Abstract
1. A study has been made of the changes in the fluorescence of desheathed rabbit cervical vagus nerves that occur during and after electrical stimulation of its non-myelinated fibres.
2. Stimulation for 5 sec at 30 shocks/sec produces a maximal decrease, of about 1% of the resting fluorescence. Stimulation for less than 0·5 sec fails to produce responses visible above the inherent noise in the recording system.
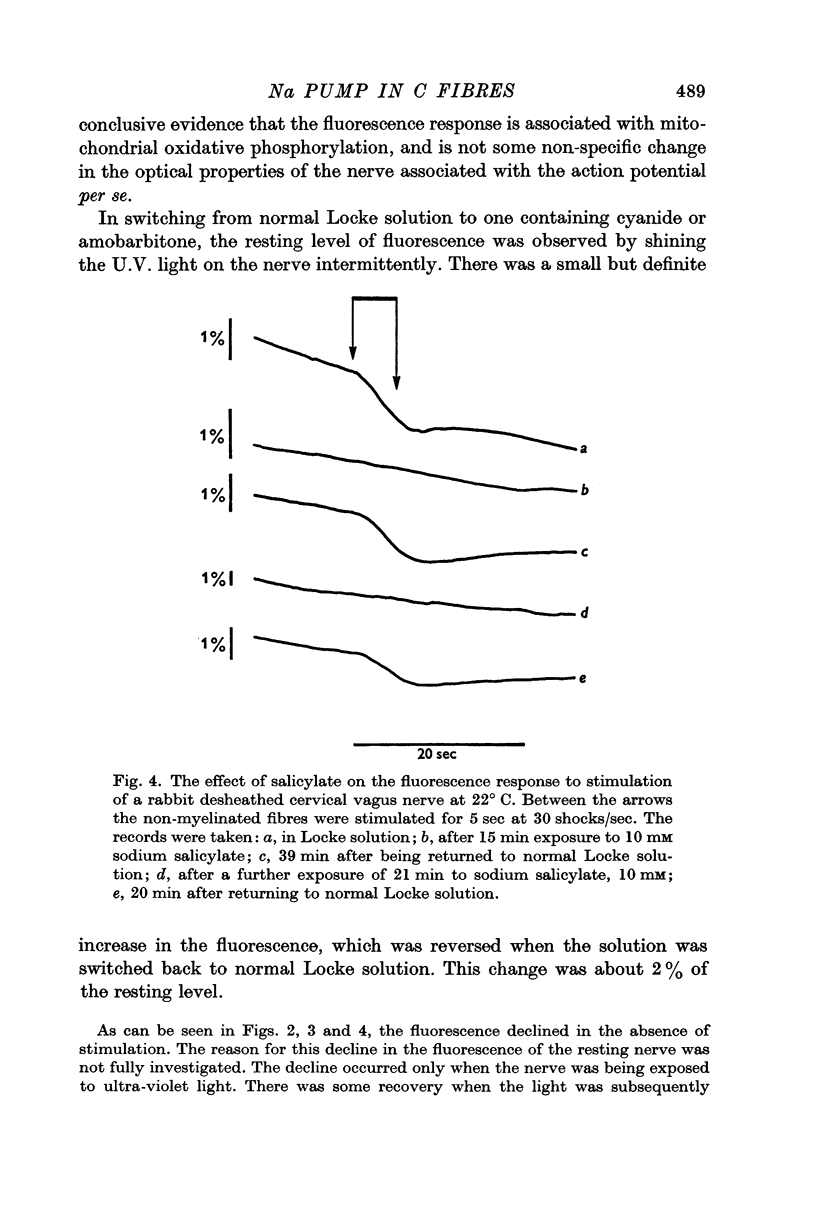
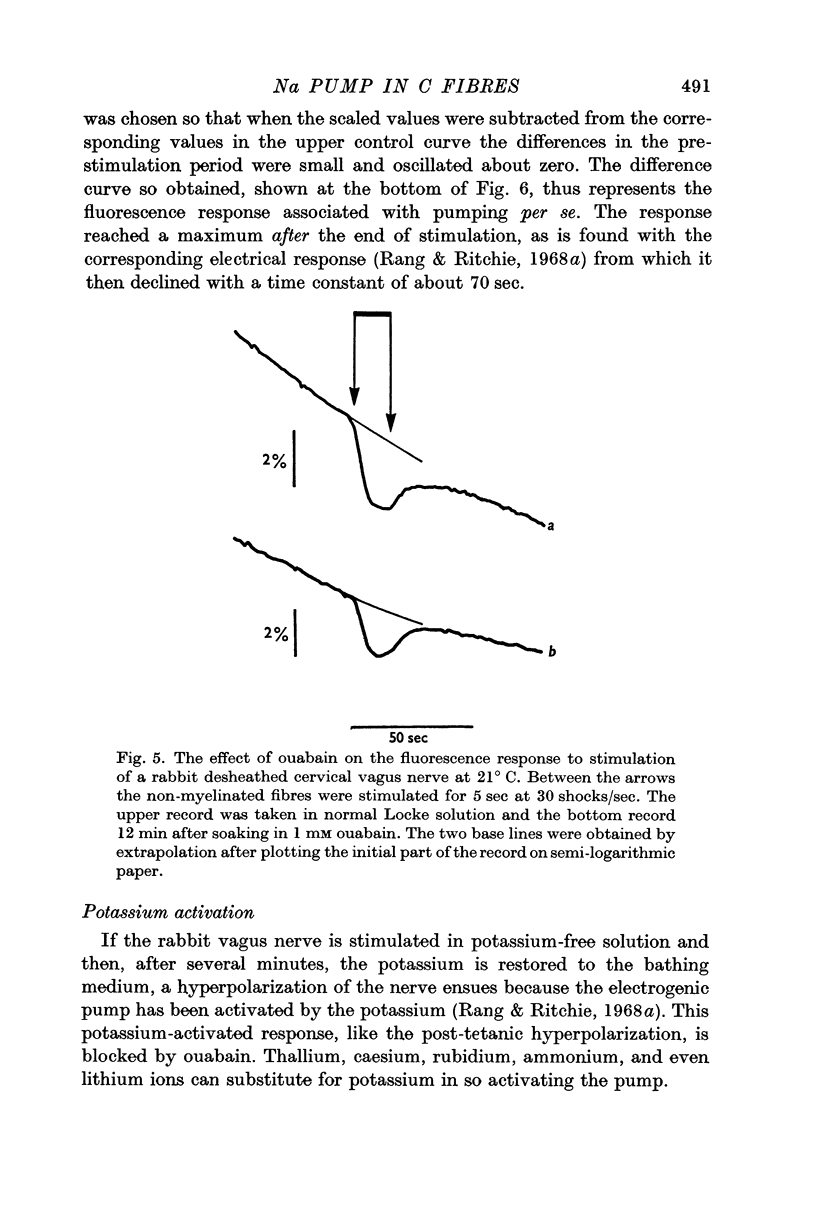
