Abstract
1. The release of γ-aminobutyric acid (GABA) from the surface of the posterior lateral gyrus of the cerebral cortex was measured by a sensitive enzymic fluorimetric assay procedure. Experiments were performed with anaesthetized cats during resting conditions and during cortical inhibition produced by electrical stimulation of the brain surface or of the lateral geniculate nucleus (l.g.n.).
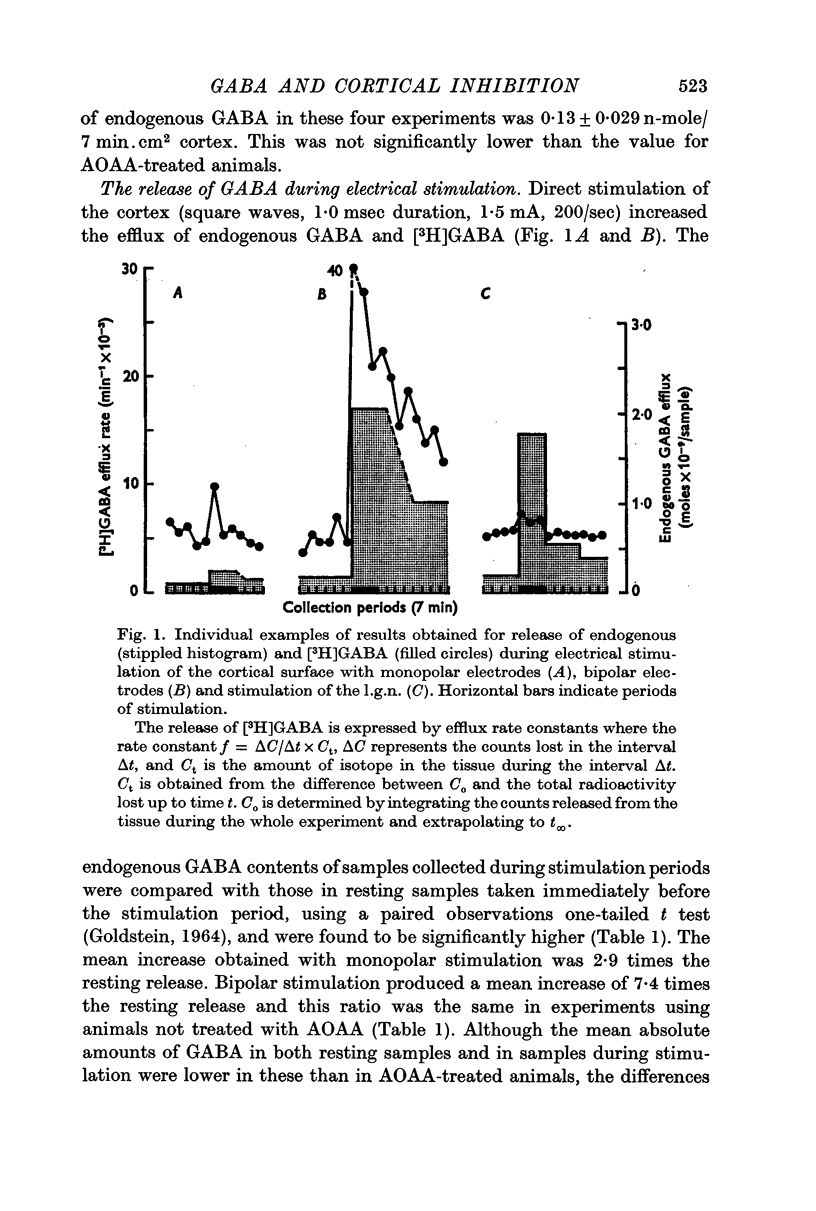
