Abstract
The expression of many gene products required during the early stages of Bacillus subtilis sporulation is regulated by sinIR operon proteins. Transcription of sinIR from the P1 promoter is induced at the end of exponential growth. In vivo transcription studies suggest that P1 induction is repressed by the transition-state regulatory protein Hpr and is induced by the phosphorylated form of Spo0A. In vitro DNase I footprinting studies confirmed that Hpr, AbrB, and Spo0A are trans-acting transcriptional factors that bind to the P1 promoter region of sinIR. We have also determined that the P1 promoter is transcribed in vitro by the major vegetative sigma factor, ςA, form of RNA polymerase.
Natural environments are oligotrophic (35). Organisms such as the common soil bacterium Bacillus subtilis frequently exist in slow- or nongrowing physiological states. The rich diversity of B. subtilis transition-state regulatory systems (50, 55, 56) confirms the biological importance of managing the transition from rapid- to slow- to nongrowing cell states. Depending on the environmental cues present, B. subtilis transition-state regulation can channel a cell toward motility, nutrient scavenging through the production of extracellular enzymes, competence, or sporulation cell fates (for a review, see references 12 and 53). The best-characterized B. subtilis transition-state regulators are the AbrB, Hpr, Spo0A, and SinR DNA-binding proteins.
Recent structural studies have shown that the AbrB protein is a tetramer of 10,500-Da subunits that interacts with a variety of specific nucleotide sequences, presumably by recognizing a particular three-dimensional DNA architecture (54, 59, 62). AbrB can function as a repressor of genes such as spo0E, spo0H, spoVG, and aprE (14, 34, 43, 64) and as an activator of genes such as hpr and the rbs operon (52, 53). Transcription of abrB is controlled by negative autoregulation and repression by Spo0A (53, 55). The hpr gene product is a 23,718-Da protein, which was originally identified as a locus (hpr, scoC, and catA) for mutations causing protease overproduction and catabolite-resistant sporulation (10, 21, 39). Hpr binds to a consensus DNA sequence RATAnTATY (25, 53). Hpr represses the expression of the protease genes aprE and nprE and oligopeptide permease operons (20, 26) and when present on a multicopy plasmid can inhibit sporulation in an as-yet-undetermined manner (39). The Spo0A 29,691-Da protein is the master controller of early developmental events (55, 56). Metabolic and environmental signals cause the autophosphorylation of sensor kinases such as KinA, KinB, and KinC (1, 24, 27, 41), which transfer phosphate groups through a phosphorelay (Spo0F and Spo0B) to generate Spo0A∼P (24, 55, 57). Spo0A∼P recognizes a 0A box DNA sequence, TGNCGAA (51). Spo0A∼P is a repressor of abrB transcription and an activator of spoIIA, spoIIG, and spoIIE operon expression (4, 45, 51, 57, 63). spo0A expression is controlled by ςA and ςH promoters (9, 44, 49). Vegetative spo0A expression originates from the ςA promoter, and catabolite-regulated postexponential expression is controlled from the ςH promoter.
The dicistronic sin operon was originally identified as a clone which could inhibit sporulation and protease production when present on a high-copy-number plasmid (16, 17). The first gene in the operon encodes a 57-amino-acid protein, SinI, that posttranslationally antagonizes the activity of SinR, the product of the second gene in the operon (3). SinR is a 111-amino-acid protein, which is a repressor of aprE, amyE, sacB, spo0A, spoIIA, spoIIG, and spoIIE (17, 18, 31, 32, 33, 38, 50) and binds to a DNA sequence whose consensus appears to be GNCNCGAAATACA. The crystal structure of SinR has revealed that the DNA-binding domain is similar to that of the bacteriophage 434 repressor proteins, CI and Cro (30). The SinR tetramer represses transcription of the spoIIG promoter by inducing DNA conformational changes, preventing activation of transcription by Spo0A∼P (7). SinR is an activator of competence, motility, and autolysin production (15, 46). The sin operon is expressed from three differentially regulated promoters (17); promoters P1 and P2 precede sinI and produce RNAs which span the operon and terminate at two rho-independent terminators; the P3 promoter abuts the sinR gene and produces a transcript which starts 15 nucleotides (nt) upstream of the first sinR codon and terminates at the rho-independent termination sites. P1 expression is downregulated in vegetative growth and increases dramatically at the onset of stationary phase (17). P3-derived RNAs are expressed in vegetative growth and during the first 2 h of stationary phase. Synthesis of sin operon mRNA originating from P2 commences 2 h after entry into stationary phase. sinR gene disruptions suppress the effects of sporulation defects caused by null mutations in kinA and missense mutations in ftsA and spo0K (31). These effects are consistent with findings (33) that sinR deletions relieve repression of spo0A and would be expected to increase the concentration of Spo0A∼P, thus bypassing the effects of kinA and spo0K mutations. sinR deletions also cause derepression of the sinIR operon (16, 32), suggesting that SinR may autogenously regulate sinIR expression.
In the studies described here, we have investigated the interplay of these genetic factors in controlling the activity of the sinIR operon. We describe in vitro transcription studies suggesting that the sinIR P1 and P3 promoters are transcribed by ςA RNA polymerase. To elucidate the genetic factors that regulate expression from these promoters, we have examined the role of transition-state and spo genes in governing the in vivo expression of sinIR. These data establish that sin operon expression is regulated by the phosphorelay and Hpr.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains used, their genotypes, and their sources are listed in Table 1. Plasmids containing the sinIR operon have been described (16, 17).
TABLE 1.
