Abstract
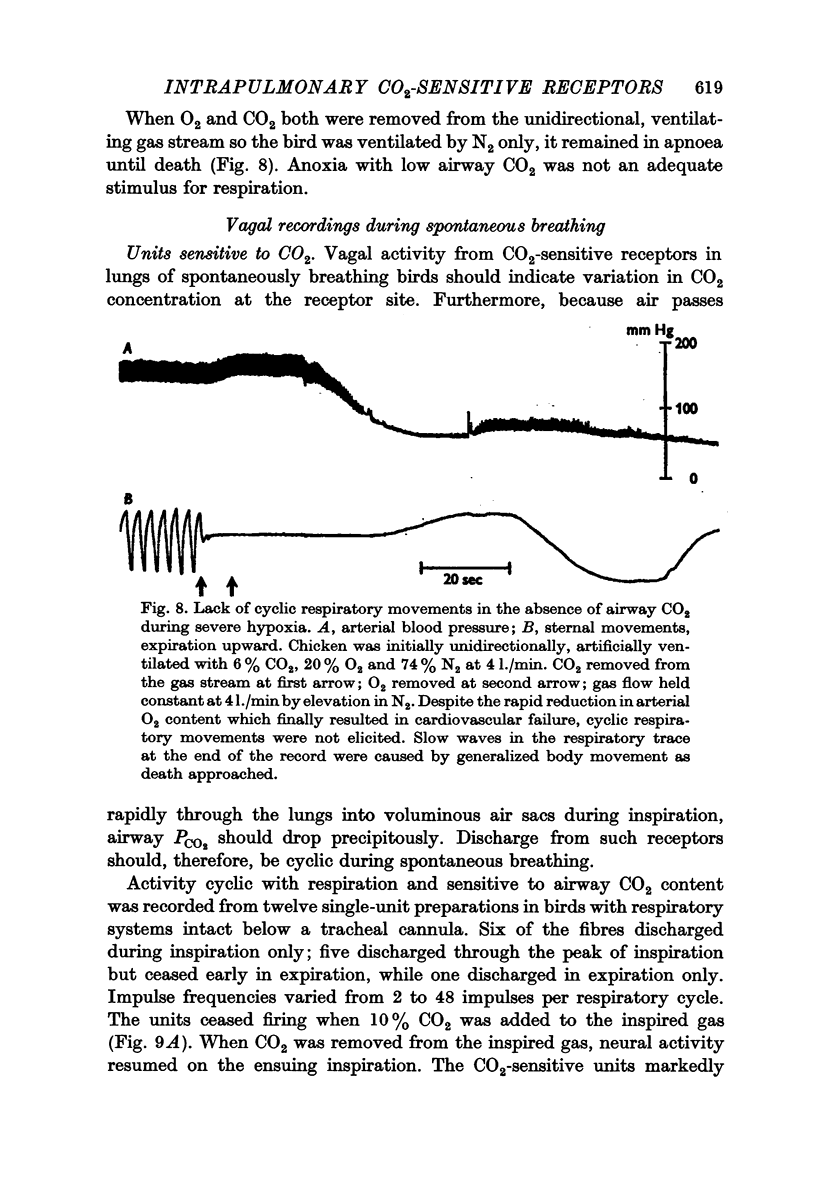
1. A unidirectional, artificial ventilating system was used to observe respiratory and neural responses to changes in CO2 concentration in pulmonary airways of the chicken.
2. Sectioning any of the three pulmonary branches of the vagus slowed the respiratory response to sudden removal of CO2 from unidirectional ventilating gas stream.
3. The impulse frequency of CO2-sensitive units was inversely related to the CO2 content in the ventilating gas stream.
4. Abrupt elimination of CO2 from the ventilatory gas stream caused a transient burst of activity from CO2-sensitive receptors, which quickly adapted to a constant discharge frequency. Fifty percent of the units responded within 0·35 sec after CO2-free gas reached the lungs. The neural response to abrupt re-addition of CO2 to the gas stream was less rapid.
