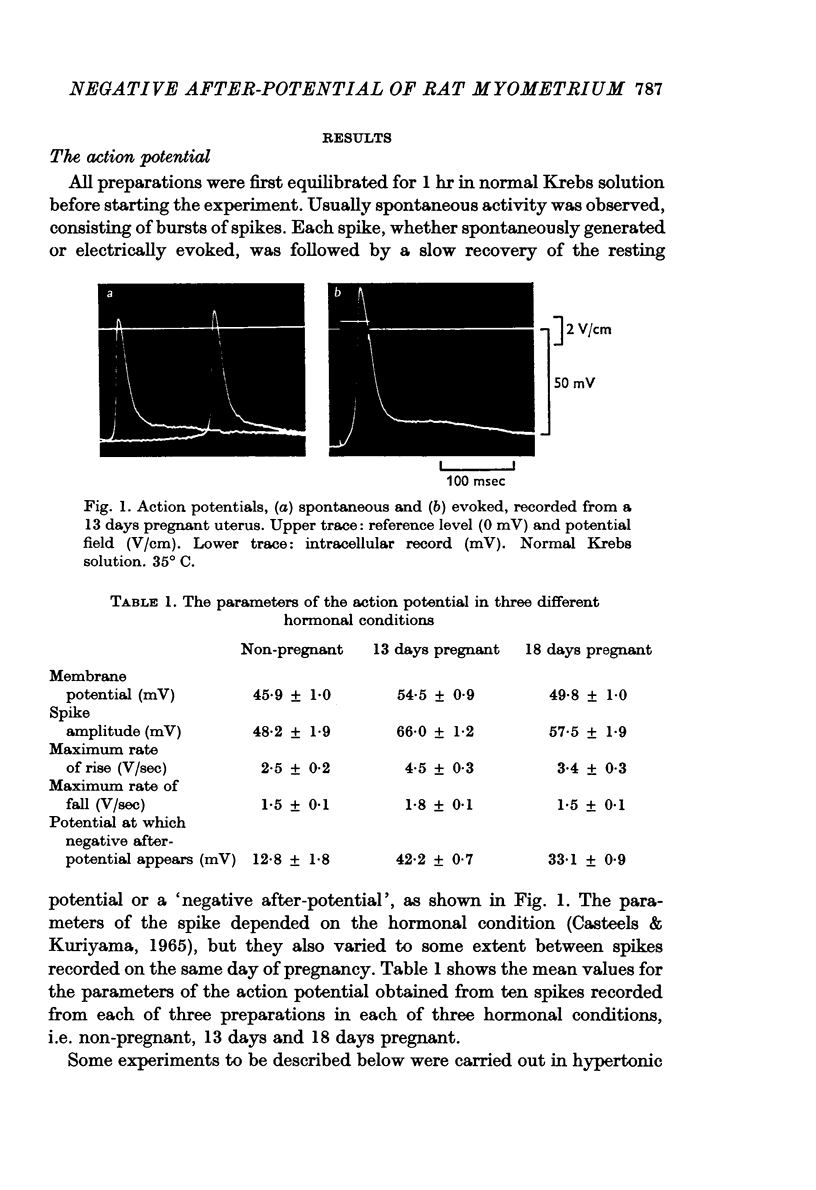
Abstract
1. The spontaneous activity of the smooth muscle of rat uterus consists of bursts of spikes, each spike being followed by a negative after-potential. The effect of changes in ionic environment on the negative after-potential was investigated at various stages of pregnancy and in the non-pregnant condition.