Bacterial strains
| Strain | Description (relevant genotype)a | Source or referenceb |
|---|---|---|
| 1A180 | hpr16 | BGSC |
| RS1000 | 168 | This study |
| RS1001 | metC2 lys-1 | This study |
| RS1004 | spo0A12 | This study |
| EE1000 | spo0E11 | This study |
| EE1001 | spo0F221 | This study |
| EE1002 | spo0H116 | This study |
| EE1003 | spo0K141 | This study |
| EE1004 | kinA::Tn917 | This study |
| EE1005 | abrB::Tn917 | This study |
| SWV119 | abrB::Tet trpC2 phe-1 | This study |
| SWV185 | abrB::Tet trpC2 phe-1 spo0E::lacZ | This study |
| RS5101 | rvtA11 | This study |
| SS11 | metC2 lys-1 sinI::lacZ | This study |
| SS12 | metC2 lys-1 sinR::lacZ | This study |
| SS13 | sinI::lacZ | This study |
| SS14 | sinR::lacZ | This study |
| SS15 | spo0A12 sinI::lacZ | SS13→RS1004(Cmr selection) |
| SS16 | spo0A12 sinR::lacZ | SS14→RS1004 (Cmr selection) |
| SS17 | spo0E11 sinI::lacZ | SS13→EE1000 (Cmr selection) |
| SS18 | spo0E11 sinR::lacZ | SS14→EE1000 (Cmr selection) |
| SS19 | spo0F221 sinI::lacZ | SS13→EE1001 (Cmr selection) |
| SS20 | spo0F221 sinR::lacZ | SS14→EE1001 (Cmr selection) |
| SS21 | spo0H116 sinI::lacZ | SS13→EE1002 (Cmr selection) |
| SS22 | spo0H116 sinR::lacZ | SS14→SS1750 (Cmr selection) |
| SS23 | spo0K141 sinI::lacZ | SS13→EE1003 (Cmr selection) |
| SS24 | spo0K141 sinR::lacZ | SS14→EE1003 (Cmr selection) |
| SS25 | kinA::Tn917 sinI::lacZ | SS13→EE1004 (Cmr selection) |
| SS26 | kinA::Tn917 sinR::lacZ | SS14→EE1003 (Cmr selection) |
| SS27 | rvtA11 sinI::lacZ | SS13→RS5101 (Cmr selection) |
| SS28 | rvtA11 sinR::lacZ | SS14→RS5101 (Cmr selection) |
| SS29 | hpr16 sinI::lacZ | SS13→1A180 (Cmr selection) |
| SS30 | hpr16 sinR::lacZ | SS14→1A180 (Cmr selection) |
| SS33 | abrB::Tn917 sinI::lacZ | SS13→EE1005 (Cmr selection) |
| SS34 | abrB::Tn917 sinR::lacZ | SS14→EE1005 (Cmr selection) |
| SS35 | abrB::Tet sinI::lacZ | SS13→SS43 (Cmr selection) |
| SS37 | abrB::Tn917 rvtA11 sinI::lacZ | SS43→SS27 (Tetr selection) |
| SS38 | abrB::Tn917 spo0A12 sinI::lacZ | EE1005→SS15 (MLSr selection) |
| SS40 | spo0F221 rvtA11 sinI::lacZ | SS27→EE1001 (Cmr selection, Tetr screening) |
| SS43 | abrB::Tet | SWV119→RS1000 (Tetr selection) |
| SS44 | abrB::Tet spo0E::lacZ | SWV185→RS1000 (Cmr selection and congression) |
| SS46 | hprOHgr;pMTL20EC | Linearized pSS60→RS1000 (Emr selection) |
| SS62 | hprOHgr;pMTL20EC sinI::lacZ | SS46→SS13 (Emr selection) |
| SS63 | hpr::pMTL20EC spo0A12 sinI::lacZ | Linearized pSS60→SS30 (Emr selection) |
| SS955 | spo0A::Em sinI::lacZ | Linearized pSS950→SS13 (Emr selection) |
| SS1750 | spo0H::Em sinI::lacZ | Linearized pSS1750→SS13 (Emr selection) |
For clarity, the auxotrophic genotypes have been omitted.
BGSC, Bacillus Genetics Stock Center. Cmr, chloramphenicol resistance; Tetr, tetracycline resistance.
β-Galactosidase synthesis with B. subtilis lacZ fusion strains.
The following lacZ fusions were used in these studies: sinI::lacZ and sinR::lacZ (17) are translational fusions and were introduced into the amyE locus as described earlier (31). The β-galactosidase expression from lacZ fusions was determined as described previously (11). Specific activity is expressed as nanomoles of o-nitrophenyl hydrolyzed per milligram of cellular protein per minute.
Cell growth, induction of sporulation, and sporulation quantitation.
Cell growth, induction of sporulation in Schaeffer’s medium, and sporulation quantitation were performed as described elsewhere (48).
DNase I footprinting analysis of the sinIR 5′ regulatory region.
DNase I footprinting experiments were performed utilizing a fragment containing the first 395 bp (XbaI-AhaIII) of the sinIR operon (16, 17). The transcribed strand was labeled at its 3′ end by using the Klenow fragment of DNA polymerase I (Bethesda Research Laboratories) and [α-32P]dATP (Amersham). Protein binding and footprinting reactions were performed as described elsewhere (25, 51, 54). The labeled fragment was also subjected to Maxam-Gilbert A+G and C+T sequencing reactions (36) to generate a reference ladder. AbrB was purified as described previously (54). Spo0A was a gift from Jim Hoch. Each protein was purified separately from different Escherichia coli strains harboring expression vectors as described previously (25). Each protein preparation was >95% homogeneous as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Spo0A was not phosphorylated.
sinIR in vitro transcription analysis.
