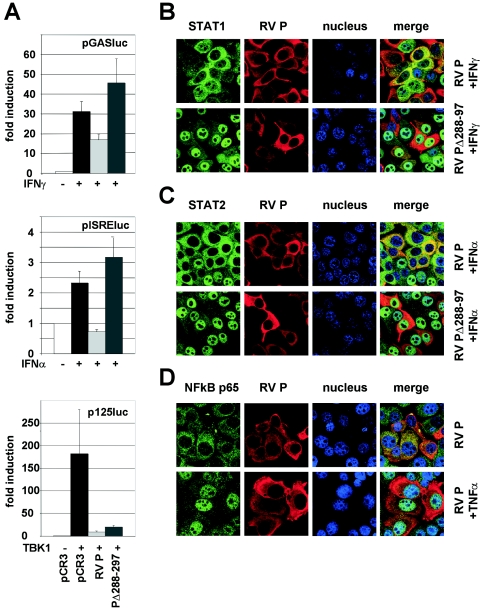
As STATs were found to be correctly activated by tyrosine phosphorylation in RV-infected cells, we examined by confocal laser scanning microscopy the subcellular localization of STAT1 and STAT2 proteins after stimulation with IFN. For this purpose, HEp-2 cells were infected at an MOI of 1 for 24 h, stimulated for 45 min with 100 ng/ml of IFN-γ or 1,000 U/ml of IFN-α, and immunostained for STAT1 and STAT2, respectively. In noninfected HEp-2 cells, IFN-γ treatment led to a redistribution of STAT1 from the cytoplasm to the nucleus (Fig. 3A), whereas IFN-treated RV-infected cells maintained the phenotype of nontreated cells, with the major portion of STAT1 in the cytoplasm (Fig. 3A). Stimulation of noninfected cells with IFN-α led to an almost complete relocalization of STAT2 from the cytoplasm to the nucleus. This translocation was precluded when cells were previously infected with RV (Fig. 3B). To exclude the possibility that RV causes a general block of nuclear import or an increase in nuclear export, we investigated the nuclear localization of another transcription factor, NF-κB p65, on stimulation with TNF-α (Fig. 3C). In contrast to STAT1 and STAT2, nuclear accumulation of NF-κB p65 was not affected in RV-infected cells, indicating that RV specifically interferes with the nuclear import of STAT1 and STAT2 (see Discussion).
FIG. 3.
RV infection prevents IFN-mediated translocation of STAT1 (A) and STAT2 (B) to the nucleus. RV-infected (MOI of 1) and mock-infected Hep-2 cells were stimulated with IFN-γ or IFN-α for 45 min at 24 h postinfection, fixed with 3% paraformaldehyde, and stained with STAT1 or STAT2 antibodies as indicated. The import of both STATs on IFN-treated cells is prevented in RV-infected cells, whereas import of NF-κB p65 on TNF-α-treated cells is not (C). Nuclei of cells were visualized by staining with TO-PRO-3 dye; RV P was visualized by using P-specific polyclonal mouse serum.
Failure of the RV SAD ΔPLP to interfere with JAK-STAT signaling.