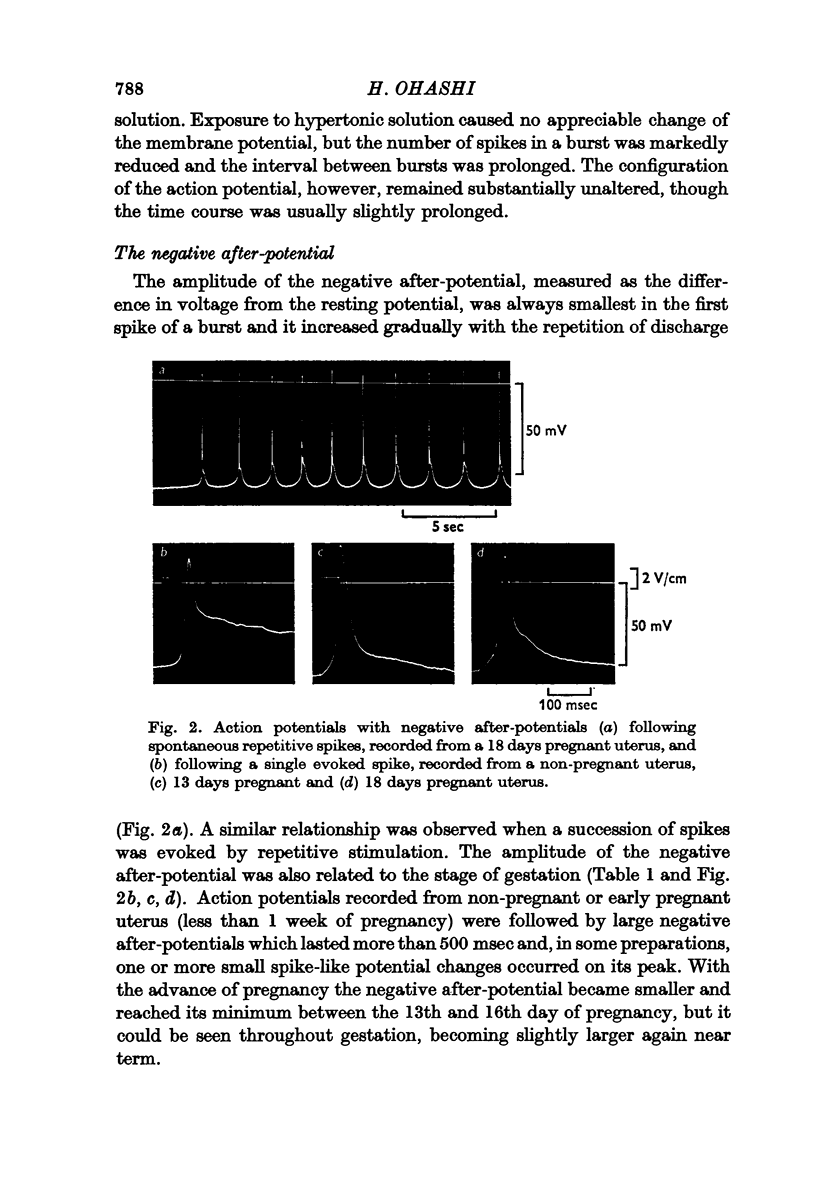
2. The amplitude of the negative after-potential was the same in spontaneously generated and electrically evoked spikes. During repetitive discharge, whether spontaneous or in response to depolarizing current application, the amplitude of the after-potential was smallest in the first spike of a burst and it increased gradually with repetition of discharge.
3. The decay of the negative after-potential was slower than the passive return of the membrane potential to its resting level.
4. The amplitude of the negative after-potential was larger in non-pregnant uterus than during late pregnancy.
