Abstract
Auxin transport is required for important growth and developmental processes in plants, including gravity response and lateral root growth. Several lines of evidence suggest that reversible protein phosphorylation regulates auxin transport. Arabidopsis rcn1 mutant seedlings exhibit reduced protein phosphatase 2A activity and defects in differential cell elongation. Here we report that reduced phosphatase activity alters auxin transport and dependent physiological processes in the seedling root. Root basipetal transport was increased in rcn1 or phosphatase inhibitor–treated seedlings but showed normal sensitivity to the auxin transport inhibitor naphthylphthalamic acid (NPA). Phosphatase inhibition reduced root gravity response and delayed the establishment of differential auxin-induced gene expression across a gravity-stimulated root tip. An NPA treatment that reduced basipetal transport in rcn1 and cantharidin-treated wild-type plants also restored a normal gravity response and asymmetric auxin-induced gene expression, indicating that increased basipetal auxin transport impedes gravitropism. Increased auxin transport in rcn1 or phosphatase inhibitor–treated seedlings did not require the AGR1/EIR1/PIN2/WAV6 or AUX1 gene products. In contrast to basipetal transport, root acropetal transport was normal in phosphatase-inhibited seedlings in the absence of NPA, although it showed reduced NPA sensitivity. Lateral root growth also exhibited reduced NPA sensitivity in rcn1 seedlings, consistent with acropetal transport controlling lateral root growth. These results support the role of protein phosphorylation in regulating auxin transport and suggest that the acropetal and basipetal auxin transport streams are differentially regulated.
INTRODUCTION
Polar auxin transport in higher plants is a directional and regulated process. Auxin transport controls a variety of important growth and developmental processes, including gravity response and the development of primary and lateral apices. Auxin is transported from cell to cell and in shoots, indoleacetic acid (IAA) moves unidirectionally from the apex to the base (reviewed by Lomax et al., 1995). In roots, transport is more complex, with two distinct polarities. IAA moves acropetally (toward the root apex) through the central cylinder and basipetally (from the root apex toward the base) through the outer layer of cells (reviewed by Lomax et al., 1995; Jones, 1998). In Arabidopsis, both of these polarities of IAA movement have been detected and linked to specific physiological processes (Reed et al., 1998; Rashotte et al., 2000). Acropetal movement of IAA from the shoot into the root has been implicated in the control of lateral root development (Reed et al., 1998), whereas the basipetal movement of IAA from the root tip back has been linked to gravity response (Rashotte et al., 2000).
Recent work has provided insight into the identities of proteins that transport auxin into and out of cells. IAA entry into cells is facilitated by an auxin uptake carrier encoded by the AUX1 gene (Marchant et al., 1999). Auxin moves out of plant cells through an efflux carrier complex that is sensitive to synthetic inhibitors of auxin transport, including N-naphthylphthalamic acid (NPA), and is thought to be composed of at least two polypeptides (Morris et al., 1991; Muday, 2001). The first polypeptide of the efflux carrier is an integral membrane transporter presumably encoded by one of the members of the PIN gene family (Palme and Gälweiler, 1999), and functional assays in yeast suggest that this protein has auxin carrier activity (Chen et al., 1998; Luschnig et al., 1998). PIN genes encode proteins with 10 membrane-spanning domains that have similarity to other membrane transport proteins (Chen et al., 1998; Gälweiler et al., 1998; Luschnig et al., 1998; Muller et al., 1998; Utsuno et al., 1998). The PIN proteins show an asymmetric localization in the plasma membrane consistent with a role in controlling the polarity of auxin movement (Gälweiler et al., 1998; Muller et al., 1998). Several members of the PIN gene family in Arabidopsis have been identified, indicating that there are multiple auxin efflux carriers with distinct expression patterns (reviewed in Palme and Gälweiler, 1999). Plants with mutations in either PIN1 or PIN2/AGR1/EIR1/WAV6 have phenotypes consistent with tissue-specific alterations in auxin transport (Okada et al., 1991; Chen et al., 1998; Luschnig et al., 1998; Muller et al., 1998; Utsuno et al., 1998) and exhibit abnormal auxin transport in the affected tissues (Okada et al., 1991; Chen et al., 1998; Rashotte et al., 2000).
Although our understanding of carrier protein function has improved, the mechanisms that regulate auxin transport during plant growth and development remain enigmatic (Lomax et al., 1995). The second protein component of the auxin efflux carrier complex appears to act as a regulatory subunit and is the binding site for synthetic inhibitors such as NPA (Rubery, 1990). This NPA binding protein seems to be a separate polypeptide from the PIN protein, because it has a peripheral membrane association and cytoplasmic localization (Cox and Muday, 1994; Dixon et al., 1996; Muday, 2000). The NPA binding protein also is believed to bind endogenous regulatory compounds such as flavonoids, which have been shown to displace NPA binding and to inhibit auxin efflux in vitro (Jacobs and Rubery, 1988; Rubery and Jacobs, 1990). Arabidopsis mutants that make no flavonoids because of a biosynthetic defect have elevated auxin transport and phenotypes consistent with altered auxin transport, including increased inflorescence and root branching (Murphy et al., 2000; Brown et al., 2001). Changes in the synthesis or localization of auxin transport proteins may result in altered rates or directions of transport, thus regulating auxin transport (Suttle, 1988, 1991; Morris and Johnson, 1990).
Reversible protein phosphorylation also may control the activity of auxin transport proteins. Inhibitor studies have implicated protein kinases in the regulation of transport. Staurosporine, a broad-spectrum kinase inhibitor, rapidly reduces auxin efflux without affecting auxin influx, although several inhibitors of protein phosphatase 1 (PP1) and 2A (PP2A) activity are unable to reverse this effect (Delbarre et al., 1998). Furthermore, tyrosine kinase inhibitors reduce the regulation of auxin efflux by NPA (Bernasconi, 1996). These reports suggest that phosphorylation controls normal auxin efflux and its sensitivity to NPA. Tyrosine phosphorylation may primarily affect the NPA regulation of auxin efflux, whereas serine/threonine phosphorylation may act at a different level to regulate auxin efflux. A recent report suggests that okadaic acid, a PP1 and PP2A inhibitor, blocks gravity response in oat pulvini (Chang and Kaufman, 2000). Together, these studies suggest that protein phosphorylation modulates both auxin transport and gravity response, but the mechanisms linking these processes are not apparent.
Additional evidence for the link between auxin transport and phosphorylation has been found in the roots curl in NPA1 (rcn1) mutant. This mutant was identified in a screen for a root-curling response in the presence of the auxin transport inhibitor NPA. The RCN1 gene encodes a regulatory A subunit of PP2A, and the rcn1 mutant exhibits reduced PP2A activity in extracts, in addition to defects in root curling and other phenotypes requiring differential cell elongation (Garbers et al., 1996; Deruère et al., 1999). The phenotypic alterations in this mutant are due to reductions in protein phosphatase activity, because treatment with the phosphatase inhibitor cantharidin produces a phenocopy of rcn1 (Deruère et al., 1999). These data suggest that PP2A activity is required for the normal regulation of auxin transport.
The goal of this study was to analyze the role of phosphorylation in auxin transport through examination of the rcn1 mutant, as well as wild-type plants treated with the phosphatase inhibitor cantharidin to mimic rcn1 phenotypes. Earlier work on rcn1 suggested that the NPA sensitivity of hypocotyl elongation and auxin accumulation is increased in mutant seedlings, but striking changes in gravity response were not reported (Garbers et al., 1996). New auxin transport assays designed for Arabidopsis roots (Reed et al., 1998; Rashotte et al., 2000) and a high resolution method for the examination of gravity response (Mullen et al., 1998a) have allowed a detailed analysis of these phenotypes. Auxin transport was measured in roots of rcn1 and phosphatase inhibitor–treated seedlings to determine the effect of reduced PP2A activity on basipetal and acropetal auxin transport. We also assayed root gravity response and lateral root growth, processes governed by the basipetal and acropetal auxin transport streams, respectively. Our results reaffirm the role of protein phosphorylation in the regulation of auxin transport. Reduced PP2A activity produces auxin transport abnormalities that impede gravity response and alter lateral root growth.