To map the in vivo and in vitro transcriptional start sites of the sinIR operon P1 and P3 promoters, primer extension with 5′ 32P-end-labeled primers and reverse transcriptase was performed as described previously, as was the purification of RNA polymerase (44). For in vivo primer extension reactions, 60 μg of RNA was harvested from cells grown in nutrient sporulation medium (NSM) at the T0 stage of growth. The in vitro start sites were determined with RNA prepared from four-times-normal-size transcription reaction mixtures. The sequence of the primer used for the P1 promoter mapping, OSIN-1 (5′-CAG CCA GTC CGG CCA TGA C-3′), corresponds to nucleotides −37 to −56 with respect to the start of the sinI coding sequence. The equivalent primer for P3 mapping, P3-1 (5′-CAG CTA GTT CTG ATA GTG AGT-3′), corresponds to nucleotide positions +270 to +249, also with respect to the start of the sinI coding sequence. The plasmid used for the in vitro primer extensions and for the dideoxy DNA sequencing reactions was constructed by subcloning the XbaI-NruI fragment, containing P1 and P3 from pIS74 (16), into a lacZ shuttle plasmid. This insert contains the MscI-NruI fragment that was removed from pIS109.
Plasmids. (i) pIS109.
Plasmid pIS109 was constructed from excision of the XbaI-HinDIII fragment from pIS90 (16, 17) and ligation into XbaI-HinDIII-restricted pUC19. In this construct, a 240-bp internal MscI (BalI)-NruI fragment was deleted. This plasmid contains the sinIR operon P1, P2, and P3 promoters and the T2 and T3 terminators 5′ to the HinDIII site. Predicted transcript sizes are as follows: P1, 455 nt when terminated at T2 and 497 nt when terminated at T3; and P3, 142 nt when terminated at T2 and 184 nt when terminated at T3.
(ii) pSS60.
Plasmid pSS60 carries a 256-bp internal sequence, +108 to +364 with respect to the start codon, from the hpr (scoC) coding region. This plasmid was used to inactivate hpr by Campbell integration.
(iii) pSS950.
Plasmid pSS950 carries a null mutation in spo0A. A 64-bp internal fragment from the spo0A-coding region, +535 to +599 with respect to the start codon, was replaced with the erythromycin-resistance (Emr) gene from pHP13 (19) and cloned into a Topo vector (Invitrogen). The plasmid was linearized with SmaI restriction enzyme and used to inactivate spo0A by gene replacement.
(iv) pSS1750.
Plasmid pSS1750 carries a null mutation in spo0H. A 30-bp internal fragment from the spo0H-coding region, +532 to +562 with respect to the start codon, was replaced with the Emr gene from pHP13 (19) and cloned into a Topo vector (Invitrogen). The plasmid was linearized with SmaI restriction enzyme and used to inactivate spo0H by gene replacement.
RESULTS
Transcriptional analysis of the sinIR promoters.
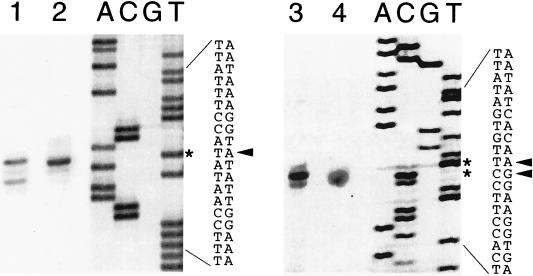
Previous in vivo studies (17) had identified three separate and individually regulated promoters involved in the transcription of the sinIR operon. Among these promoters, P1 is the most important in regulating sinIR expression at the onset of sporulation and in determining the ratio of SinI and SinR proteins (3). Although P1 has typical ςA promoter motifs, it had not been directly demonstrated that P1 could be transcribed in vitro by ςA RNA polymerase. To more accurately establish the transcriptional start sites, we performed RNA mapping studies, utilizing reverse transcriptase-based primer extension assays. Total RNA prepared from IS75 (16) cells harvested at T0 and RNA isolated from in vitro transcription reaction mixtures with B. subtilis RNA polymerase holoenzyme containing ςA were used for these experiments. The in vivo and the in vitro extension products obtained for each promoter were similar (Fig. 1, lanes 1 and 2 for P1 and lanes 3 and 4 for P2), indicating that the in vivo and the in vitro start sites for P1 and P3 are identical. A minor extension product was observed, however, in the P1 in vitro reaction (lane 1) that was not present in the in vivo RNA reaction (lane 2). Its significance (alternative start site, degradation product, or an artifact of in vitro transcription) is not known. The mapping of the P3 start is ambiguous, in that the start site could be either an A or the adjacent G (Fig. 1, lanes 3 and 4). The DNA sequences in the P1 and P3 promoter regions are presented below, with the initiating nucleotide(s) in boldface: P1, GATTATAATAAAGGTATATT; and P3, TGCTATAATATCACAAGGA. These results confirm a previous transcriptional mapping of the P1 promoter, which had been obtained by the S1 nuclease protection method, to the nucleotide, but the results with P3 presented here indicate that the actual initiating nucleotide is 3 nt downstream of the one previously described (17).
FIG. 1.