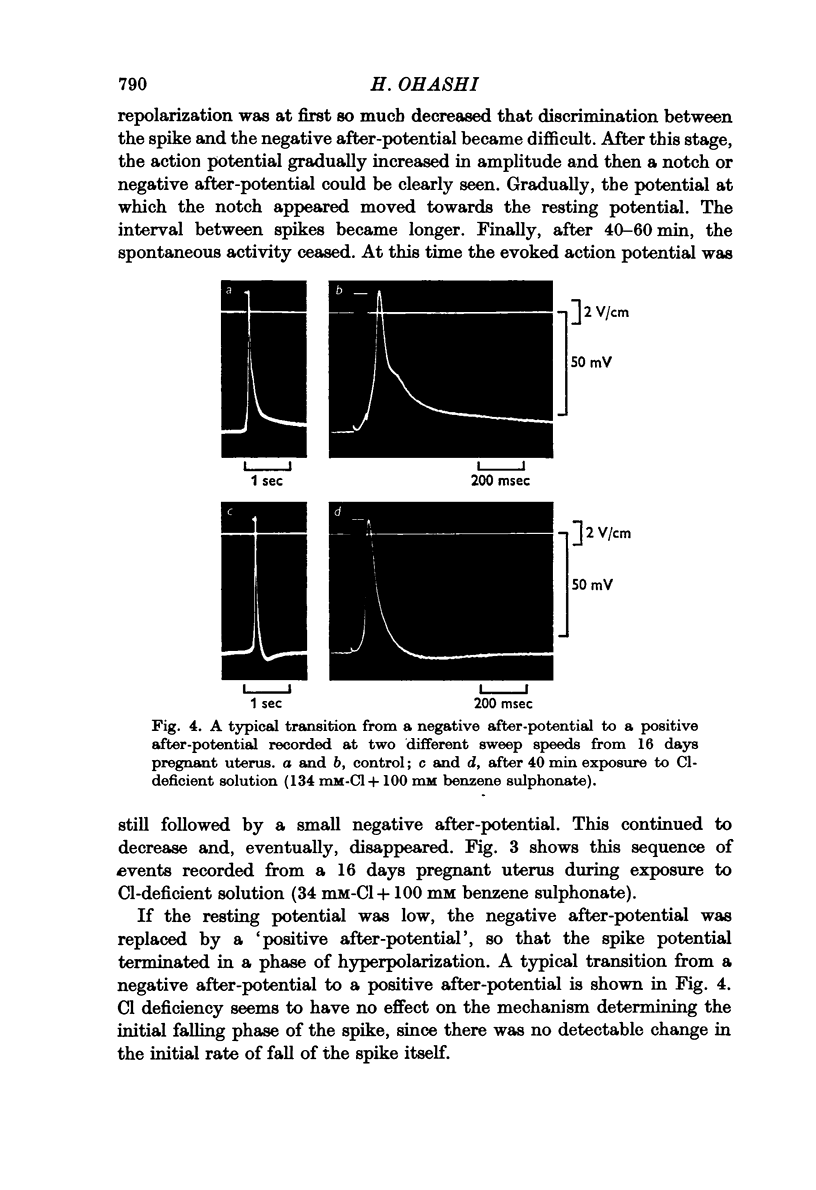
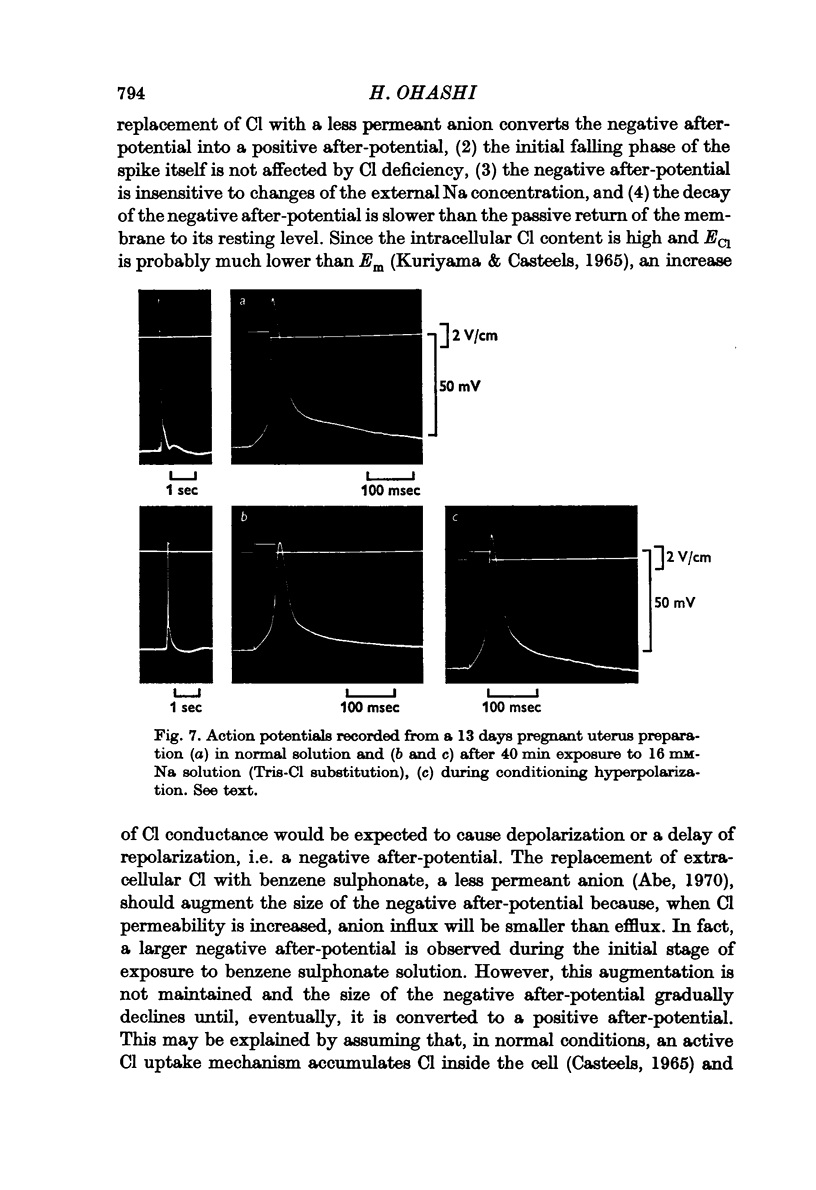
5. In pregnant uterus, the replacement of the Cl in the medium with benzene sulphonate transiently augmented the negative after-potential and then gradually reduced it. Eventually, the negative after-potential disappeared and, instead, a positive after-potential was observed. This conversion took place without a noticeable change in the resting potential or in the initial falling phase of the action potential itself. Replacement of Cl with NO3 had no appreciable effect on the negative after-potential.