RESULTS
Kinetics of Gravity Response in rcn1
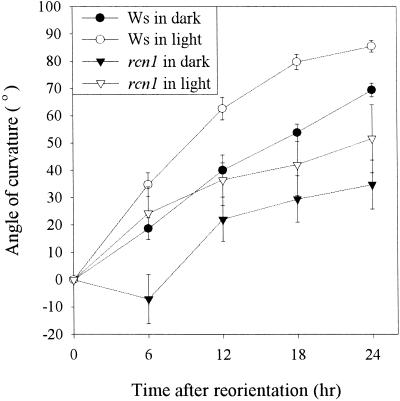
The rcn1 mutant was isolated in a screen for root curling in the presence of the auxin transport inhibitor NPA, and mutant seedlings also showed an abnormal curling growth pattern in the absence of NPA (Garbers et al., 1996). Although the factors that cause curling of roots grown on horizontal agar plates are not understood completely, root curling depends in part on root gravitropism (Okada and Shimura, 1990). Therefore, gravitropic bending in rcn1 roots was compared with that of wild-type roots to determine whether kinetic differences in the gravitropic response could contribute to the mutant phenotype. Seedlings were grown on vertically oriented agar plates, and then the plates were reoriented by 90°. The angle of root curvature was recorded every 6 hr for 24 hr and was plotted as a function of time after reorientation (Figure 1). Roots of rcn1 seedlings responded to gravity more slowly than did those of the wild type during the first 24 hr. The slowed rcn1 response was more apparent when seedlings were gravity stimulated in the dark, especially within the first 6 hr after reorientation, as a result of the absence of phototropic response (Vitha et al., 2000; Ruppel et al., 2001), as shown in Figure 1. Roots of rcn1 almost fully reoriented to the new gravity vector by 48 hr after reorientation (data not shown).
Figure 1.
Gravity Response Is Delayed in rcn1 Roots.
Six-day-old seedlings were transferred to new agar plates and allowed to grow vertically for 24 hr, at which time the plate was rotated 90°. Curvature of roots plotted as a function of time after reorientation is shown. Each value represents the average ±se for at least 70 seedlings. Ws, Wassilewskija.
The gravity responses of wild-type seedlings treated for 48 hr with the protein phosphatase inhibitor cantharidin also were examined, because growth on cantharidin produces a phenocopy of the rcn1 mutant in several growth assays (Deruère et al., 1999). Although treatment of wild-type seedlings with 3 μM cantharidin mimics rcn1 root phenotypes in several assays requiring 5 to 7 days of growth in the presence of inhibitor (Deruère et al., 1999), we found 10 μM cantharidin to be effective for the short-term treatments (48 hr) used in this work. In short-term assays, this cantharidin concentration caused ∼50% inhibition of root elongation but led to no significant radial swelling of wild-type roots, as reported previously at higher concentrations (Baskin and Wilson, 1997). The angles of gravitropic bending of rcn1, cantharidin-treated wild-type, and untreated seedlings 24 hr after reorientation are compared in Table 1. In both the dark and the light, cantharidin-treated wild-type and rcn1 seedlings showed significant reductions in the angle of curvature compared with untreated seedlings (P < 0.001). A cantharidin treatment as short as 12 hr was sufficient to reduce gravitropic bending (data not shown).
Table 1.
Tropic Bending Is Reduced in rcn1 and Cantharidin-Treated Wild-Type Roots
| Angle of Curvature after 24 hr
|
P Valuesa
|
||||
|---|---|---|---|---|---|
| WTb | rcn1b | WT + CTc | WT vs. rcn1 | WT vs. WT + CT | |
| Dark | 66 ± 2 | 27 ± 6 | 29 ± 5 | 1.1 × 10−9 | 3.0 × 10−10 |
| Light | 84 ± 1 | 59 ± 6 | 34 ± 7 | 1.3 × 10−4 | 3.1 × 10−9 |
P values were obtained by two-tailed Student's t test for unequal variance.
Each value is the average ±se for at least 90 plants from 11 experiments.
Each value is the average ±se for 50 plants from five experiments.
WT, wild type; CT, cantharidin.
A more detailed temporal examination of gravity response in rcn1 and cantharidin-treated wild-type roots was performed using Multi-ADAPT software (Mullen et al., 1998b). The absolute angles of a 160-μm segment at the root tip for wild-type, rcn1, and cantharidin-treated wild-type roots were plotted as a function of time after reorientation (Figure 2). Roots of wild-type seedlings began to curve within 1 hr after reorientation and were reoriented completely within 7 hr after reorientation. In contrast, roots of rcn1 and cantharidin-treated wild-type seedlings did not exhibit gravitropic bending until 4 or more hr after stimulation. In one rcn1 plant examined by Multi-ADAPT, the lag before gravitropic bending was as long as 16 hr (data not shown). Once rcn1 and cantharidin-treated wild-type roots initiated gravitropic curvature, bending proceeded at a rate slower than that exhibited by wild-type roots.
Figure 2.
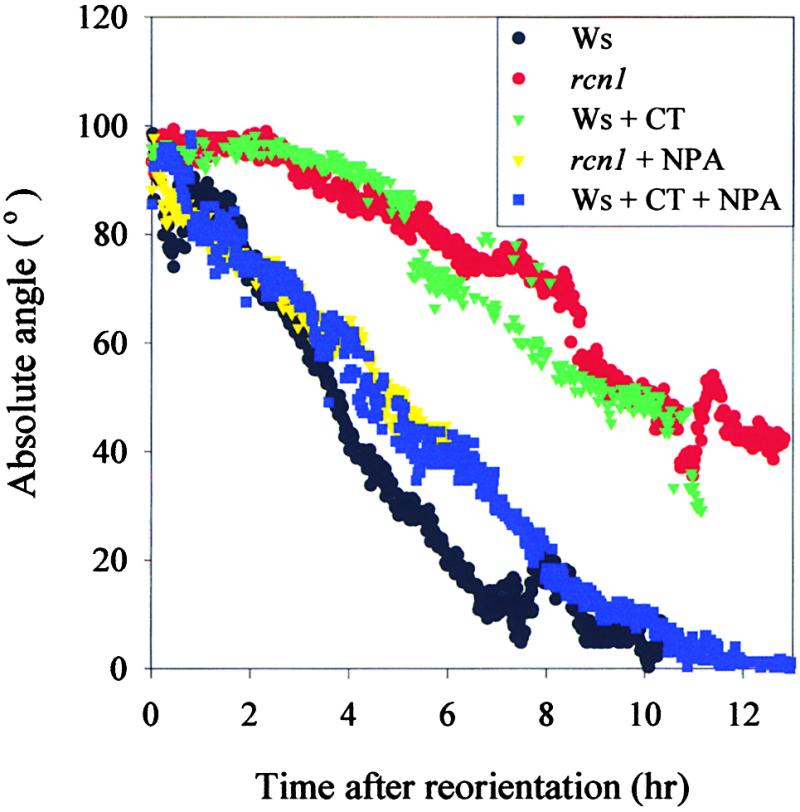
Computerized Analysis Demonstrates the Reduced Rate of Gravitropic Bending in rcn1 and Cantharidin-Treated Roots.
The absolute angles of the first 160 μm of seedling root tips were measured every minute. Wild-type, rcn1, cantharidin-treated wild-type, NPA-treated rcn1, and cantharidin- and NPA-treated wild-type roots were measured during the first 13 hr after reorientation. A representative sample of each genotype and treatment is shown. Ws, Wassilewskija; CT, cantharidin.
Multi-ADAPT software also can be used to measure elongation rates for each side of gravity-stimulated roots. Although these measurements can provide information on the relative growth rates between two plants, there is variability between experiments as a result of growth conditions. These elongation measurements are most useful for comparison of rates across sides of a gravity-stimulated root before and after gravitropic bending initiates. For a representative wild-type root, the growth rates before gravity stimulation were 5.2 and 5.6 μm/min on the two sides. During the phase of gravitropic bending, the growth rates were 5.6 μm/min on the upper side and 4.5 μm/min on the lower side. These measurements indicate that growth was inhibited on the lower side but not altered significantly on the upper side of the root. During the same time after reorientation, an rcn1 root had growth rates of 4.0 and 3.8 μm/min on the upper and lower sides, respectively, and a cantharidin-treated root had growth rates of 4.5 and 4.3 μm/min on the upper and lower sides, respectively. These similar growth rates on both sides of the root at early time points are consistent with the lack of gravitropic bending. Finally, when rcn1 roots became gravitropic, the growth rate on the upper side was unchanged at 3.8 μm/min, whereas the growth rate on the lower side of the root was reduced to 2.3 μm/min. Similarly, when cantharidin-treated wild-type roots became gravitropic, growth rates were 4.4 μm/min on the upper side and 3.9 μm/min on the lower side. Therefore, although elongation rates showed some reduction, the more striking change in rcn1 and cantharidin-treated wild-type gravitropism was a lag before initiation of the gravitropic response and a slower rate of gravitropic bending.