Mapping of in vivo and in vitro transcription start sites for sinIR promoters. Start sites were determined by reverse transcriptase extension of 32P-end-labeled primers by using in vivo RNA isolated from IS75 (16), grown in NSM, and harvested at T0 and in vitro RNA isolated from transcription reaction mixtures. B. subtilis RNA polymerase holoenzyme containing ςA was allowed to transcribe pSM109 (16), a plasmid containing sinIR P1 and P3, with nonradioactive ribonucleoside triphosphates, and RNA was isolated from the reaction mixtures. After radioactive primers were annealed to the RNAs and after extension with reverse transcriptase, the samples were separated by electrophoresis on 6% polyacrylamide gels, followed by autoradiography. Dideoxy sequencing gel lanes 1 and 2 show the primer extension products obtained from in vitro RNA (lane 1) and in vivo RNA (lane 2) using primer OSIN-1. The dideoxy sequencing ladder adjacent to lane 2 was obtained with the same primer and pSM179. The nucleotide sequence with the arrow pointing to the derived transcriptional start sites, also indicated by asterisks, is presented in the adjacent lanes. Lanes 3 (in vitro RNA) and 4 (in vivo RNA) show the equivalent primer extension products with primer P3-1, which corresponds to a region ca. 60 nt downstream from P3. Adjacent to lane 4 is the sequencing ladder obtained from pSM109 (16) with primer P3-1. The DNA sequence and derived transcription start sites are also presented.
Spo0A, Hpr, and AbrB in vitro binding to the sinIR P1 promoter region.
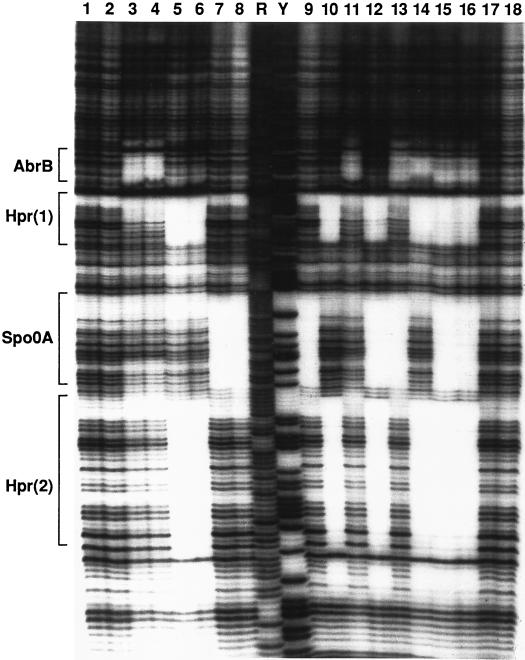
Previous studies had shown that sinI expression was regulated by Spo0A (17) and had suggested a possible regulatory role for Hpr (25). The negative effects of spo0A mutations on sinI expression could be due to a direct activation of sinI transcription by Spo0A (4, 58, 63), to an indirect effect of spo0A through control of hpr (39) or abrB (4), or to both. We examined the direct binding of these proteins by a DNase I protection assay. We found that Spo0A and AbrB bound to discrete sites at or near the sinIR P1 promoter (Fig. 2 and 3). In agreement with a previous report (25), we also found two Hpr binding sites in the sinIR upstream regulatory region (Fig. 2 and 3). P1 promoter binding by each of these proteins was independent and noncompetitive. We did not detect binding of any of these proteins to the P3 promoter region (data not shown).
FIG. 2.
DNase I footprinting of the sinIR promoter region. The regions bound by the indicated proteins are indicated in brackets at the left (also see Fig. 3). Lanes 1, 2, 17, and 18, no binding protein; lanes 3, 11, and 13 to 16, 1.5 μM AbrB; lane 4, 3 μM AbrB; lanes 5, 10, 12, and 14 to 16, 0.4 μM Hpr; lane 6, 0.8 μM Hpr; lanes 7, 9, 12, 13, 15, and 16, 2.5 μM Spo0A; lane 8, 5 μM Spo0A. Maxam-Gilbert A+G (R) and C+T (Y) sequencing ladders are shown for reference. AbrB binds the sinIR region with a Kd in the range of 40 nM (52); Hpr binds with a Kd of less than 20 nM (Strauch, unpublished); the Kd for Spo0A binding has not been determined, in part because of the recalcitrance of Spo0A-DNA complexes to be resolved by gel mobility shift assays (Strauch, unpublished).
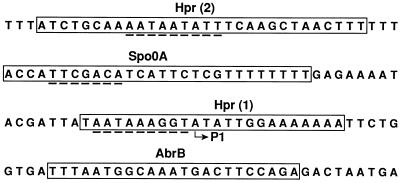
FIG. 3.
Localization of protein binding sites within the sinIR upstream region. Boxes represent the binding regions of the indicated proteins on the nontemplate strand that corresponds to the DNase I footprinting regions identified for the template strand (Fig. 2). Dashed lines beneath the Hpr binding regions are sequences with homology to the putative Hpr recognition element. The dashed line beneath the Spo0A binding region is a sequence similar to the 0A box (TGTCGAA on the strand shown). P1 indicates the transcriptional start site of the sinIR promoter, which is postexponentially activated (17).
The phosphorylated form of Spo0A regulates sinI expression.