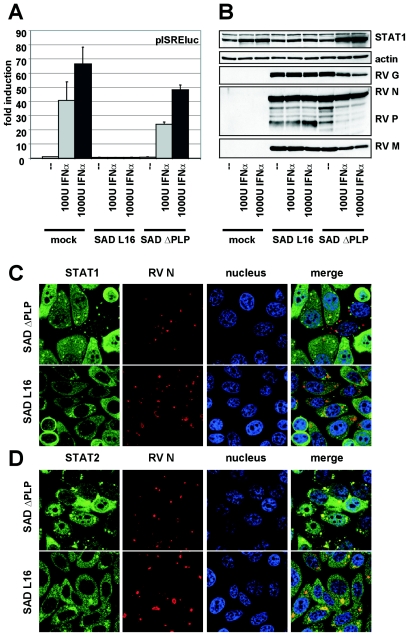
To identify the viral protein(s) responsible for interference with JAK-STAT signaling, we compared wt RV SAD L16 with the previously described SAD ΔPLP. Due to a change in the gene order from 3′-N-P-M-G-L-5′ to 3′-N-M-G-L-P-5′, SAD ΔPLP virus expresses very low amounts of P and is therefore unable to prevent activation of IRF-3 and IFN-β production (5). Reporter gene assays were therefore performed in BSR-T7/5 cells, which do not produce endogenous IFN on SAD ΔPLP infection. Cells infected at an MOI of 3 with SAD L16 and SAD ΔPLP virus for 18 h were transfected with pISRE-luc and stimulated with IFN-α, and luciferase activity was determined 24 h after stimulation. An approximately 60-fold induction of luciferase activity was observed for mock-infected cells. While infection with wt RV completely abolished luciferase activity, SAD ΔPLP infection was not able to prevent IFN signaling (Fig. 4A), suggesting that the RV P protein is involved in or responsible for counteracting IFN signaling. Indeed, SAD ΔPLP showed an increased sensitivity to the antiviral effects of IFN. Treatment of virus-infected BSR T7/5 cells with IFN-α caused a clear and dose-dependent inhibitory effect of protein expression of SAD ΔPLP, in contrast to wt RV SAD L16 (Fig. 4B). Microscopic examination of STAT localization in HEp2 cells infected with SAD ΔPLP or SAD L16 further confirmed that SAD ΔPLP has a defect in preventing nuclear accumulation of STAT1 and STAT2 (Fig. 4C and D). Thus, it appears that insufficient levels of P protein expressed from SAD ΔPLP are responsible for the failure of this virus in preventing JAK-STAT signaling.
FIG. 4.
SAD ΔPLP fails in inhibiting JAK-STAT signaling. (A) BSR-T7/5 cells infected at an MOI of 3 with wt RV SAD L16 and a mutant expressing low levels of P (SAD ΔPLP) were transfected with pISRE-luc and were stimulated with IFN-α 18 h postinfection. In contrast to wt RV, SAD ΔPLP is not able to abolished luciferase activities. (B) BSR T7/5 cells infected at an MOI of 1 with RV SAD L16 and SAD ΔPLP were treated with IFN-α at 24 h postinfection. Cell lysates were prepared 24 h after stimulation (48 h postinfection) and were analyzed by Western blotting. Accumulation of viral proteins in SAD ΔPLP-infected cells was decreased after IFN induction. (C) HEp-2 cells infected with SAD L16 or SAD ΔPLP at an MOI of 1 were immunostained for STAT1 and STAT2 24 h postinfection as described in the legend of Fig. 3. In contrast to wt SAD L16, SAD ΔPLP-infected HEp-2 cells allow nuclear accumulation of both STAT1 and STAT2. RV N was visualized using N-specific mouse monoclonal antibodies.
Rabies virus phosphoprotein P is responsible for interference with STAT functions.
In order to clarify whether P alone is able to prevent ISG expression, HEp-2 cells were transfected with an empty vector (pCR3) or an RV P-encoding plasmid (pCR3-RVP), stimulated with IFN, and assayed for GAS- and ISRE-directed luciferase expression. Expression of RV P was sufficient to considerably reduce both IFN-γ- and IFN-α-induced luciferase activity (Fig. 5A, top and middle panels). In striking contrast to full-length P, a P deletion mutant lacking the 10 C-terminal residues (PΔ288-297) did not show an inhibitory effect on either GAS- or ISRE-driven reporter gene expression. Notably, however, PΔ288-297 has retained the ability to prevent TBK-1-mediated activation of IRF-3 and IFN-β induction. Compared to HEK 293 cells transfected with empty vector plasmids, cotransfection of both P-encoding plasmids with a TBK-1-encoding plasmid reduced expression of luciferase from the IFN-β promoter (p125luc) considerably (Fig. 5A, bottom panel).