Root Basipetal Auxin Transport in rcn1
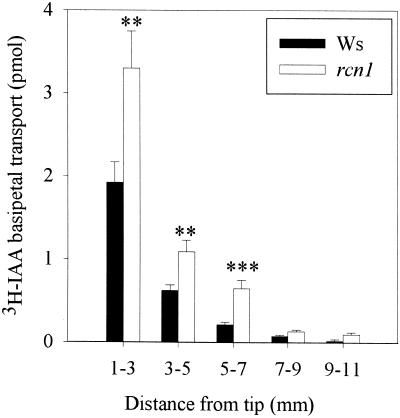
Inhibition of basipetal auxin transport, either by mutations in genes encoding auxin transport proteins (Chen et al., 1998; Marchant et al., 1999) or by treatment with auxin transport inhibitors (Rashotte et al., 2000), prevents root gravitropism. Therefore, basipetal auxin transport was measured in rcn1 roots using 3H-IAA in an in vivo assay. Agar lines containing radiolabeled IAA were applied at root tips. Five hours after application, the amount of radioactive auxin transported back from the root tip was determined by cutting each root into 2-mm segments. The amount of radioactivity in each section is reported as a function of the distance from the root tip (Figure 3). The amount of transported auxin in rcn1 and wild-type Wassilewskija roots decreased as a function of distance from the root tip, as found previously in the Landsberg erecta background (Rashotte et al., 2000). In each of the three segments into which auxin was transported, rcn1 root segments contained significantly more transported IAA, between 150 and 175% of the values for the wild type (P < 0.01).
Figure 3.
Basipetal 3H-IAA Transport in rcn1 Roots Is Increased.
An agar cylinder containing 3H-IAA was applied at the root tip of wild-type and rcn1 plants grown on control plates. After 5 hr of transport, the apical 1 mm of the root was excised and discarded, and the amount of 3H-IAA in each subsequent 2-mm segment back from the root tip was determined. Values shown are averages and se for at least 30 seedlings. The amount of auxin transported into each segment for wild type and rcn1 was compared by Student's t test with the P values indicated. **P < 0.01, ***P < 0.001. Ws, Wassilewskija.
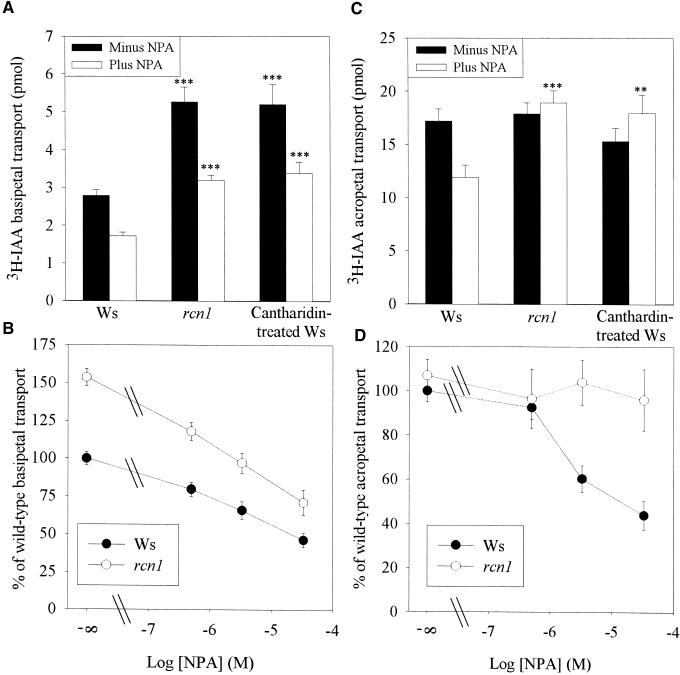
Because most (85%) of the auxin transport was found in the apical 5 mm of the root tip for both rcn1 and wild-type roots (Figure 3), additional experiments compared IAA transport into a 5-mm apical segment in rcn1 and wild-type roots. In these experiments, the amount of 3H-IAA transported in the presence and absence of NPA was compared in rcn1, wild-type, and cantharidin-treated wild-type roots, as shown in Figure 4A. Roots of both rcn1 and wild-type seedlings treated with cantharidin for 48 hr before transport measurements showed significant increases in basipetal auxin transport compared with untreated seedlings (P < 0.001). The auxin transport inhibitor NPA reduced basipetal auxin transport by 40% in rcn1, wild-type, and cantharidin-treated wild-type roots, suggesting that reduction in protein phosphatase activity does not affect the regulation of basipetal auxin transport by NPA.
Figure 4.
Reduced Phosphatase Activity Alters Basipetal and Acropetal Auxin Transport in Roots.
(A) Basipetal IAA transport was measured by application of agar containing 3H-IAA with or without 100 μM NPA to the root tip of seedlings grown on control or 10-μM cantharidin plates. After 5 hr, the apical 1 mm of the root was excised and discarded, and the amount of 3H-IAA in the apical 1 to 6 mm of the root was determined.
(B) NPA sensitivity of basipetal auxin transport was measured by placing seedlings on agar plates containing the NPA concentrations shown and assaying basipetal 3H-IAA transport as described above.
(C) Acropetal auxin transport was measured by application of agar containing 3H-IAA with or without 100 μM NPA to the root/shoot junction of plants grown on control or 10-μM cantharidin plates. After 18 hr, the amount of 3H-IAA in the apical 10 mm of the root tip was determined.
(D) NPA sensitivity of acropetal auxin transport was measured by placing seedlings on agar plates containing the NPA concentrations shown and assaying acropetal 3H-IAA transport as described above.
Each data point represents the average and se for at least 40 plants from several experiments ([A] and [C]) or for at least 10 seedlings from a representative experiment presented as a percentage relative to the Wassilewskija (Ws) minus NPA value ([B] and [D]). The amount of auxin transported into each segment for wild type, rcn1, and cantharidin-treated wild type was compared by Student's t test with the P values indicated. **P < 0.01, ***P < 0.001.
As a control for diffusion of weak acids, 14C-benzoic acid (BA) was substituted for 3H-IAA in this assay. The amount of 14C-BA movement in rcn1, wild-type, and cantharidin-treated plants is compared in Table 2 and was nearly identical in all three treatments. Therefore, the observed differences in basipetal IAA transport are not attributable to altered diffusion of weak acids.
Table 2.
14C-BA Movement in Root Auxin Transport Assays
|
14C-BA Transporteda (pmol)
|
||||||
|---|---|---|---|---|---|---|
| WT
|
rcn1
|
CT-Treated WT
|
||||
| Assay | −NPA | +NPAb | −NPA | +NPAb | −NPA | +NPAb |
| Root basipetalc | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.2 | 1.8 ± 0.1 |
| Root acropetalc | 4.3 ± 0.4 | 4.2 ± 0.5 | 4.9 ± 0.3 | 4.8 ± 0.3 | 4.3 ± 0.3 | 4.2 ± 0.3 |
The values for both rcn1 and cantharidin-treated (CT) wild-type were compared with wild-type values by Student's t test and had P values >0.05 for all tests.
The values for minus and plus NPA were compared by Student's t test for wild type, rcn1, and cantharidin-treated wild type and had P values >0.05 for all tests.
The average ±se of 29 to 42 plants from three to five experiments are reported.
WT, wild type.
Because the rcn1 mutant was isolated in a screen for altered growth in the presence of NPA, it was important to determine whether basipetal auxin transport was less sensitive to NPA. Basipetal IAA transport was measured in seedlings that had been transferred to agar plates containing a range of NPA concentrations, and IAA transport was plotted as a function of NPA concentration (Figure 4B). Basipetal auxin transport was increased in rcn1 seedlings to ∼150% the level of the wild type at each NPA concentration measured (P < 0.001). The dose dependence of NPA inhibition of auxin transport was very similar in wild-type and rcn1 roots, as shown by the similar slopes of the dose–response curves (Figure 4B). These data indicate that altered basipetal auxin transport in rcn1 is not caused by reduced NPA sensitivity. As reported by Reed et al. (1998), much less NPA was needed to inhibit auxin transport in this assay, in which plants were exposed globally to NPA on agar plates, than in the preceding experiments, in which NPA was applied only locally in the IAA-containing agar line. Global NPA application was used in these assays to allow direct comparison of these results with those of gravitropic bending assays, described below.
Effect of NPA on Root Gravitropic Bending
The results presented above show that although basipetal auxin transport was increased in roots of rcn1 seedlings, gravity response was reduced. One possible explanation for this surprising finding is that increased auxin transport may prevent either the formation or the perception of an auxin gradient across the two sides of a gravity-stimulated rcn1 root. If this is correct, then reduction of auxin transport should restore the gravity response. This hypothesis was tested by transferring dark-grown vertically oriented seedlings to agar plates containing 0.5 μM NPA. After 24 hr of growth in the dark and in the presence of NPA, the seedlings were reoriented by 90°, and the angle of curvature was measured after an additional 24 hr of growth (Table 3). Gravity response in rcn1 seedlings was increased significantly during growth on 0.5 μM NPA (P < 0.01), whereas this concentration of NPA decreased gravity response in wild-type roots. At higher NPA concentrations, gravity response was reduced in both wild-type and rcn1 seedlings (Table 3). However, NPA at all concentrations caused similar inhibition of root elongation in wild-type and rcn1 seedlings (Garbers et al., 1996; data not shown). Restoration of gravity response also was seen when rcn1 seedlings were gravity stimulated in the light after transfer to NPA (data not shown).