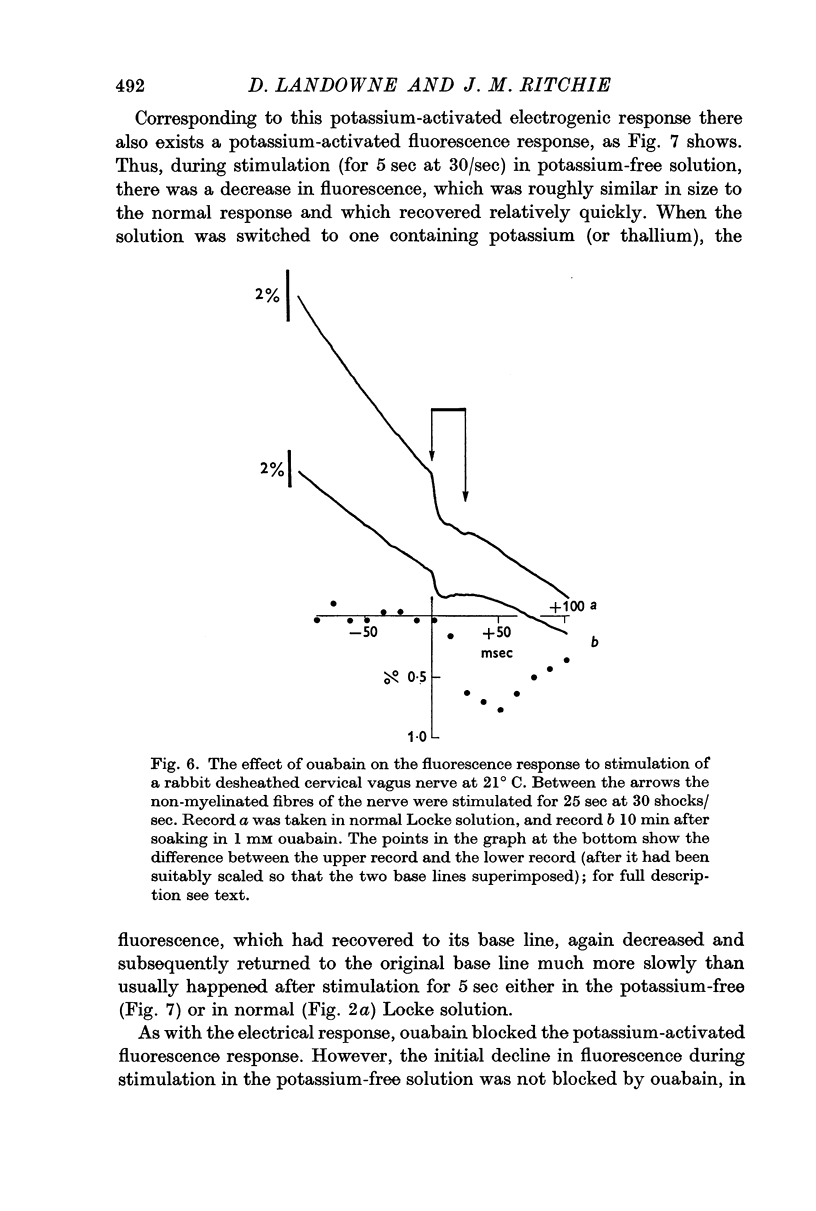
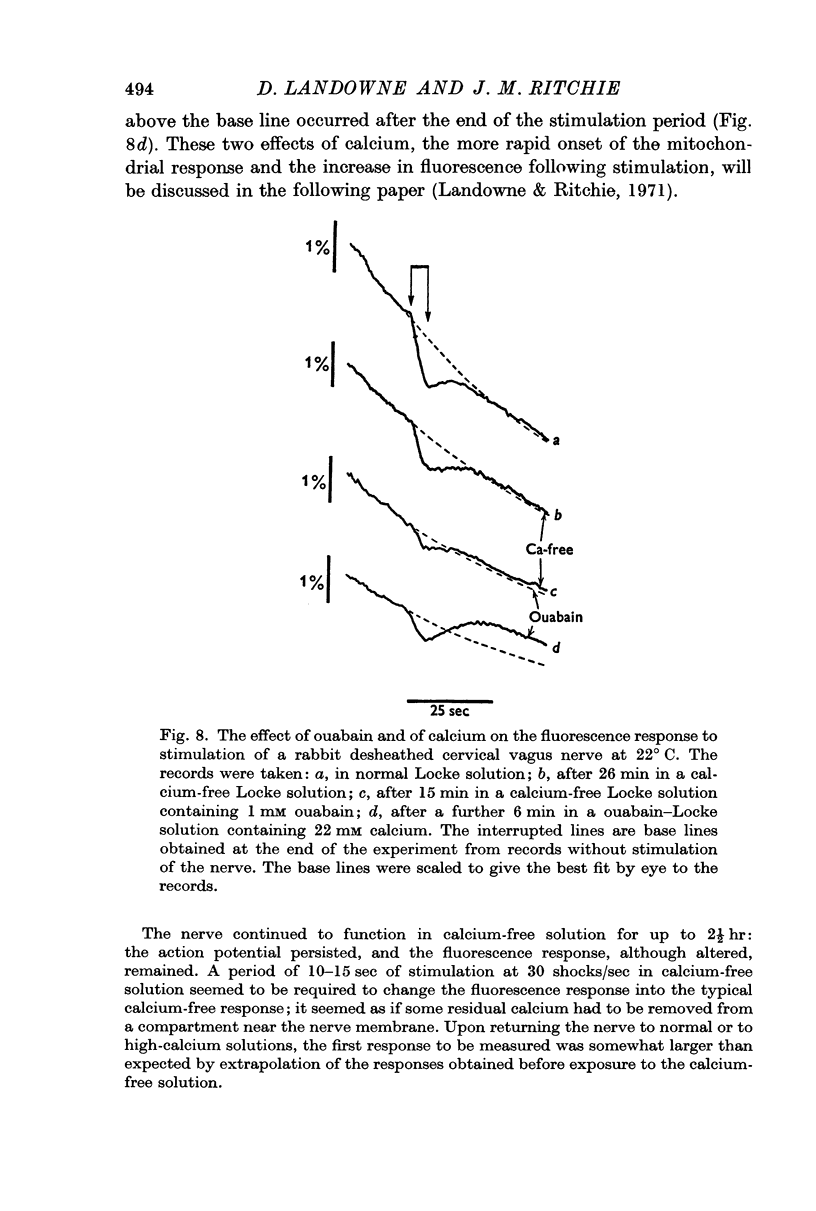
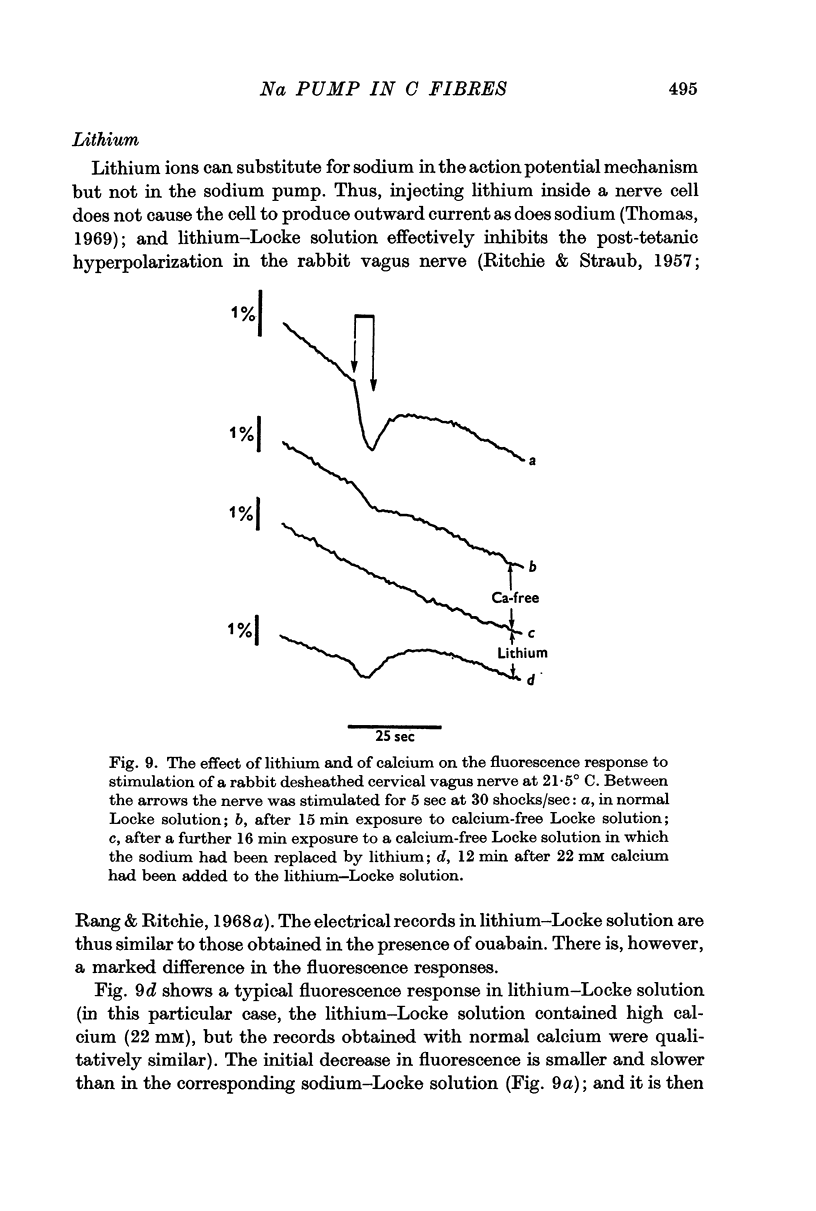
3. A pharmacological dissection (with ouabain, metabolic inhibitors, and calcium) has revealed four phases of fluorescence change:
(a) under conditions where the sodium pump is functioning, there is a prolonged decrease in the fluorescence following electrical activity;
(b) even in the absence of pumping the mere entry of sodium into the nerve causes an initial decrease in fluorescence;
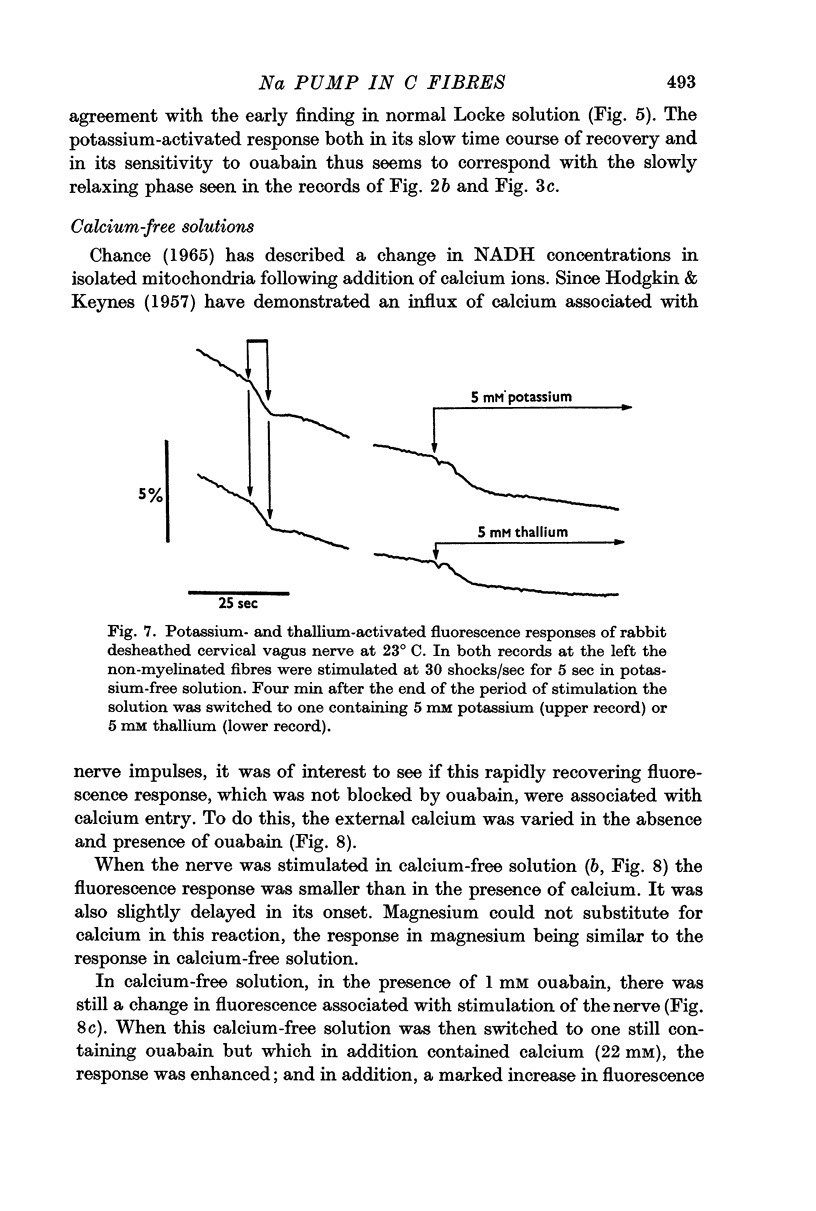
(c) the entry of calcium ions with electrical activity also causes an initial rapid decrease in fluorescence;
(d) following these phases of decreased fluorescence there is a phase of increased fluorescence.