FIG. 5.
Expression of P alone prevents STAT signaling. (A) pISRE-luc or pGAS-luc reporter constructs were cotransfected with plasmids encoding wt P (pCR3-RVP), a C-terminally truncated protein, PΔ288-297 (pCR3-RVPΔ288-297), or with empty vector (pCR3) into HEp-2 cells. After 24 h, IFN-α or IFN-γ was added, and luciferase activities were analyzed after an additional 24 h (top and middle panels). A reporter gene construct controlled by the IFN-β promoter (p125luc) was cotransfected with RV P and TBK-1 expression plasmids into HEK 293 cells as indicated. Luciferase activities were determined at 48 h posttransfection (lower panel). The average of at least two independent experiments is shown with error bars. (B to D) RV P prevents nuclear accumulation of STATs. HEp-2 cells were transfected with the indicated expression constructs. After 24 h cells were stimulated for 45 min and were subsequently stained for STAT1 (B), STAT2 (C), and NF-κB p65 (D) as described in the legend of Fig. 3. In contrast to PΔ288-297, expression of wt P prevents nuclear import of STAT1 and STAT2 by IFN but not of NF-κB p65 by TNF-α.
To verify the results from the reporter gene assays and to address the question of how expression of RV P and PΔ288-297 affect the nuclear import of STATs, transfected HEp-2 cells were treated with IFN and processed for confocal microscopy as described above. In cells expressing P, neither STAT1 nor STAT2 could accumulate in the nucleus upon IFN stimulation. In contrast, cells expressing even abundant PΔ288-297 showed efficient nuclear accumulation of STAT1 and STAT2 (Fig. 5B and C). As observed previously for RV-infected cells, nuclear import of NF-κB p65 was not impaired in P-expressing cells (Fig. 5D). Thus, RV P alone is sufficient to specifically and efficiently prevent STAT1 and STAT2 nuclear import and ISG induction by IFN JAK-STAT signaling.
Activation-dependent binding of STATs by RV P.
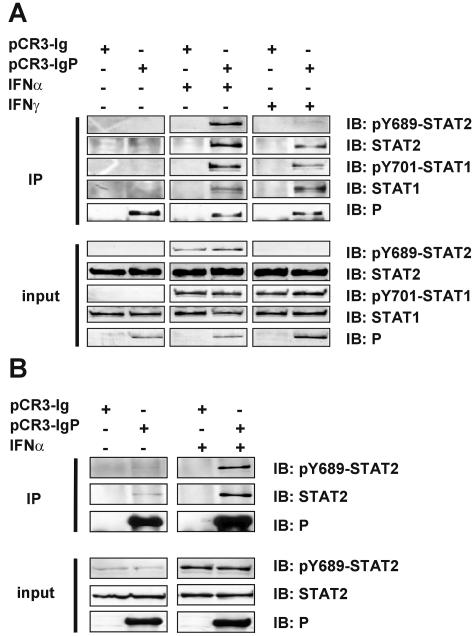
To assay whether RV P is able to physically associate with STATs and thereby preclude their nuclear import, precipitation assays were performed using a P in which an Ig tag was fused to the N terminus of P (pCR3-IgP) or of PΔ288-297 (pCR3-Ig PΔ288-297). As we considered the possibility that activated STATs represent the targets of P (see Discussion), the experiments were designed in a way to include IFN-treated cells. HEK 293 cells were transfected with pCR3-IgP or a control plasmid expressing the Ig tag alone (pCR3-Ig) and were stimulated with 1,000 U/ml of IFN-α or 100 ng/ml of IFN-γ after 48 h for 45 min. Ig-containing complexes were precipitated from cell extracts using Sepharose A beads (Amersham Biosciences), and precipitates were analyzed by Western blotting.