Table 3.
A Low NPA Dose Restores rcn1 Gravity Response
| Angle of Curvaturea
|
|||
|---|---|---|---|
| NPA Concentration (μM) | Wild Type | rcn1 | P Valuesb |
| 0 | 62 ± 4 | 23 ± 8 | 1.4 × 10−5 |
| 0.5 | 18 ± 7 | 50 ± 10c | 0.0056 |
| 33 | 11 ± 11 | 2 ± 24 | 0.37 |
The angle of curvature 24 hr after gravity stimulation in at least 27 plants from three experiments. The average ±se are reported.
P values are compared for wild type vs. rcn1 at each concentration by Student's t test.
The P value for rcn1 at 0 NPA vs. rcn1 at 0.5 μM NPA compared by Student's t test is 0.020.
Similarly, when wild-type roots were treated with cantharidin, NPA was able to reverse the delay in gravity response. Seedlings were grown in the presence of 10 μM cantharidin for 24 hr and transferred to medium containing both cantharidin and 0.5 μM NPA for an additional 24 hr before gravity stimulation. The angle of curvature from several experiments was recorded 24 hr after gravity stimulation, and the average angles of curvature were 74 ± 2°, 26 ± 7°, and 64 ± 4° for wild-type plants that were untreated, treated with cantharidin, or treated with both cantharidin and NPA, respectively. In these experiments, the inhibition of gravitropic bending by cantharidin treatment relative to untreated roots and the restoration of gravity response of cantharidin-treated seedlings by NPA treatment were statistically significant (P < 0.001).
The ability of NPA to restore gravity response in both rcn1 and cantharidin-treated wild-type roots also was examined using Multi-ADAPT software. Seedlings of rcn1 were grown vertically in the dark and transferred to agar plates containing 0.5 μM NPA 24 hr before reorientation. Figure 2 shows data for representative roots; the absolute angles were plotted after reorientation. Again, NPA treatment restored gravity response of rcn1 to wild-type levels during the first hours after gravitropic stimulation. This result was obtained in five separate experiments. Additionally, when the elongation rates of rcn1 roots in the presence or absence of NPA were compared, it was clear that the rate of growth was not the major determinant in the reduced gravitropic response of rcn1 roots. For the first 250 min after gravity stimulation, an untreated rcn1 root had growth rates of 4.0 and 3.8 μm/min on the upper and lower sides, respectively. An NPA-treated rcn1 root analyzed during the same period after gravity stimulation had growth rates of 3.6 and 2.7 μm/min on the upper and lower sides, respectively. Although the NPA treatment led to a slight reduction in growth rate, root gravity response was increased. These results show that reducing auxin transport can restore gravity response to seedlings with impaired PP2A activity. NPA rescues the gravity defect by restoring differential growth, not by correcting elongation rates overall.
To examine the effect of NPA on cantharidin-treated seedlings, plants were grown as described above. In three separate experiments, NPA restored the gravitropic bending of cantharidin-treated roots, as shown for a representative experiment in Figure 2. The elongation rates of NPA- and cantharidin-treated wild-type roots were similar to those of untreated wild-type roots before gravity stimulation, 5.4 and 5.5 μm/min on the upper and lower sides, compared with 5.2 and 5.6 μm/min for the upper and lower sides of untreated roots, respectively. The elongation rates of NPA- and cantharidin-treated roots also were similar to those of untreated roots during gravity stimulation, 5.5 and 4.9 μm/min on the upper and lower sides, compared with 5.6 and 4.5 μm/min for the upper and lower sides of untreated roots, respectively. These results show that NPA treatment can restore gravity response to seedlings with impaired PP2A activity by restoration of differential growth.
Expression of an Auxin-Responsive Reporter
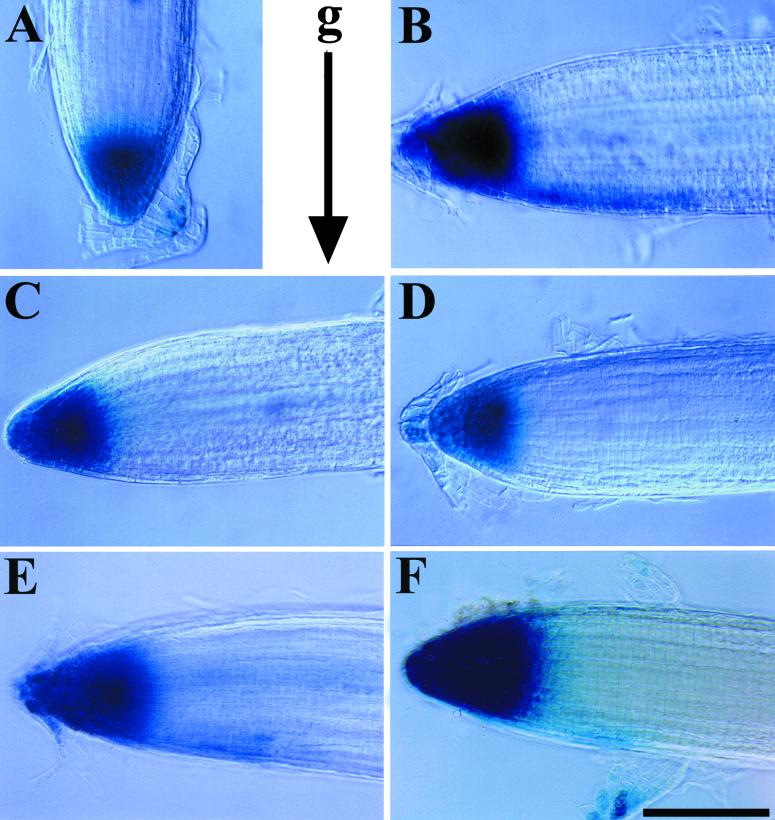
The experiment described above suggested that phosphatase inhibition may impair the formation or the perception of an auxin gradient in the root tip. We asked whether the auxin-responsive reporter construct DR5–β-glucuronidase (DR5-GUS; Ulmasov et al., 1997) might reveal such a defect in roots treated with cantharidin. The DR5-GUS reporter has been used to detect changes in auxin-induced gene expression in several mutant lines and after auxin transport inhibitor treatment (Sabatini et al., 1999; Casimiro et al., 2001). The expression of DR5-GUS was examined after gravity stimulation in wild-type roots grown in the absence and presence of cantharidin or NPA, as shown in Figure 5. DR5-GUS activity produced intense staining in the first 100 μm of the root tip in vertical, unstimulated roots (Figure 5A; Sabatini et al., 1999). Six hours after gravity stimulation, GUS activity was observed in the same region of the root tip and in a streak extending basipetally from the tip along the lower side of the root (Figure 5B). The asymmetric expression of DR5-GUS was first detectable at 4 hr after stimulation, and it persisted until the root was more than 50% reoriented (usually by 8 hr after stimulation). The asymmetric expression was even more tightly correlated with gravitropic angle, in that asymmetry was detected only in roots that had bent between 30 and 60° after gravity stimulation, and it was found in a majority of roots with this angle of curvature.
Figure 5.
Expression of an Auxin-Responsive Reporter Is Increased on the Lower Side of Gravity-Stimulated Wild-Type Roots.
GUS expression was visualized in 7-day-old seedlings homozygous for the DR5-GUS construct. Root tips are shown for a vertically grown seedling on control medium (A), a seedling 6 hr after gravity (g) stimulation on control medium (B), 1 μM NPA (C), 10 μM cantharidin (D), and 10 μM cantharidin and 0.5 μM NPA (F), and a seedling 14 hr after gravity stimulation on 10 μM cantharidin (E). Bar = 100 μm.
The asymmetric GUS expression was observed in the region between 100 and 200 μm from the root tip, which is the apical end of the distal elongation zone in Arabidopsis roots, where gravitropic bending occurs (Mullen et al., 1998a). To determine whether asymmetric DR5-GUS expression required auxin transport, roots were transferred to NPA immediately before gravity stimulation and DR5-GUS expression was analyzed 6 hr after stimulation (Figure 5C). NPA prevented both root gravitropism and formation of the asymmetric DR5-GUS expression pattern, indicating that redistribution of auxin is required for this asymmetric expression. The limited exposure of these roots to NPA treatment did not alter GUS activity at the tip, in contrast to long term exposure to NPA, which alters root development and DR5-GUS expression (Sabatini et al., 1999; Casimiro et al., 2001).