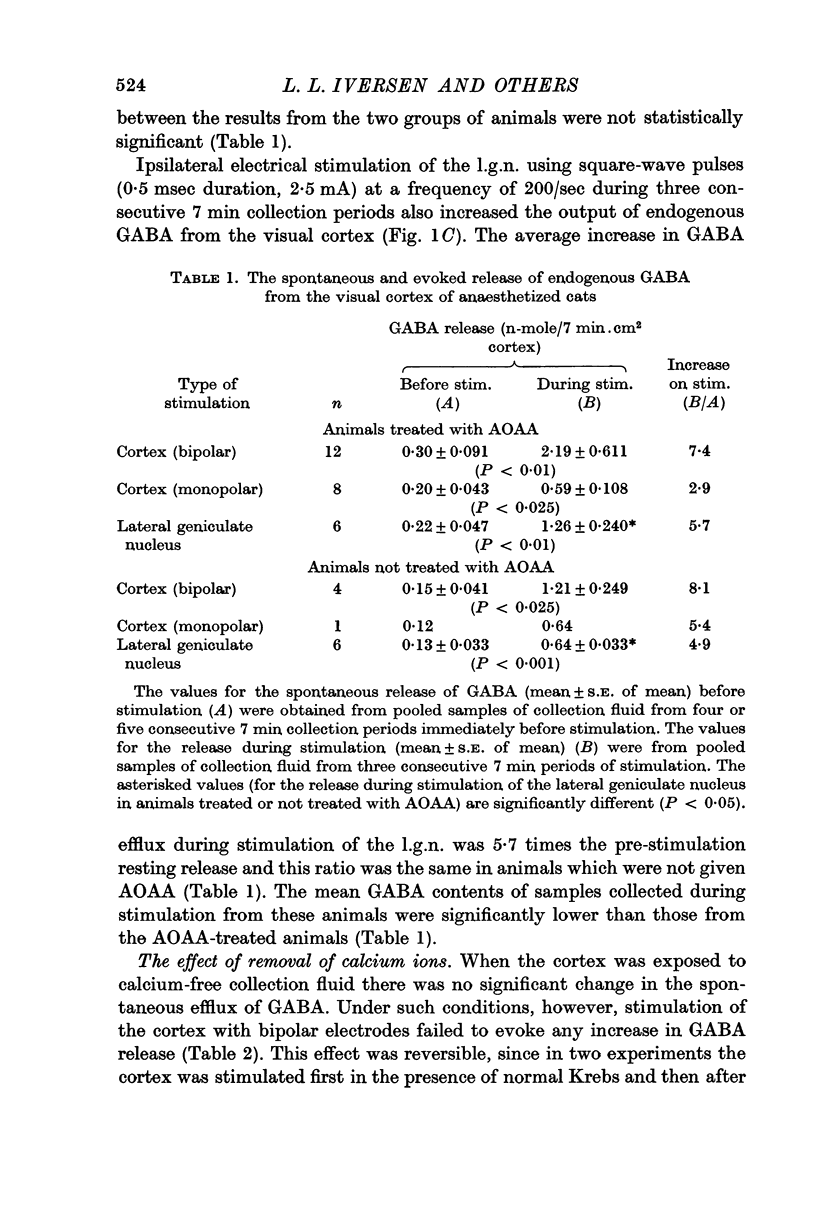
2. The average resting release of endogenous GABA was 0·20 n-mole/ 7 min.cm2 cortex; this was increased during stimulation of both the cortical surface (2·9 times resting release during monopolar stimulation and 7·4 times resting release during bipolar stimulation) and the l.g.n. (5·7 times resting release).
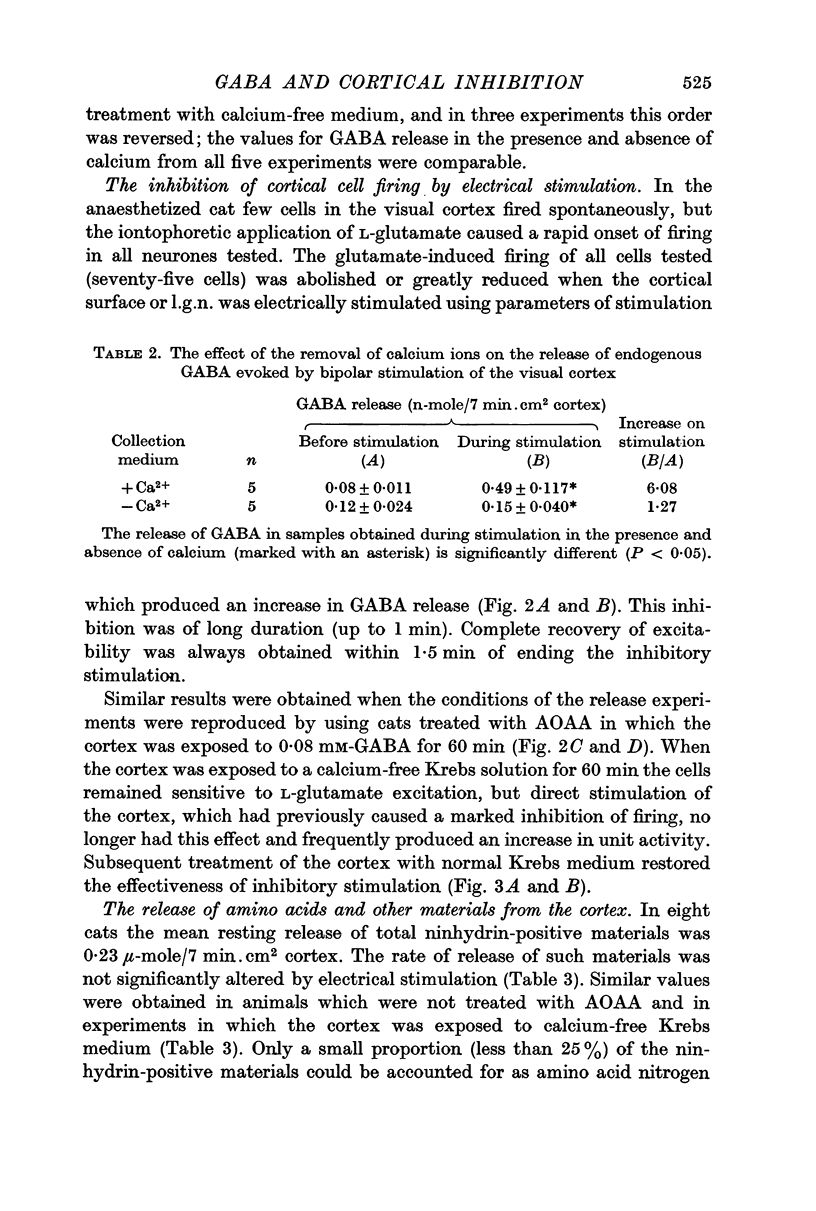