4. These changes in fluorescence are related to changes in the NADH concentration in the nerve resulting from:
(a) the splitting of ATP during sodium extrusion;
(b) the initial binding of sodium to the sodium- and potassium-dependent ATPase, which is the sodium pump;
(c) the stimulation of mitochondrial respiration by calcium that has entered during the spike; and
(d) an increased glycogenolysis as a result of the calcium entry during activity.
Full text
PDF

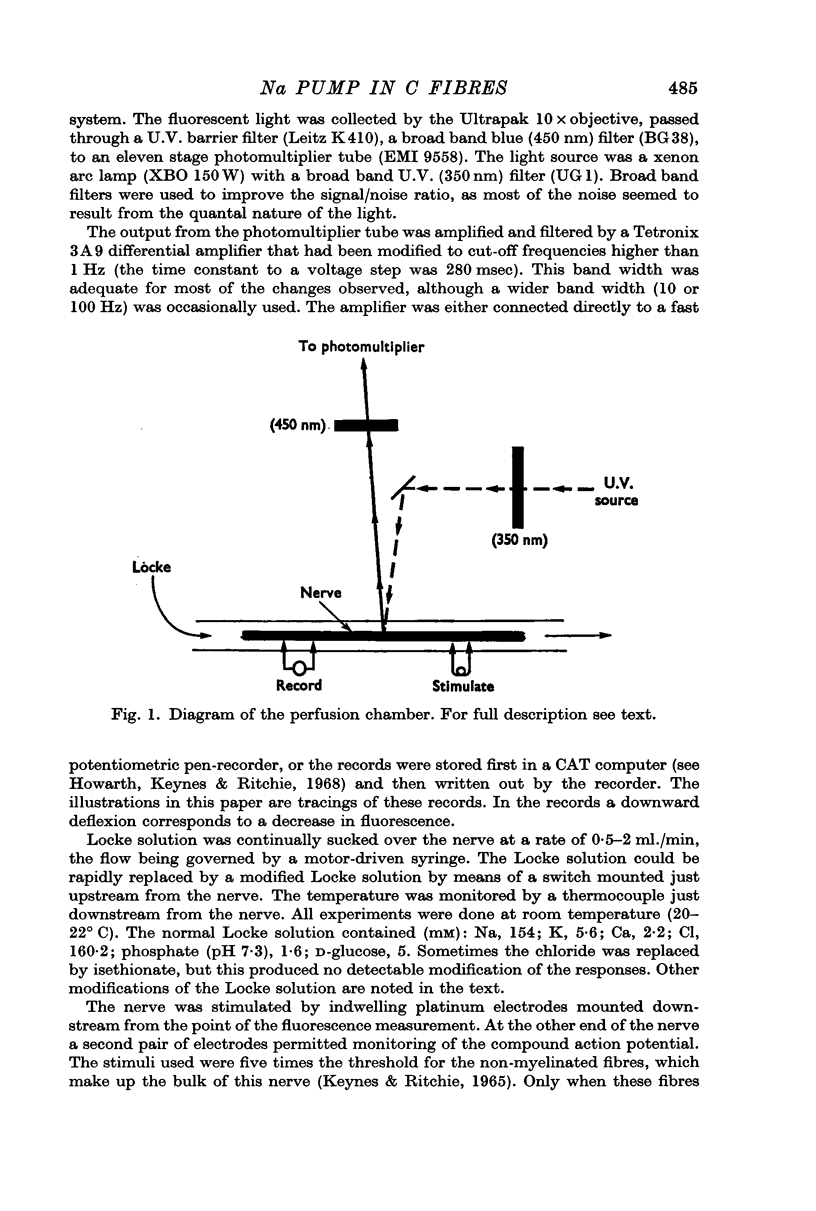

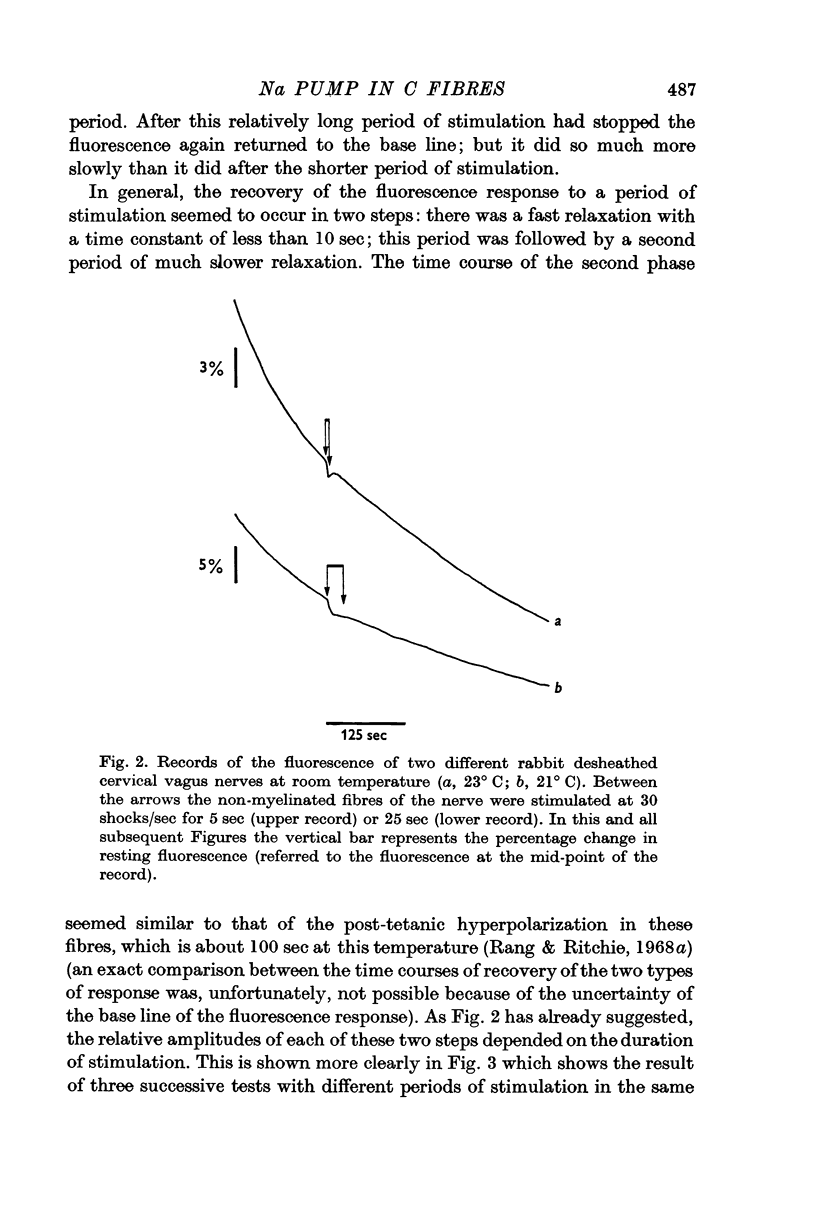
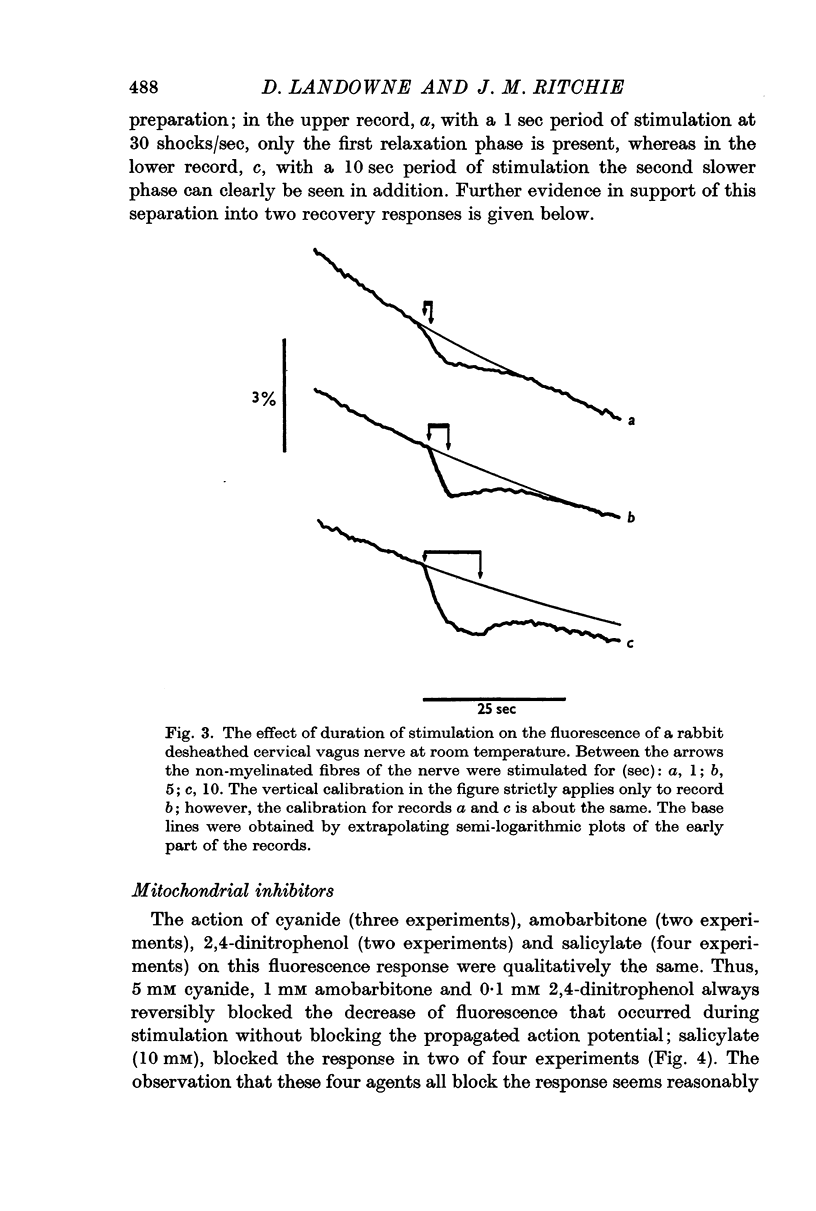








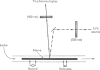
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Chmouliovsky M., Schorderet M., Straub R. W. Effect of electrical activity on the concentration of phosphorylated metabolites and inorganic phosphate in mammalian non-myelinated nerve fibres. J Physiol. 