No asymmetric DR5-GUS expression was detectable in cantharidin-treated roots 6 hr after gravity stimulation (Figure 5D). This result was obtained in six separate experiments with 20 plants per experiment. Thus, differential expression of an auxin-responsive reporter was observed during gravitropic bending, but cantharidin or NPA treatment blocked this differential expression. No obvious differences in the size or shape of the region of GUS staining at the root tip were observed relative to untreated, unstimulated seedlings. To verify that this region of staining was similar, vertically grown DR5-GUS seedlings were stained and visualized by light microscopy, and images were captured with a cooled charge-coupled device camera. Image Pro Plus software was used to measure the length and two-dimensional area of GUS staining for at least 15 seedlings, and the average and standard error were determined. The length of the zone of GUS expression was 88 ± 4 and 96 ± 3 μm, and the total area was 5627 ± 340 and 5765 ± 297 μm2 for cantharidin-treated seedlings and untreated seedlings, respectively. Therefore, treatment with cantharidin did not alter the basal expression pattern of this reporter, only the formation of the asymmetric expression pattern across the root tip.
Because cantharidin-treated roots ultimately become gravitropic, the expression of the DR5-GUS reporter was examined at times later than 6 hr after gravity stimulation and the expression was correlated with the onset of gravity responsiveness. Although cantharidin-treated plants were more random in their response to gravity, as seen in Table 1, asymmetric DR5-GUS expression was observed in the majority of roots that had bent between 30 and 60° at 12 to 14 hr after gravity stimulation. The asymmetric expression pattern observed in a representative root is shown in Figure 5E. Therefore, the delay in gravitropic bending was accompanied by a delay in asymmetric auxin-induced gene expression. Additionally, rcn1 plants carrying the DR5-GUS reporter did not exhibit asymmetric expression at 6 hr, but they did show asymmetric expression between 12 and 14 hr after gravity stimulation (data not shown).
Because treatment with NPA restored gravity response in cantharidin-treated wild-type plants, the ability of NPA to restore asymmetric DR5-GUS expression in gravity-stimulated and cantharidin-treated DR5-GUS seedlings was examined. DR5-GUS seedlings were transferred to agar medium containing 10 μM cantharidin 48 hr before gravity stimulation and then were transferred to agar medium containing both 10 μM cantharidin and 0.5 μM NPA 24 hr before reorientation. Seedlings were then reoriented, and after 6 hr of gravity stimulation they were stained for GUS expression, as shown in Figure 5F. Cantharidin-treated DR5-GUS seedlings that were grown in the presence of NPA exhibited asymmetric DR5-GUS expression, although the asymmetric expression was usually less striking than in the untreated controls at 6 hr after gravity stimulation. The difference in asymmetric DR5-GUS expression after cantharidin and NPA treatment may be due to a slight reduction in the rate of gravitropic bending, compared with untreated wild-type plants; both the angle of gravitropism and the intensity of the DR5-GUS gradient were less at 6 hr. Throughout these experiments, asymmetric auxin-induced gene expression correlated directly with the gravity responsiveness of seedling roots.
Effects of Phosphatase Inhibition on Basipetal Auxin Transport in Auxin Carrier Mutants
The EIR1 gene is essential for gravitropic bending in the root and is thought to encode an auxin efflux carrier that controls basipetal auxin transport (Luschnig et al., 1998). Root basipetal transport is reduced in the eir1-1 mutant (Rashotte et al., 2000), and RCN1 and AGR1/EIR1/PIN2/WAV6 are expressed in the same region of the root tip (Chen et al., 1998; Deruère et al., 1999). To determine whether RCN1-regulated dephosphorylation acts directly on a putative auxin efflux carrier expressed in the root tip, an eir1-1 rcn1 double mutant was constructed (see Methods). Basipetal auxin transport was measured in the double mutant. The results are compared with those from the single mutants and the wild type in Table 4. If RCN1 acts directly on the carrier protein, then the eir1-1 rcn1 double mutant would be expected to exhibit reduced basipetal auxin transport, like eir1-1. Instead, basipetal auxin transport in the eir1-1 rcn1 double mutant was similar to that in rcn1 and was increased above the levels observed in eir1-1, wild type, and the eir1-1 RCN1 sibling (P < 0.001). This suggests that the rcn1 effect on auxin transport does not require the AGR1/EIR1/PIN2/WAV6 efflux carrier and in fact overcomes the effect of the eir1-1 mutation.
Table 4.
Increased Basipetal Auxin Transport in rcn1 Does Not Require EIR1 Function
| Transport of 3H-IAA
|
|||
|---|---|---|---|
| Plant | pmola | % of WT | P Value |
| Ws | 2.9 ± 0.3 | 100 | NA |
| rcn1b | 5.1 ± 0.3 | 176 | 3.6 × 10−6 |
| Columbia | 2.8 ± 0.2 | 100 | NA |
| eir1-1c | 1.9 ± 0.1 | 68 | 2.8 × 10−6 |
| eir1-1 RCN1b | 1.9 ± 0.1 | 68 | 3.9 × 10−5 |
| eir1-1 rcn1c | 4.8 ± 0.3d | 171 | 2.8 × 10−7 |
The number of plants examined is at least 27 from three experiments, and the average ±se are reported.
P value is compared with the Ws wild-type background by Student's t test.
P values are compared with the Columbia wild-type background by Student's t test.
The P value for eir1-1 rcn1 vs. rcn1 compared by Student's t test is 0.48.
WT, wild type; Ws, Wassilewskija; NA, not applicable.
Because eir1-1 is a relatively weak allele, root basipetal transport also was assayed in cantharidin-treated seedlings carrying a stronger allele at the AGR1/EIR1/PIN2/WAV6 locus. Table 5 shows that in cantharidin-treated seedlings, basipetal auxin transport was increased to ∼150% of the level in non-cantharidin-treated seedlings for eir1-1, agr1-7 (a strong allele), and wild-type roots. A similar result was obtained with the null allele agr1-5 (data not shown). These results confirm that RCN1 does not act directly on the AGR1/EIR1/PIN2/WAV6 protein to regulate basipetal auxin transport in the root.
Table 5.
Increased Basipetal Auxin Transport in rcn1 Does Not Require AGR1/EIR1/PIN2/WAV6 or AUX1 Function
| Transport of 3H-IAA (pmol)
|
||||
|---|---|---|---|---|
| Plant | −CTa | +CTa | +ΨΤ −CT (%) | P Valueb |
| EST-0 | 2.7 ± 0.2 | 4.3 ± 0.4 | 157 | 0.00020 |
| agr1-7 | 2.3 ± 0.2 | 4.2 ± 0.2 | 180 | 6.5 × 10−10 |
| Columbia | 2.8 ± 0.2 | 4.2 ± 0.2 | 150 | 6.9 × 10−6 |
| eir1-1 | 2.3 ± 0.1 | 3.8 ± 0.2 | 161 | 2.3 × 10−8 |
| aux1-7 | 2.2 ± 0.1 | 3.3 ± 0.1 | 148 | 6.2 × 10−8 |
The number of plants examined is at least 28 from three experiments, and the average ±se are reported.
P value is for the comparison of each genotype with and without CT and was determined by Student's t test.
CT, cantharidin.
We also tested the hypothesis that the target of phosphatase action is the AUX1 auxin influx carrier. Basipetal auxin transport in aux1-7 roots was reduced relative to wild-type roots, as shown in Table 5. As in the wild type, cantharidin treatment of aux1-7 caused an increase in auxin transport relative to untreated seedlings. These data suggest that neither the influx carrier, AUX1, nor the efflux carrier, AGR1/EIR1/PIN2/WAV6, is a target for RCN1-regulated dephosphorylation.
Root Acropetal Auxin Transport in rcn1
To determine whether the rcn1 lesion alters acropetal as well as basipetal auxin transport in roots, acropetal transport was assayed. Acropetal auxin transport was measured in wild-type, rcn1, and cantharidin-treated wild-type seedlings by placing an agar line containing 3H-IAA with or without NPA on the root just below the root/shoot junction. After 18 hr, the apical 1 cm of the root was excised and the amount of radioactivity in the tip was determined; the results are shown in Figure 4C. In the absence of NPA, acropetal auxin transport was similar in all three sets of plants. However, auxin transport was not inhibited by local application of 100 μM NPA in rcn1 and cantharidin-treated seedlings, whereas a statistically significant reduction in transport occurred when NPA was applied locally to wild-type seedlings (P < 0.01). Diffusion of 14C-BA in this assay was unaffected in mutant and wild-type seedlings treated with either NPA or cantharidin, indicating that transport differences were not caused by differences in weak acid diffusion (Table 2).
These data suggest that root acropetal auxin transport is not regulated by NPA in rcn1 and cantharidin-treated seedlings. To examine this possibility in greater detail, acropetal transport was measured in plants transferred to agar plates containing a range of NPA concentrations. The amount of acropetal IAA transport in rcn1 and the wild type was plotted as a function of NPA concentration (Figure 4D). Wild-type seedlings showed a statistically significant reduction in transport as a result of global applications of NPA concentrations greater than 1 μM (P < 0.001), whereas transport was not reduced at NPA concentrations up to 33 μM in rcn1 seedlings (P > 0.5). These results indicate that the NPA sensitivity of acropetal auxin transport was reduced in rcn1 seedlings. Additionally, this experiment confirmed that low concentrations of globally applied NPA were effective in transport inhibition, as discussed above.