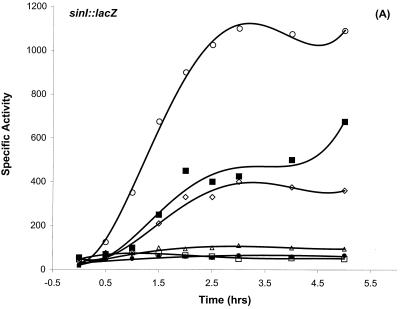
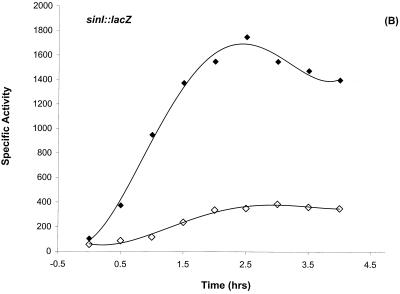
DNase I footprinting results (Fig. 2 and 3) established that Hpr, AbrB, and Spo0A can bind to the sinIR upstream regulatory region. The region protected by Spo0A contained a perfect match to the 0A box consensus sequence (51). The location (−43 to −49) of the 0A box relative to P1 is similar to cases in which binding of Spo0A∼P has been shown to activate transcription (4, 45, 57, 63). These data suggested a dual regulatory mechanism for P1 transcription: activation by Spo0A∼P and repression by Hpr. We examined sinI::lacZ expression in spo0A mutant strains and in a number of other spo gene mutations that are known to prevent phosphorylation of the Spo0A protein. Postexponential induction of sinI was not observed in spo0A12, spo0A::Em, spo0F221, spo0H::Em, or spo0K141 mutant backgrounds and was substantially reduced in a kinA mutant background (Fig. 4A and data not shown). The rvtA11 allele of spo0A bypasses the requirement for many of the normal phosphorelay gene products and can suppress sporulation defects such as spo0F mutations (48). The rvtA11 allele restored sinI expression in a spo0F mutant background and elevated sinI expression in a wild-type background (Fig. 4A). The spo0E11 gain-of-function mutation encodes an overactive phosphatase that inhibits sporulation by specifically dephosphorylating Spo0A∼P (37, 43). sinI expression was also substantially reduced in this mutant background (Fig. 4A). These results indicate that the phosphorylated form of Spo0A is required for sinI induction.
FIG. 4.
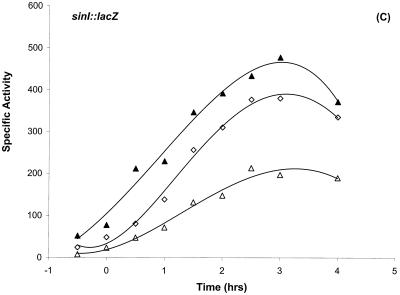
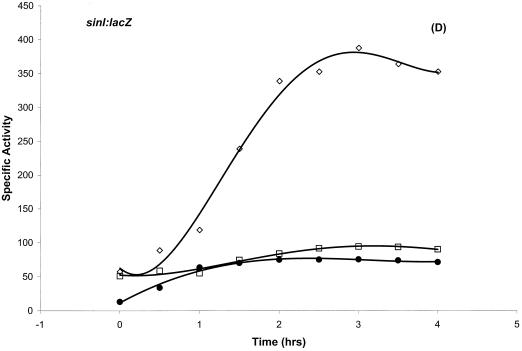
Differential regulation of sinI::lacZ expression. (A) Dependence of sinI::lacZ expression on the phosphorelay. The indicated strains were grown in Schaeffer’s sporulation medium and analyzed as described previously (11). T0 denotes the end of exponential growth. (A) β-Galactosidase expression of sinI::lacZ in SS13 (wild type) (◊), SS15 (spo0A12) (•), SS17 (spo0E11) (▵), SS19 (spo0F221) (□), SS40 (spo0F221 rvtA11) (▪), and SS27 (rvtA11) (○) strains. (B) The effect of a loss-of-function mutation in hpr on sinI::lacZ expression. β-Galactosidase expression of sinI::lacZ in SS13 (wild type) (◊) and in SS30 (hpr16) (⧫) strains. (C) The negative effect of a loss-of-function mutation in abrB on sinI::lacZ expression is suppressed by rvtA11 mutation. β-Galactosidase expression of sinI::lacZ in SS13 (wild type) (◊), SS33 (abrB::Tn917) (▵), and SS37 (abrB::Tn917 rvtA11) (▴) strains. (D) The epistatic relationship between Spo0A and Hpr in the regulation of sinI expression. β-Galactosidase expression of sinI::lacZ in SS13 (wild type) (◊), SS15 (spo0A12) (•), and SS63 (hprOHgr;pMTL20EC spo0A12) (□) strains.
Hpr is a negative regulator of sinI expression.
P1 expression is downregulated in vegetative growth and increases dramatically at the onset of stationary phase (17). We have examined whether the binding of Hpr near P1 can account for repression of this promoter. Loss-of-function mutations in hpr (hpr16 and hprOHgr; pMTL20EC) caused substantial overexpression of sinI::lacZ (Fig. 4B and data not shown), indicating that Hpr binding near the P1 promoter (Fig. 2 and 3) is repressive in nature. We have characterized the nature of the hpr16 mutation by DNA sequencing. This mutation is caused by a missense mutation (T→A) at position +205, with respect to the start codon, resulting in a Phe69-Ile amino acid substitution. sinI::lacZ expression remained inhibited in a spo0A hpr double-mutant background (Fig. 4D), indicating that Spo0A is epistatic to Hpr in regulating sinI gene expression.
Role of AbrB in the regulation of sinI and sporulation.
sinI::lacZ expression is unexpectedly decreased in the abrB::Tn917 or abrB::Tet null mutant backgrounds (Fig. 4C and data not shown). Expression was restored to wild-type levels when the rvtA11 mutation was introduced into these abrB mutant backgrounds (Fig. 4C and data not shown).