3. Removal of calcium ions from the collection fluid did not affect the resting release of endogenous GABA but prevented the increase in GABA release normally evoked by stimulation of the cortical surface.
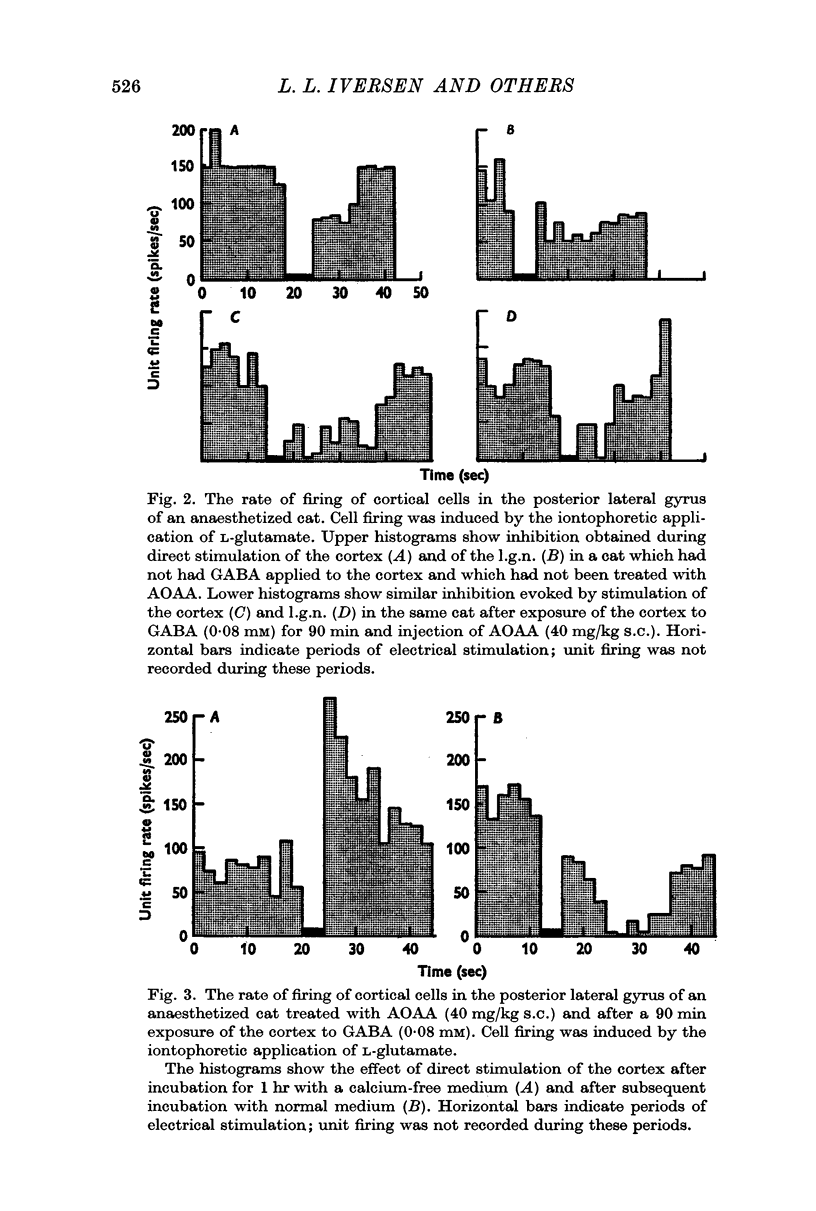
4. The stimulus parameters used to increase the release of GABA also inhibited the glutamate-induced firing of single cells in the visual cortex and this inhibition was abolished in the absence of calcium ions.