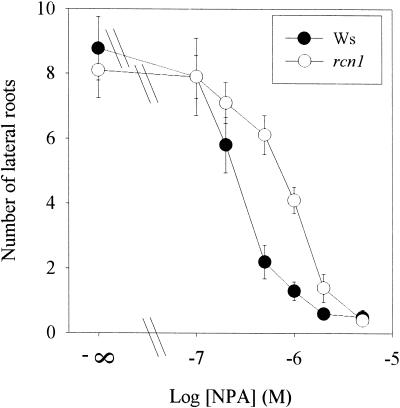
NPA Sensitivity of Lateral Root Growth in rcn1
Auxin transport inhibitors reduce the number of elongated lateral roots in a dose-dependent manner (Reed et al., 1998) and arrest lateral root development at one of the earliest cell divisions (Casimiro et al., 2001). Root acropetal auxin transport has been implicated specifically in lateral root development (Reed et al., 1998), although it also has been suggested that basipetal auxin transport supplies auxin needed for the earliest steps in lateral root initiation (Casimiro et al., 2001). Therefore, lateral root growth and the ability of NPA to prevent lateral root development were examined in rcn1 seedlings, in which acropetal auxin transport was at wild-type levels and unaffected by NPA and basipetal auxin transport was increased and showed normal regulation by NPA. The number of elongated lateral roots in wild-type and rcn1 seedlings, reported as a function of NPA concentration, is shown in Figure 6. In the absence of NPA, wild-type and rcn1 plants had similar numbers of lateral roots. However, at 0.2 μM NPA, rcn1 seedlings had more lateral roots than did wild-type seedlings (P < 0.05). At NPA concentrations greater than 5 μM, no lateral roots emerged on either wild-type or rcn1 plants. Lateral root growth was threefold less sensitive to NPA in rcn1 seedlings than in wild-type seedlings (50% inhibitory concentration were 0.33 and 0.91 μM for wild-type and rcn1 seedlings, respectively). A similar reduction in NPA sensitivity of lateral root growth was observed for cantharidin-treated wild-type roots (data not shown).
Figure 6.
Lateral Root Growth in rcn1 Shows Reduced NPA Sensitivity.
Four-day-old wild-type and rcn1 seedlings were transferred to agar plates containing NPA at the concentrations indicated and allowed to grow vertically. After 6 days, the number of lateral roots emerging from each primary root was counted. Each data point shown gives the average ±se for 10 seedlings. Ws, Wassilewskija.
Growth on medium containing 1 × Murashige and Skoog (1962) (MS) salts (our standard growth medium) can prevent elongation of initiated lateral roots, but all lateral root primordia emerged and elongated on 0.25 × MS medium (Mary Williams, personal communication; data not shown). To differentiate between the effects on lateral root formation and elongation, rcn1 and wild-type seedlings were grown on agar plates containing 0.25 × MS salts in the presence or absence of NPA. As in the experiments described above, the number of lateral roots formed in the presence of NPA was decreased in wild-type seedlings but not in rcn1 seedlings. These data show that NPA reduced the number of lateral roots in the wild type, whereas rcn1 roots were less sensitive to the effect of NPA.
DISCUSSION
One major goal of this work was to test directly whether reduced phosphatase activity in the rcn1 mutant affected root auxin transport and gravity response. Roots of rcn1 seedlings were found to have a reduced rate of gravitropic bending that could be mimicked in wild-type plants with the phosphatase inhibitor cantharidin. Although rcn1 roots reoriented completely to a new gravity vector by 48 hr after gravity stimulation, they exhibited a delay in bending during the first hours after a gravitropic stimulus and bent at a slower rate than did wild-type plants. In vivo auxin transport assays revealed significantly increased basipetal transport in roots of the rcn1 mutant, although the NPA sensitivity of basipetal transport was normal. These changes in auxin transport also were observed in cantharidin-treated roots and thus were the result of reduced phosphatase activity. Phosphatase inhibition also reduces gravity response in oat pulvini (Chang and Kaufman, 2000).
The reduced gravitropic response and increased auxin transport in rcn1 seem paradoxical, because chemical treatments or genetic lesions that reduce auxin transport have been shown to reduce gravity response as well (Bennett et al., 1996; Chen et al., 1999; Rashotte et al., 2000). One explanation for this finding is that increased basipetal auxin movement may prevent either the normal formation of an auxin gradient or the perception of the gradient; both may be required for gravitropic bending (Trewavas, 1992; Chen et al., 1999). We tested this hypothesis by assaying gravity responses after an NPA treatment that reduces basipetal IAA transport. Gravitropism was increased by this NPA treatment in rcn1 plants. Interestingly, the Arabidopsis hyl1 mutant exhibits reduced gravitropic bending that can be restored partially by growth on low concentrations of the auxin transport inhibitor 2,3,5-triiodobenzoic acid, although NPA did not have a similar effect (Lu and Fedoroff, 2000).
Additional support for the hypothesis that increased basipetal auxin movement may prevent the normal formation or perception of an auxin gradient required for gravitropic bending comes from the asymmetric expression pattern of the auxin-responsive DR5-GUS reporter construct in gravity-stimulated roots and the sensitivity of this expression pattern to both cantharidin and NPA. Normal wild-type plants showed high levels of GUS activity on the lower sides of gravity-stimulated roots in the region immediately proximal to the root tip, whereas GUS activity was undetectable in this same region on the upper side. Because NPA prevented formation of the asymmetric DR5-GUS expression pattern, redistribution of auxin was responsible, at least in part, for the observed differential gene expression. Cantharidin treatment prevented the asymmetric expression of the DR5-GUS construct 6 hr after gravity stimulation, although basal expression was unchanged by this treatment. When cantharidin-treated roots became gravitropic 12 to 14 hr after gravity stimulation, asymmetric expression of DR5-GUS was detectable. Additionally, the asymmetry in DR5-GUS expression and gravitropic bending were restored 6 hr after gravity stimulation in cantharidin-treated roots by exposure to NPA. These results demonstrate a strong correlation between gravitropic bending and asymmetric auxin-induced gene expression across the root tip. The ability of auxin transport inhibitor treatment to prevent asymmetric DR5-GUS expression clearly indicates that redistribution of auxin is required for this response. However, DR5-GUS expression provides information on both auxin concentration and auxin responsiveness, and asymmetric expression also may require changes in auxin responsiveness resulting from the activity of other transcriptional regulatory proteins needed for DR5-GUS expression.
It is important to ask whether reductions in protein phosphatase alter IAA transport and root gravitropism directly or whether alterations in activity lead to altered root morphology that affects IAA transport indirectly. Several protein kinase and phosphatase inhibitors, including cantharidin, can alter the morphology of Arabidopsis roots when applied at high concentrations (Baskin and Wilson, 1997). However, the cantharidin concentration used for these experiments did not cause increases in root diameter, as can be seen in Figures 5D, 5E, and 5F. Additionally, no obvious morphological changes in the root were observed up to 6 days after transfer to cantharidin-containing plates, although minor morphological changes were observed in roots grown on cantharidin for more than 10 days (A.M. Rashotte and G.K. Muday, unpublished observation). No obvious morphological differences have been observed in rcn1 roots (A. DeLong, unpublished observation). Therefore, altered gravity response and auxin transport in rcn1 and cantharidin-treated plants do not appear to result from altered root morphology.
Although phosphatase inhibition alters both basipetal and acropetal root auxin transport, there are striking differences in the effects on the two distinct polarities of auxin movement. Basipetal auxin transport was increased when phosphatase activity was reduced by either genetic lesions or inhibitor treatments, whereas the regulation by NPA was unchanged. In contrast, acropetal auxin transport was not increased, but its regulation by NPA was abrogated. The physiological processes that are linked to each polarity of auxin movement show abnormalities that correlate well with the observed auxin transport defects. Gravitropic changes are consistent with altered basipetal transport (Rashotte et al., 2000), and the reduced NPA sensitivity of lateral root growth is consistent with the altered sensitivity of acropetal transport to inhibition by NPA (Reed et al., 1998). Our results further demonstrate that these two auxin transport streams are distinct in their function and regulation and that it is necessary to examine both transport polarities to understand the role of auxin transport in root growth and development.