DISCUSSION
Inactivation of spo0A, spo0F, spo0H, spo0K, or kinA substantially reduced the postexponential expression of sinI::lacZ. These results suggest that sinI expression is controlled by the spo0A phosphorelay. Two other lines of genetic evidence support this interpretation. The spo0E11 mutation, a gain-of-function mutation which causes increased dephosphorylation of Spo0A∼P (37, 43), also diminished sinI::lacZ expression (Fig. 4A). On the other hand, the rvtA11 mutation in spo0A, which bypasses the requirement for other phosphorelay gene products (27), restored expression of sinI in a spo0F mutant background and elevated sinI expression in a wild-type background (Fig. 4A). Loss-of-function mutations in hpr (hpr16 and hprΩpMTL20EC) caused substantial overexpression of sinI::lacZ (Fig. 4B and data not shown). sinI expression remained low in the hpr mutant background during vegetative growth (although reproducibly two- to threefold higher than the corresponding time points in the wild-type background) but was substantially derepressed as the cells entered postexponential growth phase. These data suggest that the absence of high-level expression from the P1 promoter during vegetative growth time points might be due to the absence of the phosphorylated form of Spo0A. This interpretation is supported by the finding that postexponential derepression of sinI, observed in an hpr mutant background (Fig. 4B), is abolished in hpr spo0A double-mutant backgrounds (Fig. 4D), indicating that Spo0A∼P binding may be essential for expression from the P1 promoter. Moreover, the −35 region of the P1 promoter is significantly different from the ςA RNA polymerase consensus sequence, supporting the interpretation that the P1 promoter may be weakly transcribed in the absence of its activator, Spo0A∼P.
sinI::lacZ expression was diminished in both abrB::Tn917 and abrB::Tet loss-of-function mutations (Fig. 4C and data not shown). The downregulation of sinI::lacZ expression in abrB mutants could be due to AbrB functioning as an activator of sinIR or as a repressor of another gene(s), such as spo0E, whose product directly or indirectly inhibits sinI::lacZ expression. Spo0E phosphatase inhibits sporulation by removing the phosphate group from Spo0A∼P (37, 43). Moreover, spo0E expression is inhibited by AbrB during the vegetative phase and dramatically increases during transition into stationary phase (43). The expression of sinI was fully restored in an abrB rvtA11 double-mutant background (Fig. 4C), suggesting that the in vivo levels of Spo0A∼P may be reduced in abrB null mutant backgrounds. The exact role of AbrB in regulation of the sinIR operon, however, cannot be unambiguously determined from these results and is currently under investigation.
Expression of the sinR::lacZ fusion driven from the P3 promoter was not affected by any of the mutations (spo, abrB, hpr, and rvtA) shown here to regulate sinI::lacZ expression (data not shown).
In vitro DNase I footprinting experiments (Fig. 2 and 3) confirmed that the proteins implicated by in vivo genetic studies of sinIR regulation (Spo0A, AbrB, and Hpr) were able to bind to regions upstream of the P1 promoter. The binding motifs for Hpr and Spo0A are typical of sites where negative (Hpr) (25; M. A. Strauch, unpublished results) and positive (Spo0A) (4, 45, 57, 63) regulation is exerted. In vitro primer extension transcription studies with sinIR templates and ςA RNA polymerase established that the RNA transcripts seen in vivo originated from the P1 (sinI proximate) and P3 (sinR proximate) promoters (Fig. 1). The P2 promoter site, which resembles a ςE sequence (17, 50), was not used in vitro by ςA RNA polymerase (data not shown).
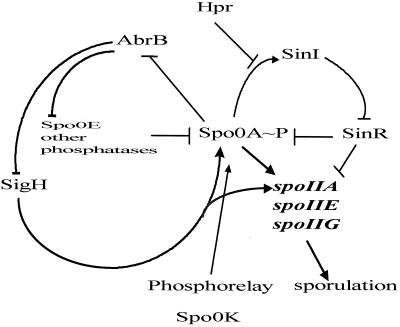
Analysis of sin operon transcription provides an opportunity to investigate the genetic strategies used to control complex postexponential regulons. A diagram summarizing these interlocking control circuits is shown in Fig. 5. The combinatorial alternative states provided by positive and negative regulator interactions are more than sufficient to account for the effects of the sin operon on postexponential cell state regulation.
FIG. 5.
A diagram depicting the sinIR transcriptional control circuit. The arrows indicate a positive interaction at either a transcriptional or later step; the T bars denote a negative interaction. For clarity, several proteins and regulatory pathways affecting the phosphorelay have been omitted.
The balance of Hpr and Spo0A∼P effects on sin operon transcription may determine the set point of the system in terms of selecting sporulation cell fate. Hpr would appear to be a checkpoint for the commitment to sporulation. If insufficient levels of Spo0A∼P are present, Hpr repression will downregulate sinI transcription, allowing SinR to repress spo0A, spoIIA, spoIIG, and spoIIE expression (18, 31, 32, 33, 50) and to upregulate genes involved in motility and competence (15, 46). The finding that a sinR deletion can suppress the sporulation phenotypes caused by mutations in spo0K and the cell cycle control gene ftsA (31) is consistent with this interpretation. The principal regulatory interplay dominating the expression of the sin operon is thus determined by Spo0A∼P and Hpr. As critical nutrients become limiting, a sequence of transition state interlocks guide the cells to a “postexponential soft landing.” Exponential-phase cells can enter motility, nutrient-scavenging, or competence postexponential differentiation pathways (for a review, see references 12 and 53). Cells committed to sporulation traverse these interlock pathways by inactivation of the Hpr system and culminate in the activation of the SinI interlock, which in turn relieves SinR repressive effects on sporulation, allowing subsequent progression to stage II and irreversible commitment to sporulation.
Acknowledgments
We thank Gopalan Nair, Patrick Lau, Allan Tang, and Ben Wen for technical assistance. We also thank Ehab El-Helow for useful comments on the manuscript.
This research was supported by NIH grants GM32651 (I.S.) and GM46700 (M.A.S.), DOE grant DOE 4976-75 (T.L.), and U.S. Army Corps of Engineers contract DACA399520005 (T.L.).