Notably, neither STAT1 nor STAT2 was coprecipitated with IgP from nonstimulated cells (Fig. 6A, left panel). However, when cells were pretreated with IFN-α, both STAT1 and STAT2 molecules were effectively pulled down with IgP but not by the Ig control construct (Fig. 6A, middle panel). The presence of activated, tyrosine-phosphorylated STAT2 (pY689) and STAT1 (pY701) in the precipitates was confirmed by phospho-specific antibodies.
FIG. 6.
RV P interacts with STATs in an IFN-dependent manner. (A) HEK 293 cells were transfected with constructs encoding Ig-tagged P (pCR3-IgP) or the Ig moiety alone (pCR3-Ig). Precipitation was performed using Sepharose A beads binding the Ig tag at 48 h postinfection from extracts of cells that were treated for 45 min with 1,000 U/ml IFN-α or 100 ng/ml IFN-γ or were not treated. Precipitates (IP) and 3% of cell extracts (input) were analyzed by Western blotting with the indicated antibodies. Only from IFN-treated cells were STATs coprecipitated with Ig-P. (B) The experiment shown in panel A was performed with U3A cells that lack STAT1. STAT2 is precipitated from IFN-treated cells independent of STAT1.
After treatment of cells with IFN-γ, pY701-STAT1 was readily detected in the cell extracts in contrast to pY689-STAT2 (Fig. 6A, right panel, input). Again, IgP coprecipitated STAT1 exclusively and effectively from stimulated cells. Furthermore, not only STAT1 (pY701-STAT1) was present in the IgP precipitates but also STAT2 (pY689-STAT2), although pY689-STAT2 was below the detection limit in the input cell extracts (Fig. 6A, lower panel). Coprecipitation of STATs with IgPΔ288-297 was not observed in either IFN-stimulated or nonstimulated cells (data not shown).
These experiments confirmed that RV P associated with STAT2 and/or STAT1 only after IFN activation; however, they did not reveal whether the interaction with STAT2 occurs via STAT1 or independently of STAT1. We therefore repeated the precipitation experiments with cell extracts of U3A cells that lack STAT1. IgP clearly coprecipitated pY689-STAT2 from cell extracts of IFN-α-stimulated U3A cells, as well as from nonstimulated cells, in which low amounts of pY689-STAT2 were already detectable (Fig. 6B). Again, no precipitation was observed with the C-terminally truncated IgPΔ288-297 (data not shown). Thus, full-length RV P is able to independently interact with pY-STAT1 and pY-STAT2 in IFN-stimulated cells.
DISCUSSION
In this work, we have demonstrated that RV has the ability to interrupt the IFN-stimulated JAK-STAT signaling pathways and thereby to prevent the detrimental effects of type I and type II IFN. This activity could be attributed entirely to the P protein. The mechanism by which RV P interrupts IFN JAK-STAT signaling involves a specific association of P with STAT1 and STAT2 exclusively after activation by IFN, which is unique for viral IFN antagonists.
The existence of RV proteins able to counteract IFN signaling was first suggested by the observation that treatment of cells previously infected with RV had no detectable effects on virus gene expression and infectious virus titers, whereas pretreatment of cells with IFN completely prohibits RV replication. The resistance of RV to both IFN-α/β and IFN-γ further pointed toward STAT1 as a target, since it is a common factor of the IFNAR and IFNGR/JAK-STAT pathways. In RV-infected cells an almost complete inhibition of ISRE-controlled luciferase activity was observed, consistent with the later observation that RV P binds both STAT1 and STAT2. The observed residual leakiness of RV-infected cells for IFN-γ-stimulated and GAS-mediated reporter gene expression may be due to different reasons, e.g., activation of STAT3 in addition to STAT1 at the IFN-γ receptor (44).