Earlier studies indicated that serine/threonine phosphorylation modulates auxin efflux, whereas tyrosine phosphorylation controls NPA sensitivity (Bernasconi, 1996; Delbarre et al., 1998). Our results suggest that auxin efflux and its NPA sensitivity are both affected by serine/threonine phosphorylation in planta. Although it is clear that RCN1-regulated PP2A activity plays a role in controlling auxin transport, the protein targets of its action are unknown. RCN1 may act directly through dephosphorylation of an auxin efflux carrier protein. However, basipetal auxin transport assays of both double mutants and cantharidin-treated seedlings show that increased transport in the rcn1 mutant is independent of the AGR1/EIR1/PIN2/WAV6 auxin efflux carrier, which is believed to control root basipetal auxin transport. An effect on AUX1 influx carrier activity is unlikely to account for increased transport, because cantharidin treatment also increases basipetal transport in aux1 seedlings. Because AGR1/EIR1/PIN2/WAV6 is a member of a large gene family (Palme and Gälweiler, 1999), it is possible that RCN1 acts on another member of this family of auxin efflux carriers. For instance, loss of RCN1 function might allow activation of an efflux carrier that is normally inactive in basipetal transport. This hypothesis is consistent with elevated auxin transport in the eir1-1 rcn1 double mutant over the wild type.
In conclusion, reduction of protein phosphatase activity in rcn1 mutant and in wild-type plants treated with cantharidin led to increased root basipetal auxin transport, reduced root gravity response, and a delay in asymmetric auxin-induced gene expression across a gravity-stimulated root tip. Gravity response and asymmetric auxin-induced gene expression were restored through reduction of the increased IAA transport in rcn1 or chemically mimicked cantharidin-treated wild-type seedlings by growth on NPA. Root acropetal auxin transport in rcn1 showed a loss of regulation by NPA, as did the growth of lateral roots, which depends on acropetal auxin transport. These results suggest that normal PP2A activity regulated by RCN1 is required for the proper regulation of root auxin transport, gravity response, and lateral root development in Arabidopsis. Furthermore, these results support the idea that auxin gradients across roots are necessary for gravitropic bending in roots.
METHODS
Chemicals
Naphthylphthalamic acid (NPA) was purchased from Chemical Services (West Chester, PA). Absolute ethanol was purchased from McCormick Distilling (Weston, MO). 3-5(n)-3H-Indoleacetic acid (IAA; 27 and 25 Ci/mmol) and ring-U-14C-benzoic acid (BA; 126 mCi/mmol) were purchased from Amersham (Arlington Heights, IL). All other chemicals were purchased from Sigma (St. Louis, MO).
Seed Germination and Plant Growth
Wild-type Arabidopsis thaliana seed (ecotypes Wassilewskija and Columbia) and aux1-7 and eir1-1 seed were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). The agr1-5 and agr1-7 seed and their parental background ecotype EST-0 were generously provided by Patrick Masson (University of Wisconsin, Madison) (Chen et al., 1998). Transgenic DR5–β-glucuronidase (DR5-GUS) seed were from Jane Murfett (University of Missouri, Columbia) (Ulmasov et al., 1997). Seed were soaked in distilled water for 30 min and surface sterilized with 95% ethanol for 5 min and 20% bleach with 0.01% Triton X-100 for 5 min. After five washes in sterile distilled water, seed were germinated and grown on 9-cm Petri plates containing sterile control medium (0.8% agar [Sigma type M, plant tissue culture], 1 × Murashige and Skoog salts, pH 6.0, 1.5% Suc, 1 μg·mL−1 thiamine, 1 μg·mL−1 pyridoxine HCl, and 0.5 μg·mL−1 nicotinic acid). Seed were grown in vertically oriented Petri dishes in continuous fluorescent light (90 μmol·m−2· sec−1) at room temperature (22°C) for light-grown root auxin transport, gravity, or lateral root experiments. Seedlings used in assays lacking light were grown at room temperature (22°C) either entirely in the absence of light or were transferred to this condition from the light conditions described above at least 2 days before assays were performed.
Analysis of Gravitropism
Gravity response was measured using 6-day-old seedlings transferred to plates containing either control agar or agar supplemented with 10 μM cantharidin and/or NPA at the concentrations indicated (Figure 2, Table 3). After 24 hr of vertical growth, control or supplemented control plates were reoriented by 90°. Angles and lengths were measured using a protractor and ruler at specific times after reorientation. Compounds on supplemented plates were added to 50°C molten control agar and poured onto plates. NPA and cantharidin were dissolved in DMSO; the final DMSO concentration was 0.1% in agar medium. Fresh agar containing these compounds was made for each experiment to minimize breakdown of the compounds.
For automated video digitizer analysis of root gravitropism, plants were germinated and grown as described above. Immediately before analysis, a seedling was transferred to a new agar plate and was covered with liquid agar cooled to 35°C. Both the agar covering the seedling and the new agar plate were supplemented or not as treated previously. The seedling remained on the agar plate for ∼5 min until the agar had solidified before image analysis began. Images were captured with a charge-coupled device camera connected to a computer by a frame-grabber circuit board. The seedling was illuminated from behind with an infrared light–emitting diode. The Petri dish was oriented vertically and held in place with a micromanipulator. The charge-coupled device camera, computer, infrared light–emitting diode, and software were purchased from the Plant Growth Imaging Facility at Ohio State University. Images were analyzed using Multi-ADAPT software (Mullen et al., 1998a). This software divides the root into segments of consistent length on the basis of user-defined reference points at the tip and in the nonelongating region of the root. The angle of each segment and the length on both sides of the root from the tip to the nonelongating region were recorded at user-specified time intervals. For this analysis, segments 160 μm long were examined at 60-sec intervals.
Expression of the Auxin-Responsive Reporter Gene
Seedlings containing the auxin-responsive construct DR5-GUS were grown on agar plates in the presence or absence of 10 μM cantharidin and/or 0.5 or 1 μM NPA and gravity stimulated for various times between 2 and 24 hr. Seedlings were left on agar plates during histochemical GUS staining so that the position of GUS staining could be compared with the angle and position of root gravicurvature. At the times indicated (Figure 5), seedlings were washed gently with 100 mM sodium phosphate buffer, pH 7.0, and then stained for 2 to 3 hr at 37°C in 2 mM X-glucuronide dissolved in 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 0.5% Triton X-100, and 100 mM sodium phosphate buffer, pH 7.0 (Craig, 1992). After staining was complete, tissue was washed in 100 mM sodium phosphate buffer followed by 95% ethanol and then cleared in an 8:2:1 chloral-hydrate:water:glycerol solution (Berleth and Jurgens, 1993) for differential interference microscopy. Microscopy was performed with a Zeiss (Jena, Germany) Axioplan microscope with a ×40 objective, and images were captured on 35-mm film, scanned, and processed to remove background inconsistencies in Adobe (Mountain View, CA) Photoshop. Analysis of the area and length of the zone of GUS staining at the root tip was performed in seedlings grown, treated, stained, and cleared for microscopy as described above. At least 15 seedlings for each treatment were examined with a Zeiss Axioplan microscope with a ×20 objective, and images of each root were captured digitally and analyzed for both the length back from the root tip and the total area of GUS staining using Image Pro Plus software. Measurements were transferred to Microsoft (Redmond, WA) Excel for determination of averages, standard error, and P values by Student's t tests.
Auxin Transport Assays
Basipetal auxin transport was measured in 7-day-old vertically grown seedlings as described by Rashotte et al. (2000). Seedlings were transferred to control plates with their root tips aligned. Mixtures containing 1% agar, 100 nM 3H-IAA, and either 100 μM NPA or 1% DMSO were prepared in 3-mL scintillation vials. A narrow stem transfer pipette was inserted carefully into the hardened agar mixture such that a 1-mm-diameter cylinder of agar was removed. This cylinder containing 3H-IAA agar was applied such that the agar just touched the root tip of the seedlings. Plates remained oriented vertically in the dark to avoid IAA or NPA degradation, and 3H-IAA transport was measured after 5 hr by first removing the apical 1 mm in contact with the agar line and then cutting 2- or 5-mm segments back from the apical tip. Each root segment was placed into 2.5 mL of scintillation fluid, and the amount of radioactivity within each sample was determined using a Beckman LS6500 scintillation counter for 2 min. To control for diffusion of a weak organic acid like IAA, 14C-BA also was used in this assay in place of 3H-IAA in a 1% agar cylinder containing 4 μM 14C-BA applied to the root tip. In assays designed to measure the concentration dependence of NPA inhibition for basipetal auxin transport, seedlings were transferred to either control plates or plates containing NPA 24 hr before initiation of the transport assay. Assays were performed as described above, but no additional NPA was added to the agar lines for these experiments.
Acropetal auxin transport was measured in 7-day-old vertically grown seedlings as modified from Reed et al. (1998). Seedlings were transferred to plates containing control agar such that their root/shoot junctions were aligned. Mixtures containing 1% agar, 100 nM 3H-IAA, 10 μM cold IAA, and either 100 μM NPA or 1% DMSO were prepared in a 3-mL scintillation vial. Agar cylinders were made using narrow stem transfer pipettes as described above and placed on the root just below the root/shoot junction. Seedlings were oriented vertically in the dark to avoid IAA or NPA degradation. IAA transport was measured after 18 hr by excising a 5- or 10-mm segment of the root tip, placing it into 2.5 mL of scintillation fluid, and determining the amount of radioactivity, as described above. 14C-BA also was used in this assay in place of IAA as a weak acid diffusion control in a 1% agar cylinder containing 4 μM 14C-BA applied to the root just below the root/shoot junction. In assays designed to measure the concentration dependence of NPA inhibition of acropetal auxin transport, seedlings were transferred to either control plates or plates with various concentrations of NPA 24 hr before initiation of the transport assay. Assays were performed as described above, but no additional NPA was added to the agar lines for these experiments.