6. In non-pregnant uterus, the conversion of the negative to a positive after-potential was never observed. However, in Cl-deficient solution the size and duration of the negative after-potential were reduced.
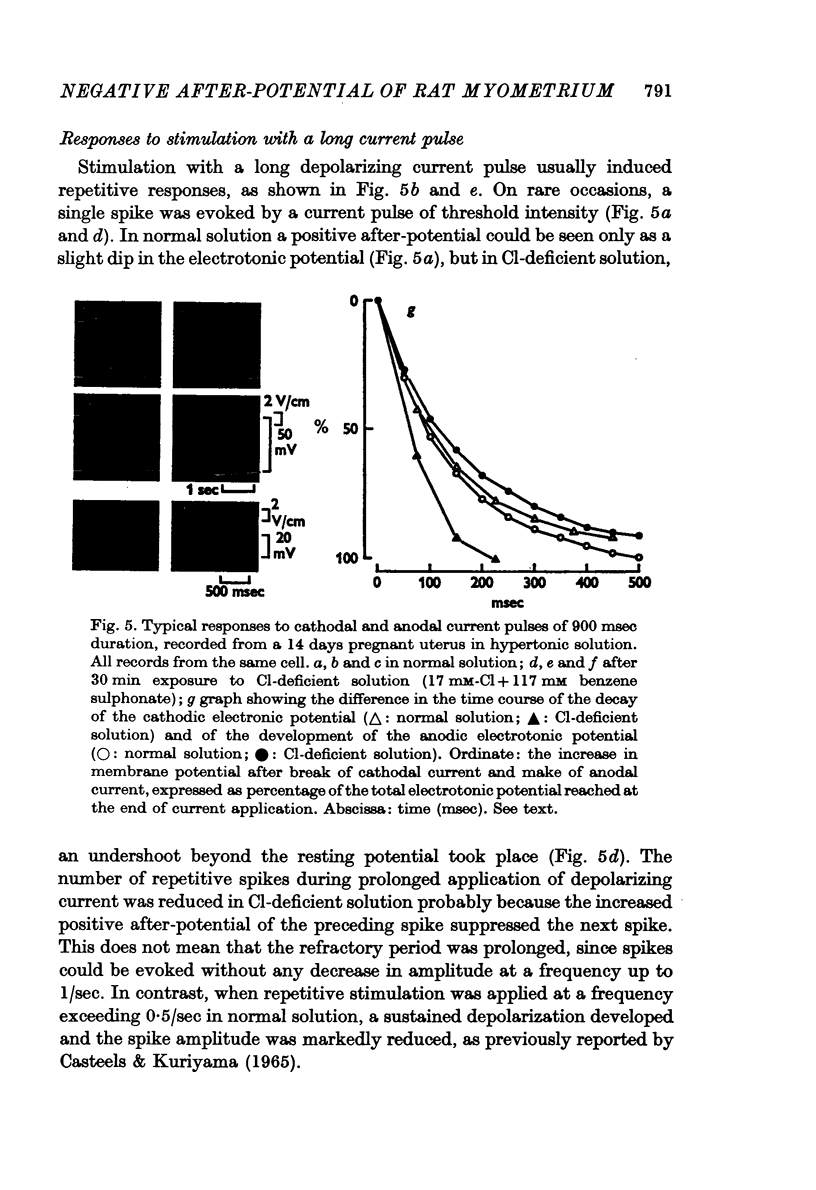
7. In Cl-deficient solution (benzene sulphonate substitution), the decay of the electrotonic potential following the break of cathodal current became faster than that in normal solution. On the other hand the development of the anodic electrotonic potential became slower.