Upon stimulation of the IFN receptors by IFN, STATs are phosphorylated by the receptor-associated Janus kinases at tyrosine residues 689 (STAT2) and 701 (STAT1) (19, 22, 23, 46). The STATs then dimerize through SH2-phosphotyrosine interactions, translocate to the nucleus, and bind to ISRE or GAS sequences. Additional phosphorylation of C-terminal Ser residues by kinases such as protein kinase C δ may improve the transcriptional activity of tyrosine-phosphorylated STATs by facilitating binding of nuclear factors, such as CBP/p300, but this is not a requirement for STAT transcriptional activity (12, 47). The analysis of STAT proteins with phospho-specific antibodies revealed that the critical tyrosine phosphorylation of STATs is not precluded in RV-infected cells. Rather, an accumulation of tyrosine-phosphorylated STATs was observed over time, whereas in mock-infected cells these molecules rapidly disappeared (Fig. 2B). In contrast to the initial tyrosine phosphorylation, however, the following serine phosphorylation of STATs appeared to be somewhat hampered in RV cells, as suggested by poor accumulation of pS727-STAT1 (Fig. 2B). These observations pointed toward an interference of RV P with a step following the initial receptor-mediated STAT activation.
Indeed, in spite of correct tyrosine phosphorylation, neither STAT1 nor STAT2 was able to accumulate in the nucleus of RV-infected cells. A very clear-cut alternative distribution was observed for STAT2. IFN-α treatment of mock-infected cells led to a rapid and almost complete relocalization of STAT2 from the cytoplasm to the nucleus, whereas in RV-infected or RV P-expressing and IFN-treated cells, virtually no STAT2 was detectable in the nucleus (Fig. 3 and 5). In case of STAT1, a readily detectable portion of the protein showed nuclear localization even in nonstimulated cells. This reflects the well-known shuttling of nonactivated STAT1 between the cytoplasm and nucleus (24, 28) and indicated that cycling of nonactivated STAT1 is not affected by RV P. Nevertheless, an efficient inhibition of IFN-triggered nuclear accumulation of STAT1 by RV was obvious, indicating that RV P retains activated STAT1 in the cytoplasm and prevents its nuclear import. It should be noted that pY-STAT1 is incapable of nuclear exit and has to be dephosphorylated first by nuclear phosphatases (26-28). Confocal live microscopy experiments involving photo-bleaching of cytoplasmic STAT1-GFP (where GFP is green fluorescent protein) revealed that the nuclear export rate of nonphosphorylated STAT1-GFP is not changed in the presence of RV P (not shown). The lack of nuclear import of activated STATs and of their dephosphorylation in the nucleus therefore explains the overall accumulation of pY-STATs over time in RV-infected cells.
Direct evidence for activation-dependent binding of STAT1 and STAT2 by P was obtained from precipitation assays with Ig-tagged P protein. Effective coprecipitation of both STATs with RV P was dependent on stimulation of cells with IFN. We were unable to detect substantial coprecipitation of STAT1 or STAT2 in nonstimulated HEK 293 cells, suggesting that nonphosphorylated STATs are not recognized, or only poorly recognized, and bound by P. After submission of this work, Vidy et al. published a report on binding of the RV P of the CVS strain to STAT1 (49). These authors were able to coprecipitate STAT1 from cell extracts using a glutathione transferase-P fusion construct. It was not revealed whether this represented activated or nonactivated STAT1. However, the observation that STAT1 and P coexpression was able to activate gene transcription in a yeast two-hybrid system indicates some basic association of P with STAT.
Only IFN stimulation, however, resulted in effective coprecipitation of both STAT1 and STAT2 in our hands. The presence of tyrosine-phosphorylated STATs in the precipitates strongly argues in favor of the idea that P binds exclusively to tyrosine-phosphorylated STATs, although we cannot formally exclude some coprecipitation of nonphosphorylated STATs via pY-STATs, due to the lack of antibodies that positively identify nonphosphorylated STATs. Recognition and precipitation of pY689-STAT2 by P appear to be very specific, as suggested by the precipitation of these molecules from cell extracts of IFN-γ-stimulated cells, in which they were not detectable by Western blotting or were present in very small amounts (53) among a bulk of nonphosphorylated STAT2 (Fig. 6A and B). We could further show that recognition of activated STAT2 is STAT1 independent, as shown by coprecipitation experiments using U3A cells that lack STAT1 (25) (Fig. 6B). Thus, RV P interacts with both STAT1 and STAT2 only upon activation, either directly or in a complex with other proteins, and this interaction explains the retention of activated STATs in the cytoplasm of infected cells.