Double Mutant Analyses
The double mutant eir1-1 rcn1 was generated by crossing the rcn1 mutant (as male) onto eir1-1 plants. F3 progeny families from two independent crosses were screened for the eir1-1 agravitropic root phenotype. Two agravitropic F3 families segregating for kanamycin resistance (a marker carried on the rcn1 T-DNA) were identified, and F4 families were screened for homozygous kanamycin resistance or sensitivity. rcn1 genotypes were confirmed by polymerase chain reaction analysis. The double mutant, an eir1 RCN1 sibling, the parental single mutants, and the wild-type background of each mutant were assayed for basipetal auxin transport as described above.
Lateral Root Analyses
The lateral root development of wild type and rcn1 was examined by transferring 4-day-old seedlings germinated on control agar plates to vertically oriented agar plates with varying concentrations of NPA or cantharidin. After 6 more days of growth, the length of the primary root was measured to the nearest millimeter with a ruler. The number of lateral roots on each primary root was determined by counting all lateral roots that had emerged from the primary root using a dissecting microscope. Averages and standard errors are reported.
Statistics
Statistical analyses of the data were performed using Microsoft Excel. Multiple experiments were analyzed simultaneously by comparing averages using each root as an independent sample. The data were analyzed by a one-tailed Student's t test for equal variance for NPA treatments, because the assumption being tested was that NPA treatment would reduce IAA movement, and by a two-tailed Student's t test for equal variance when comparing the wild-type seedlings with mutant or inhibitor-treated seedlings, with no previous assumption to this data. P values are reported.
Acknowledgments
The assistance of Shari Brady and Jennifer Waters Shuler with image capture and analysis is appreciated. We thank Candace Waddell and Jean Deruère for comments on the manuscript and Jane Murfett and Patrick Masson for seed stocks. This work was supported by grants from the National Aeronautics and Space Administration Specialized Center for Research and Training (North Carolina State University) to A.M.R. and G.K.M. and from the National Science Foundation (IBN9604782) to A.D.
References
- Baskin, T.I., and Wilson, J.E. (1997). Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 113, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273, 948–950. [DOI] [PubMed] [Google Scholar]
- Berleth, T., and Jurgens, G. (1993). The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118, 575–587. [Google Scholar]
- Bernasconi, P. (1996). Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol. Plant. 96, 205–210. [Google Scholar]
- Brown, D.E., Rashotte, A.M., Murphy, A.S., Normanly, J., Tague, B.W., Peer, W.A., Taiz, L., and Muday, G.K. (2001). Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126, 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro, I., Marchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inze, D., Sandberg, G., Casero, P.J., and Bennett, M. (2001). Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S., and Kaufman, P. (2000). Effects of staurosporine, okadaic acid and sodium fluoride on protein phosphorylation in graviresponding oat shoot pulvini. Plant Physiol. Biochem. 38, 315–323. [DOI] [PubMed] [Google Scholar]
- Chen, R., Hilson, P., Sedbrook, J., Rosen, E., Caspar, T., and Masson, P.H. (1998). The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 95, 15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R., Rosen, E., and Masson, P.H. (1999). Gravitropism in higher plants. Plant Physiol. 120, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.N., and Muday, G.K. (1994). NPA binding activity is peripheral to the plasma membrane and is associated with the cytoskeleton. Plant Cell 6, 1941–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, S. (1992). The GUS reporter gene: Application to light and transmission electron microscopy. In GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression, S. Gallagher, ed (San Diego: Academic Press), pp. 115–124.
- Delbarre, A., Muller, P., and Guern, J. (1998). Short-lived and phosphorylated proteins contribute to carrier-mediated efflux, but not to influx, of auxin in suspension-cultured tobacco cells. Plant Physiol. 116, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruère, J., Jackson, K., Garbers, C., Soll, D., and DeLong, A. (1999). The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J. 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Dixon, M.W., Jacobson, J.A., Cady, C.T., and Muday, G.K. (1996). Cytoplasmic orientation of the naphthylphthalamic acid-binding protein in zucchini plasma membrane vesicles. Plant Physiol. 112, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler, L., Guan, C., Muller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Garbers, C., DeLong, A., Deruère, J., Bernasconi, P., and Soll, D. (1996). A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 15, 2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Jacobs, M., and Rubery, P.H. (1988). Naturally occurring auxin transport regulators. Science 241, 346–349. [DOI] [PubMed] [Google Scholar]
- Jones, A.M. (1998). Auxin transport: Down and out and up again. Science 282, 2201–2203. [DOI] [PubMed] [Google Scholar]
- Lomax, T.L., Muday, G.K., and Rubery, P. (1995). Auxin transport. In Plant Hormones: Physiology, Biochemistry, and Molecular Biology, P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic Press), pp. 509–530.
- Lu, C., and Fedoroff, N. (2000). A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12, 2351–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig, C., Gaxiola, R.A., Grisafi, P., and Fink, G.R. (1998). EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, A., Kargul, J., May, S.T., Muller, P., Delbarre, A., Perrot- Rechenmann, C., and Bennett, M.J. (1999). AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 18, 2066–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, D.A., and Johnson, C.F. (1990). The role of auxin efflux carriers in the reversible loss of polar auxin transport in the pea (Pisum sativum L.) stem. Planta 181, 117–124. [DOI] [PubMed] [Google Scholar]
- Morris, D.A., Rubery, P.H., Jarman, J., and Sabater, M. (1991). Effects of inhibitors of protein synthesis on transmembrane auxin transport in Cucurbita pepo L. hypocotyl segments. J. Exp. Bot. 42, 773–783. [Google Scholar]
- Muday, G. (2000). Maintenance of asymmetric cellular localization of an auxin transport protein through interaction with the actin cytoskeleton. J. Plant Growth Regul. 19, 385–396. [DOI] [PubMed] [Google Scholar]
- Mullen, J.L., Ishikawa, H., and Evans, M.L. (1998. a). Analysis of changes in relative elemental growth rate patterns in the elongation zone of Arabidopsis roots upon gravistimulation. Planta 206, 598–603. [DOI] [PubMed] [Google Scholar]
- Mullen, J.L., Turk, E., Johnson, K., Wolverton, C., Ishikawa, H., Simmons, C., Soll, D., and Evans, M.L. (1998. b). Root-growth behavior of the Arabidopsis mutant rgr1: Roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol. 118, 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, A., Guan, C., Gälweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998). AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Murphy, A., Peer, W., and Taiz, L. (2000). Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211, 315–324. [DOI] [PubMed] [Google Scholar]
- Okada, K., and Shimura, Y. (1990). Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250, 274–276. [DOI] [PubMed] [Google Scholar]
- Okada, K., Ueda, J., Komaki, M.K., Bell, C.J., and Shimura, Y. (1991). Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme, K., and Gälweiler, G. (1999). PIN-pointing the molecular basis of auxin transport. Curr. Opin. Plant Biol. 2, 375–381. [DOI] [PubMed] [Google Scholar]
- Rashotte, A., Brady, S., Reed, R., Ante, S., and Muday, G. (2000). Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, R.C., Brady, S.R., and Muday, G.K. (1998). Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 118, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery, P., and Jacobs, M. (1990). Auxin transport and its regulation by flavonoids. In Plant Growth Substances 1988, R. Pharis and S. Rood, eds (Berlin: Springer Verlag), pp. 428–440.
- Rubery, P.H. (1990). Phytotropins: Receptors and endogenous ligands. Symp. Soc. Exp. Biol. 44, 119–146. [PubMed] [Google Scholar]
- Ruppel, N.J., Hangarter, R.P., and Kiss, J.Z. (2001). Red-light-induced positive phototropism in Arabidopsis roots. Planta 212, 424–430. [DOI] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelt, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, P., Leyser, O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Suttle, J.C. (1988). Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 88, 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle, J.C. (1991). Biochemical bases for the loss of basipetal IAA transport with advancing physiological age in etiolated Helianthus hypocotyls. Plant Physiol. 96, 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas, A.J. (1992). What remains of the Cholodny-Went theory? Plant Cell Environ. 15, 759–794. [PubMed] [Google Scholar]
- Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuno, K., Shikanai, T., Yamada, Y., and Hashimoto, T. (1998). AGR, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol. 39, 1111–1118. [DOI] [PubMed] [Google Scholar]
- Vitha, S., Zhao, L., and Sack, F.D. (2000). Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol. 122, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]