REFERENCES
- 1.Antoniewski, C., B. Savelli, and P. Stragier. 1990. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 172:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, U., M. Lewandoski, E. Dubnau, and I. Smith. 1990. Temporal regulation of the Bacillus subtilis early sporulation gene spo0F. J. Bacteriol. 172:5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, U., I. Mandic-Mulec, and I. Smith. 1993. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 7:139–148. [DOI] [PubMed] [Google Scholar]
- 4.Bird, T. H., J. K. Grimsley, J. A. Hoch, and G. B. Spiegelman. 1993. Phosphorylation of Spo0A activates its stimulation of in vitro transcription from the Bacillus subtilis spoIIG operon. Mol. Microbiol. 9:741–749. [DOI] [PubMed] [Google Scholar]
- 5.Brown, N. C. 1971. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J. Mol. Biol. 59:1–16. [DOI] [PubMed] [Google Scholar]
- 6.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. Initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552. [DOI] [PubMed] [Google Scholar]
- 7.Cervin, M. A., R. J. Lewis, J. Brannigan, and G. Spiegelman. 1998. The Bacillus subtilis regulator SinR inhibits spoIIG promoter transcription in vitro without displacing RNA polymerase. Nucleic Acids Res. 26:3806–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambliss, G. H. 1993. Carbon source-mediated catabolite repression, p.213–219. A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 9.Chibazakura, T., F. Kawamura, and H. Takahashi. 1991. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J. Bacteriol. 173:2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dod, B., and G. Balassa. 1978. Spore control (sco) mutations of Bacillus subtilis. Regulation of extracellular protease synthesis in the spore control mutations scoC. Mol. Gen. Genet. 163:57–63. [Google Scholar]
- 11.Donnelly, C. E., and A. L. Sonenshein. 1984. Promoter-probe plasmid for Bacillus subtilis. J. Bacteriol. 157:965–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabret, C., V. A. Feher, and J. A. Hoch. 1999. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J. Bacteriol. 181:1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari, E., D. J. Henner, M. Perego, and J. A. Hoch. 1988. Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants. J. Bacteriol. 170:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredrick, K., and J. D. Helmann. 1996. FlgM is a primary regulator of sigD activity, and its absence restores motility to a sinR mutant. J. Bacteriol. 178:7010–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaur, N. K., E. Dubnau, and I. Smith. 1986. Characterization of a cloned Bacillus subtilis gene that inhibits sporulation in multiple copies. J. Bacteriol. 168:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaur, N. K., K. Cabane, and I. Smith. 1988. Structure and expression of the Bacillus subtilis sin operon. J. Bacteriol. 170:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaur, N. K., J. Oppenheim, and I. Smith. 1991. The Bacillus subtilis sin gene, a regulator of alternate developmental processes, codes for a DNA-binding protein. J. Bacteriol. 173:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haima, P., S. Bron, and G. Venema. 1987. The effect of restriction on shotgun cloning and plasmid stability in Bacillus subtilis Marburg. Mol. Gen. Genet. 209:335–342. [DOI] [PubMed] [Google Scholar]
- 20.Henner, D. J., E. Ferrari, M. Perego, and J. A. Hoch. 1988. Location of the targets of the hpr-97, sacU32(Hy), and sacQ36(Hy) mutations in upstream regions of the subtilisin promoter. J. Bacteriol. 170:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higerd, T. B., J. A. Hoch, and J. Spizizen. 1972. Hyperprotease-producing mutants of Bacillus subtilis. J. Bacteriol. 112:1026–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ireton, K., and A. D. Grossman. 1992. Coupling between gene expression and DNA synthesis early during development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 89:8808–8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ireton, K., and A. D. Grossman. 1994. A developmental checkpoint couples the initiation of sporulation to DNA replication in Bacillus subtilis. EMBO J. 13:1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, M., W. Shao, M. Perego, and J. A. Hoch. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535–542. [DOI] [PubMed] [Google Scholar]
- 25.Kallio, P. T., J. E. Fagelson, J. A. Hoch, and M. A. Strauch. 1991. The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein. J. Biol. Chem. 266:13411–13417. [PubMed] [Google Scholar]
- 26.Koide, A., M. Perego, and J. A. Hoch. 1999. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. J. Bacteriol. 181:4114–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDeaux, J. R., N. Yu, and A. D. Grossman. 1995. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 177:861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leighton, T., G. G. Khachatourians, and N. C. Brown. 1974. The role of semiconservative DNA replication in bacterial cell development, p.677–687. In DNA synthesis and its regulation. W. A. Benjamin, Menlo Park, Calif.