1969 Jun;202(2):90P–92P. [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D. Changes in light scattering associated with the action potential in crab nerves. J Physiol. 1971 Jan;212(1):259–275. doi: 10.1113/jphysiol.1971.sp009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Doane M. G. Fluorometric measurement of pyridine nucleotide reduction in the giant axon of the squid. J Gen Physiol. 1967 Dec;50(11):2603–2632. doi: 10.1085/jgp.50.11.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Glynn I. M., Hoffman J. F. Nucleotide requirements for Na:Na exchange by the Na pump. J Physiol. 1970 Mar;207(1):34P–35P. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Duffield J. C. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–1047. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. On the control of glycogenolysis in mammalian nervous tissue by calcium. J Physiol. 1971 Jan;212(2):503–517. doi: 10.1113/jphysiol.1971.sp009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. The binding of tritiated ouabain to mammalian non-myelinated nerve fibres. J Physiol. 1970 Apr;207(2):529–537. doi: 10.1113/jphysiol.1970.sp009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Estrada C. Fluorometric determinations of NADH2 levels in dorsal root ganglion following peripheral nerve stimulation. Brain Res. 1967 Oct;6(2):217–227. doi: 10.1016/0006-8993(67)90192-8. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Sen A. K., Tobin T. A cycle for ouabain inhibition of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1969 Dec 25;244(24):6596–6604. [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]