5. Acetylcholine or NaCN injected into the pulmonary artery had no apparent effect on the discharge of CO2-sensitive receptors. Veratridine temporarily reduced discharge and occasionally produced short, rapid bursts of activity.
6. Hypoxia and hyperoxia (5% and 80% O2) produced no significant change in the discharge of CO2-sensitive receptors.
7. Carbon dioxide-sensitive units in spontaneously breathing chickens were silenced by adding 10% CO2 to the inspired gas; the units responded vigorously to inflation of the respiratory system with a gas containing no CO2 but remained silent during inflation with gas containing 15% CO2. Units insensitive to CO2, which increased their impulse frequency during inflation regardless of the CO2 content of the inflating gas, also were observed.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton R. R., Smith A. H. Blood and air volumes in the avian lung. Poult Sci. 1968 Jan;47(1):85–91. doi: 10.3382/ps.0470085. [DOI] [PubMed] [Google Scholar]
- Butler P. J. Effect of progressive hypoxia on the respiratory & cardiovascular system of chickens. J Physiol. 1967 Jul;191(2):309–324. doi: 10.1113/jphysiol.1967.sp008252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., COMROE J. H., Jr Chemoreflexes from the heart and lungs. Physiol Rev. 1954 Apr;34(2):167–201. doi: 10.1152/physrev.1954.34.2.167. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of hypoxia, hypercapnia, and pH on the chemoreceptor activity of the carotid body in vitro. J Physiol. 1965 Jun;178(3):385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J Physiol. 1965 Jun;178(3):410–437. doi: 10.1113/jphysiol.1965.sp007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedde M. R., DeWet P. D., Kitchell R. L. Motor unit recruitment pattern and tonic activity in respiratory muscles of Gallus domesticus. J Neurophysiol. 1969 Nov;32(6):995–1004. doi: 10.1152/jn.1969.32.6.995. [DOI] [PubMed] [Google Scholar]
- Graham J. D. Respiratory reflexes in the fowl. J Physiol. 1940 Feb 14;97(4):525–532. doi: 10.1113/jphysiol.1940.sp003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. S., McLelland J., Molony V., Mortimer M. F. Respiratory afferent activity in the avian vagus: eupnoea, inflation and deflation. J Physiol. 1969 Mar;201(1):35P–36P. [PubMed] [Google Scholar]
- King A. S., Molony V., McLelland J., Bowsher D. R., Mortimer M. F. Afferent respiratory pathways in the avian vagus. Experientia. 1968 Oct 15;24(10):1017–1018. doi: 10.1007/BF02138718. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. The influence of certain chemical substances on the initiation of sensory discharges in pulmonary and gastric stretch receptors and atrial receptors. J Physiol. 1957 Mar 11;135(3):486–510. doi: 10.1113/jphysiol.1957.sp005725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. F., Fedde M. R. Receptors sensitive to carbon dioxide in lungs of chicken. Science. 1968 Dec 27;162(3861):1499–1501. doi: 10.1126/science.162.3861.1499. [DOI] [PubMed] [Google Scholar]
- Ray P. J., Fedde M. R. Responses to alterations in respiratory Po2 and Pco2 in the chicken. Respir Physiol. 1969 Feb;6(2):135–143. doi: 10.1016/0034-5687(69)90051-6. [DOI] [PubMed] [Google Scholar]
- Richards S. A., Sykes A. H. The effects of hypoxia, hypercapnia and asphyxia in the domestic fowl (Gallus domesticus). Comp Biochem Physiol. 1967 Jun;21(3):691–701. doi: 10.1016/0010-406x(67)90463-x. [DOI] [PubMed] [Google Scholar]
- Richards S. A. Vagal control of thermal panting in mammals and birds. J Physiol. 1968 Nov;199(1):89–101. doi: 10.1113/jphysiol.1968.sp008640. [DOI] [PMC free article] [PubMed] [Google Scholar]