- 29.Lewandoski, M., and I. Smith. 1988. Use of a versatile lacZ vector to analyze the upstream region of the Bacillus subtilis spo0F gene. Plasmid 20:148–154. [DOI] [PubMed] [Google Scholar]
- 30.Lewis, R. J., J. A. Brannigan, W. A. Offen, I. Smith, and A. J. Wilkinson. 1998. An evolutionary link between sporulation and prophage induction in the structure of a repressor-anti-repressor complex. J. Mol. Biol. 283:907–912. [DOI] [PubMed] [Google Scholar]
- 31.Louie, P., A. Lee, K. Stansmore, R. Grant, C. Ginther, and T. Leighton. 1992. Roles of rpoD, spoIIF, spoIIJ, spoIIN, and sin in regulation of Bacillus subtilis stage II sporulation-specific transcription. J. Bacteriol. 174:3570–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandic-Mulec, I., N. Gaur, U. Bai, and I. Smith. 1992. Sin, a stage-II specific repressor of cellular differentiation. J. Bacteriol. 174:3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandic-Mulec, I., L. Doukhan, and I. Smith. 1995. The Bacillus subtilis SinR protein is a repressor of the key sporulation gene spo0A. J. Bacteriol. 177:4619–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marahiel, M. A., P. Zuber, G. Czekay, and R. Losick. 1987. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation. J. Bacteriol. 169:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matin, A. 1994. Starvation promoters of Escherichia coli: their function, regulation, and use in bioprocessing and bioremediation. Ann. N. Y. Acad. Sci. 721:277–291. [DOI] [PubMed] [Google Scholar]
- 36.Maxam, A. M., and W. Gilbert. 1980. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 65:499–560. [DOI] [PubMed] [Google Scholar]
- 37.Ohlsen, K. L., J. K. Grimsley, and J. A. Hoch. 1994. Deactivation of the sporulation specific transcription factor Spo0A by the Spo0E protein phosphatase. Proc. Natl. Acad. Sci. USA 91:1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olmos, J., R. De Anda, E. Ferrari, F. Bolivar, and F. Valle. 1997. Effects of the sinR and degU32 (Hy) mutations on the regulation of the aprE gene in Bacillus subtilis. Mol. Gen. Genet. 253:562–567. [DOI] [PubMed] [Google Scholar]
- 39.Perego, M., and J. A. Hoch. 1988. Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J. Bacteriol. 170:2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perego, M., and J. Hoch. 1991. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J. Bacteriol. 173:2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perego, M., S. P. Cole, D. Burbulys, K. Trach, and J. A. Hoch. 1989. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J. Bacteriol. 171:6187–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perego, M., C. F. Higgins, S. R. Pearce, M. P. Gallagher, and J. A. Hoch. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173–185. [DOI] [PubMed] [Google Scholar]
- 43.Perego, M., and J. A. Hoch. 1991. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J. Bacteriol. 173:2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Predich, M., G. Nair, and I. Smith. 1992. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing sigma H. J. Bacteriol. 174:2771–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satola, S., P. A. Kirchman, and C. P. Moran, Jr. 1991. Spo0A binds to a promoter used by sigma A RNA polymerase during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 88:4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekiguchi, J., B. Ezaki, K. Kodoma, and T. Akamatsu. 1988. Molecular cloning of the gene affecting autolysin level and flagellation in Bacillus subtilis. J. Gen. Microbiol. 134:1611–1621. [DOI] [PubMed] [Google Scholar]
- 47.Sharrock, R. A., and T. Leighton. 1981. Intergenic suppressors of temperature-sensitive sporulation in Bacillus subtilis are allele non-specific. Mol. Gen. Genet. 183:532–537. [DOI] [PubMed] [Google Scholar]
- 48.Sharrock, R. A., S. Rubenstein, M. Chan, and T. Leighton. 1984. Intergenic suppression of spo0 phenotypes by the Bacillus subtilis mutation rvtA. Mol. Gen. Genet. 194:260–264. [DOI] [PubMed] [Google Scholar]
- 49.Siranosian, K. J., and A. D. Grossman. 1994. Activation of spo0A transcription by sigma H is necessary for sporulation but not for competence in Bacillus subtilis. J. Bacteriol. 176:3812–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, I. 1989. Initiation of sporulation, p.185–210. In I. Smith, R. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, D.C.
- 51.Strauch, M., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87:1801–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauch, M. A. 1995. AbrB modulates expression and catabolite repression of a Bacillus subtilis ribose transport operon. J. Bacteriol. 177:6727–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauch, M. A. 1993. Regulation of Bacillus subtilis gene expression during the transition from exponential growth to stationary phase. Prog. Nucleic Acid Res. Mol. Biol. 46:121–153. [DOI] [PubMed] [Google Scholar]
- 54.Strauch, M. A., G. B. Spiegelman, M. Perego, W. C. Johnson, D. Burbulys, and J. A. Hoch. 1989. The transition state transcription regulator abrB of Bacillus subtilis is a DNA-binding protein. EMBO J. 8:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strauch, M. A., and J. A. Hoch. 1993. Signal transduction in Bacillus subtilis sporulation. Curr. Opin. Genet. Dev. 3:203–212. [DOI] [PubMed] [Google Scholar]
- 56.Strauch, M. A., and J. A. Hoch. 1993. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol. Microbiol. 7:337–342. [DOI] [PubMed] [Google Scholar]
- 57.Trach, K., D. Burbulys, M. Strauch, J. J. Wu, N. Dhillon, R. Jonas, C. Hanstein, P. Kallio, M. Perego, and T. Bird. 1991. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res. Microbiol. 142:815–823. [DOI] [PubMed] [Google Scholar]
- 58.Tzeng, Y., V. A. Feher, J. Cavanagh, M. Perego, and J. A. Hoch. 1998. Characterization of interactions between a two-component response regulator, Spo0F, and its phosphatase, RapB. Biochemistry 37:16538–16545. [DOI] [PubMed] [Google Scholar]
- 59.Vaughn, J. L., V. Feher, S. Naylor, M. A. Strauch, and J. Cavanagh. 2000. Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator abrB. Nat. Struct. Biol. 7:1139–1146. [DOI] [PubMed] [Google Scholar]
- 60.Wang, L., R. Grau, M. Perego, and J. A. Hoch. 1997. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes Dev. 11:2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weir, J., M. Predich, E. Dubnau, G. Nair, and I. Smith. 1991. Regulation of spo0H, a gene coding for the Bacillus subtilis ςH factor. J. Bacteriol. 173:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu, K., and M. A. Strauch. 1996. In vitro selection of optimal AbrB-binding sites: comparison to known in vivo sites indicates flexibility in AbrB binding and recognition of three-dimensional DNA structures. Mol. Microbiol. 19:145–158. [DOI] [PubMed] [Google Scholar]
- 63.York, K., T. J. Kenney, S. Satola, C. P. Moran, Jr., H. Poth, and P. Youngman. 1992. Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J. Bacteriol. 174:2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]