The described conditional, activation-dependent targeting of STAT1 and STAT2 by RV P to interrupt IFN JAK-STAT signaling is unique among viruses. Constitutive targeting of bulk nonactivated STATs is a common strategy of the related paramyxoviruses and is due to the activities of their nonessential V proteins (for review see references 13, 14, 18, and 29). Rubulavirus V proteins assemble STAT-specific ubiquitin-ligase complexes from cellular components and target either STAT1 or STAT2 for proteasomal degradation (35, 48). The V proteins from other paramyxovirus genera do not lead to degradation of STATs. Henipaviruses, for example, in fact prevent phosphorylation of STATs and sequester STAT1 and STAT2 in high-molecular-mass complexes (39, 40). For Nipah virus it has been shown that the V, W, and P proteins, which are all encoded by the same viral gene and share an identical 407-amino-acid N-terminal region but have distinct C-terminal sequences, have anti-STAT function. This confirmed the finding that the common N-terminal domain is involved in the antagonist activity (38, 43). However, it was also found that activity of the P protein is not as strong as that of V or W, perhaps explaining why Nipah virus has evolved to express additionally two edited products (43). The V protein of measles virus was reported to copurify with STAT1, STAT2, STAT3, and IRF-9, to bind to the IFNAR, and to recruit STATs to viral inclusion bodies (33, 45).
We have here identified RV P as an inhibitor of IFN signaling and have previously shown that RV P is active in counteracting transcriptional induction of type I IFN (5). As demonstrated by PΔ288-297, these are independent functions of P. A region within the 10 C-terminal amino acid residues of RV P is required for counteracting STAT signaling but not for inhibiting TBK-1. Interestingly, paramyxovirus V proteins also combine inhibitory activities in IFN signaling and in IFN induction (16) but with different targets in either function. Whereas V C-terminal domains bind and inhibit the double-stranded RNA receptor mda-5 which relays signals to activation of IRF-3 and NF-kB (3), RV P acts farther downstream and specifically prevents phosphorylation of IRF-3 by TBK-1 (5). This finding stresses the importance for a virus of having means to target different steps within the powerful host IFN response. In particular, it is obvious that viral IFN antagonists are insufficient to prevent some early IFN synthesis or to prevent synthesis in certain tissue or cell types. Indeed, we have identified a variety of cell types in which active IFN is being expressed upon RV infection with SAD L16, though at lower magnitudes than the “IFN-inducing” SAD ΔPLP. In particular, and in striking contrast to measles virus and respiratory syncytial virus (41), RV SAD L16 is not able to prevent IFN induction in human plasmacytoid dendritic cells (unpublished data), which represent the major IFN producers in vivo. Consistent with these observations, IFN has been detected upon RV infection in vitro and in vivo (21, 30, 31, 36, 50). Since IFN-β and, in particular, IFN-γ have a major role in the noncytotoxic clearance of viruses from neurons and the central nervous system (8, 15), it is predicted that the ability of RV to counteract IFN-α/β and IFN-γ signaling is crucial for RV infection in vivo. Further experiments should be directed to further dissociate RV P functions in IFN induction and response. The use of recombinant RV with defined defects in either function should help to study the contribution of the innate immune response to the control of RV and to reveal a possible correlation of IFN antagonistic activities with viral pathogenicity. Viral antagonists targeting particular functions of the JAK-STAT pathways, such as RV P, may further help in studying details of STAT signaling.
.