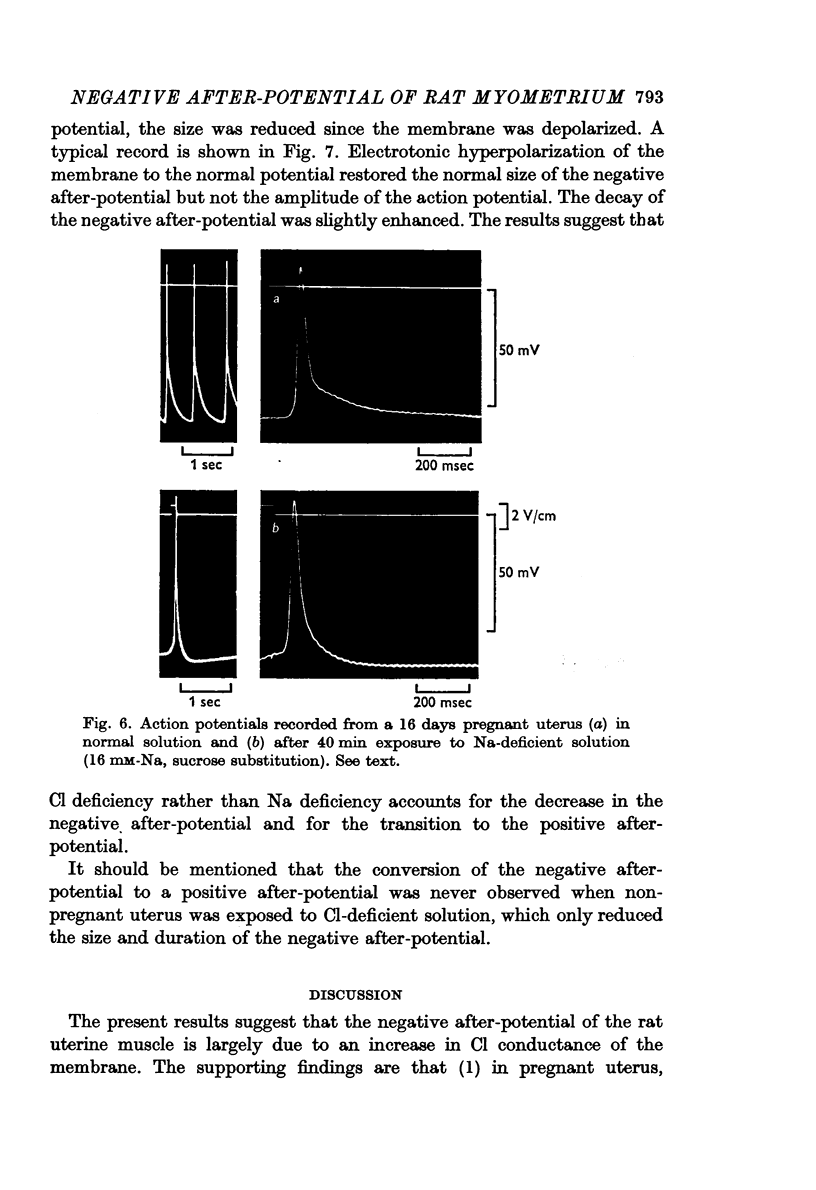
8. Replacement of the NaCl in the medium with sucrose converted the negative after-potential to a positive after-potential. On the other hand, reduction of Na only by replacement of NaCl with Tris-Cl had no noticeable effect on the negative after-potential.
9. It is concluded that the negative after-potential of the spike in rat uterine muscle is largely due to an increase of Cl conductance of the membrane.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK G. B. Negative after-potential of frog's skeletal muscle. J Neurophysiol. 1957 Nov;20(6):602–614. doi: 10.1152/jn.1957.20.6.602. [DOI] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. The effect of tetraethylammonium chloride on the muscle membrane examined with an intracellular microelectrode. J Physiol. 1955 Sep 28;129(3):513–527. doi: 10.1113/jphysiol.1955.sp005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., GRUNDFEST H., FREYGANG W. Low level impedance changes following the spike in the squid giant axon before and after treatment with "veratrine" alkaloids. J Gen Physiol. 1953 Sep;37(1):39–51. doi: 10.1085/jgp.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Potassium movement in relation to nerve activity. J Gen Physiol. 1951 Jul;34(6):795–807. doi: 10.1085/jgp.34.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Spike propagation in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Aug;191(3):517–527. doi: 10.1113/jphysiol.1967.sp008265. [DOI] [PMC free article] [PubMed] [Google Scholar]