Acknowledgments
We thank J. Cox (FLI, Tübingen, Germany) and D. Blondel (CNRS, Gif-sur-Yvette, France) for providing RV antibodies, S. Goodbourn (St. George's University London, United Kingdom) for ISRE and GAS reporter gene plasmids, and I. Kerr (CR-UK, London, United Kingdom) for U3A cells. We are grateful to N. Hagendorf for perfect technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 455, “Viral functions and immune modulation.”
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowie, A. G., and I. R. Haga. 2005. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42:859-867. [DOI] [PubMed] [Google Scholar]
- 5.Brzozka, K., S. Finke, and K. K. Conzelmann. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 79:7673-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubeck, A., M. Wagner, Z. Ruzsics, M. Lotzerich, M. Iglesias, I. R. Singh, and U. H. Koszinowski. 2004. Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J. Virol. 78:8026-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdeinick-Kerr, R., and D. E. Griffin. 2005. Gamma interferon-dependent, noncytolytic clearance of Sindbis virus infection from neurons in vitro. J. Virol. 79:5374-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenik, M., M. Schnell, K. K. Conzelmann, and D. Blondel. 1998. Mapping the interacting domains between the rabies virus polymerase and phosphoprotein. J. Virol. 72:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conzelmann, K. K. 2005. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J. Virol. 79:5241-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conzelmann, K. K., J. H. Cox, L. G. Schneider, and H. J. Thiel. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485-499. [DOI] [PubMed] [Google Scholar]
- 12.Deb, D. K., A. Sassano, F. Lekmine, B. Majchrzak, A. Verma, S. Kambhampati, S. Uddin, A. Rahman, E. N. Fish, and L. C. Platanias. 2003. Activation of protein kinase C delta by IFN-gamma. J. Immunol. 171:267-273. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 14.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3:493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 17.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 18.Horvath, C. M. 2004. Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. Eur. J. Biochem. 271:4621-4628. [DOI] [PubMed] [Google Scholar]
- 19.Horvath, C. M., G. R. Stark, I. M. Kerr, and J. E. Darnell, Jr. 1996. Interactions between STAT and non-STAT proteins in the interferon-stimulated gene factor 3 transcription complex. Mol. Cell. Biol. 16:6957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 21.Lafon, M. 2005. Modulation of the immune response in the nervous system by rabies virus. Curr. Top. Microbiol. Immunol. 289:239-258. [DOI] [PubMed] [Google Scholar]
- 22.Lau, J. F., J. P. Parisien, and C. M. Horvath. 2000. Interferon regulatory factor subcellular localization is determined by a bipartite nuclear localization signal in the DNA-binding domain and interaction with cytoplasmic retention factors. Proc. Natl. Acad. Sci. USA 97:7278-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, X., S. Leung, I. M. Kerr, and G. R. Stark. 1997. Functional subdomains of STAT2 required for preassociation with the alpha interferon receptor and for signaling. Mol. Cell. Biol. 17:2048-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marg, A., Y. Shan, T. Meyer, T. Meissner, M. Brandenburg, and U. Vinkemeier. 2004. Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. J. Cell Biol. 165:823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKendry, R., J. John, D. Flavell, M. Muller, I. M. Kerr, and G. R. Stark. 1991. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc. Natl. Acad. Sci. USA 88:11455-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer, T., L. Hendry, A. Begitt, S. John, and U. Vinkemeier. 2004. A single residue modulates tyrosine dephosphorylation, oligomerization, and nuclear accumulation of STAT transcription factors. J. Biol. Chem. 279:18998-19007. [DOI] [PubMed] [Google Scholar]
- 27.Meyer, T., A. Marg, P. Lemke, B. Wiesner, and U. Vinkemeier. 2003. DNA binding controls inactivation and nuclear accumulation of the transcription factor Stat1. Genes Dev. 17:1992-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer, T., and U. Vinkemeier. 2004. Nucleocytoplasmic shuttling of STAT transcription factors. Eur. J. Biochem. 271:4606-4612. [DOI] [PubMed] [Google Scholar]
- 29.Nagai, Y., and A. Kato. 2004. Accessory genes of the Paramyxoviridae, a large family of nonsegmented negative-strand RNA viruses, as a focus of active investigation by reverse genetics. Curr. Top. Microbiol. Immunol. 283:197-248. [DOI] [PubMed] [Google Scholar]
- 30.Nakamichi, K., S. Inoue, T. Takasaki, K. Morimoto, and I. Kurane. 2004. Rabies virus stimulates nitric oxide production and CXC chemokine ligand 10 expression in macrophages through activation of extracellular signal-regulated kinases 1 and 2. J. Virol. 78:9376-9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamichi, K., M. Saiki, M. Sawada, M. Takayama-Ito, Y. Yamamuro, K. Morimoto, and I. Kurane. 2005. Rabies virus-induced activation of mitogen-activated protein kinase and NF-κB signaling pathways regulates expression of CXC and CC chemokine ligands in microglia. J. Virol. 79:11801-11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann, H., H. Schmidt, E. Wilharm, L. Behrens, and H. Wekerle. 1997. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J. Exp. Med. 186:2023-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palosaari, H., J. P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platanias, L. C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375-386. [DOI] [PubMed] [Google Scholar]
- 35.Precious, B., K. Childs, V. Fitzpatrick-Swallow, S. Goodbourn, and R. E. Randall. 2005. Simian virus 5 V protein acts as an adaptor, linking DDB1 to STAT2, to facilitate the ubiquitination of STAT1. J. Virol. 79:13434-13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prehaud, C., F. Megret, M. Lafage, and M. Lafon. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J. Virol. 79:12893-12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raux, H., F. Iseni, F. Lafay, and D. Blondel. 1997. Mapping of monoclonal antibody epitopes of the rabies virus P protein. J. Gen. Virol. 78:119-124. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez, J. J., C. D. Cruz, and C. M. Horvath. 2004. Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J. Virol. 78:5358-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez, J. J., J. P. Parisien, and C. M. Horvath. 2002. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 76:11476-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez, J. J., L. F. Wang, and C. M. Horvath. 2003. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 77:11842-11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw, M. L., A. Garcia-Sastre, P. Palese, and C. F. Basler. 2004. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 78:5633-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi, K., S. I. Kadota, M. Takeda, N. Miyajima, and K. Nagata. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545:177-182. [DOI] [PubMed] [Google Scholar]
- 46.Uddin, S., A. Chamdin, and L. C. Platanias. 1995. Interaction of the transcriptional activator Stat-2 with the type I interferon receptor. J Biol. Chem. 270:24627-24630. [DOI] [PubMed] [Google Scholar]
- 47.Uddin, S., A. Sassano, D. K. Deb, A. Verma, B. Majchrzak, A. Rahman, A. B. Malik, E. N. Fish, and L. C. Platanias. 2002. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol. Chem. 277:14408-14416. [DOI] [PubMed] [Google Scholar]
- 48.Ulane, C. M., A. Kentsis, C. D. Cruz, J. P. Parisien, K. L. Schneider, and C. M. Horvath. 2005. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J. Virol. 79:10180-10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidy, A., M. Chelbi-Alix, and D. Blondel. 2005. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J. Virol. 79:14411-14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Z. W., L. Sarmento, Y. Wang, X. Q. Li, V. Dhingra, T. Tseggai, B. Jiang, and Z. F. Fu. 2005. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J. Virol. 79:12554-12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber, F., G. Kochs, and O. Haller. 2004. Inverse interference: how viruses fight the interferon system. Viral Immunol. 17:498-515. [DOI] [PubMed] [Google Scholar]
- 52.Young, D. F., L. Didcock, S. Goodbourn, and R. E. Randall. 2000. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann, A., M. Trilling, M. Wagner, M. Wilborn, I. Bubic, S. Jonjic, U. Koszinowski, and H. Hengel. 2005. A cytomegaloviral protein reveals a dual role for STAT2 in IFN-γ signaling and antiviral responses. J. Exp. Med. 201:1543-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]