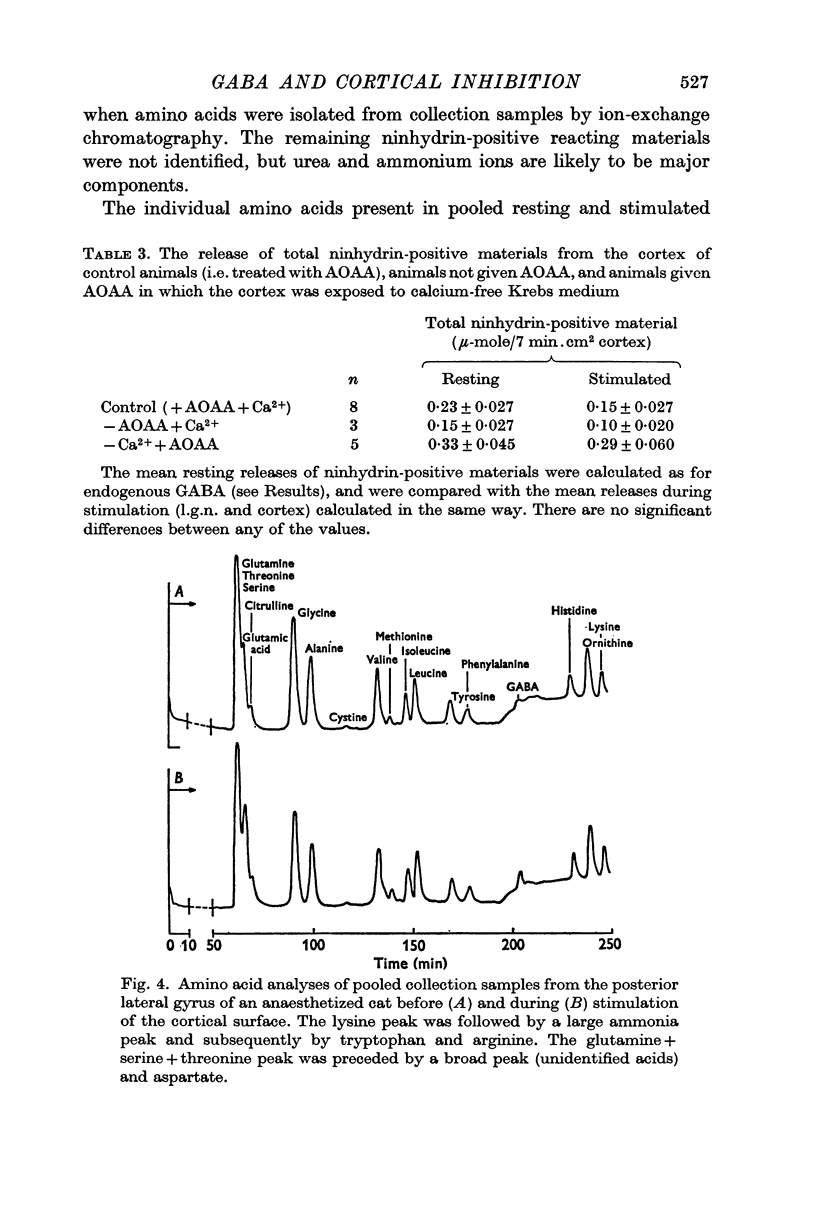
5. In three experiments the total amino acid content of cortical samples was examined using an amino acid analyser. With the exception of GABA, there were no significant differences between the rates of release of any other detected amino acids during periods with and without electrical stimulation of the cortex.
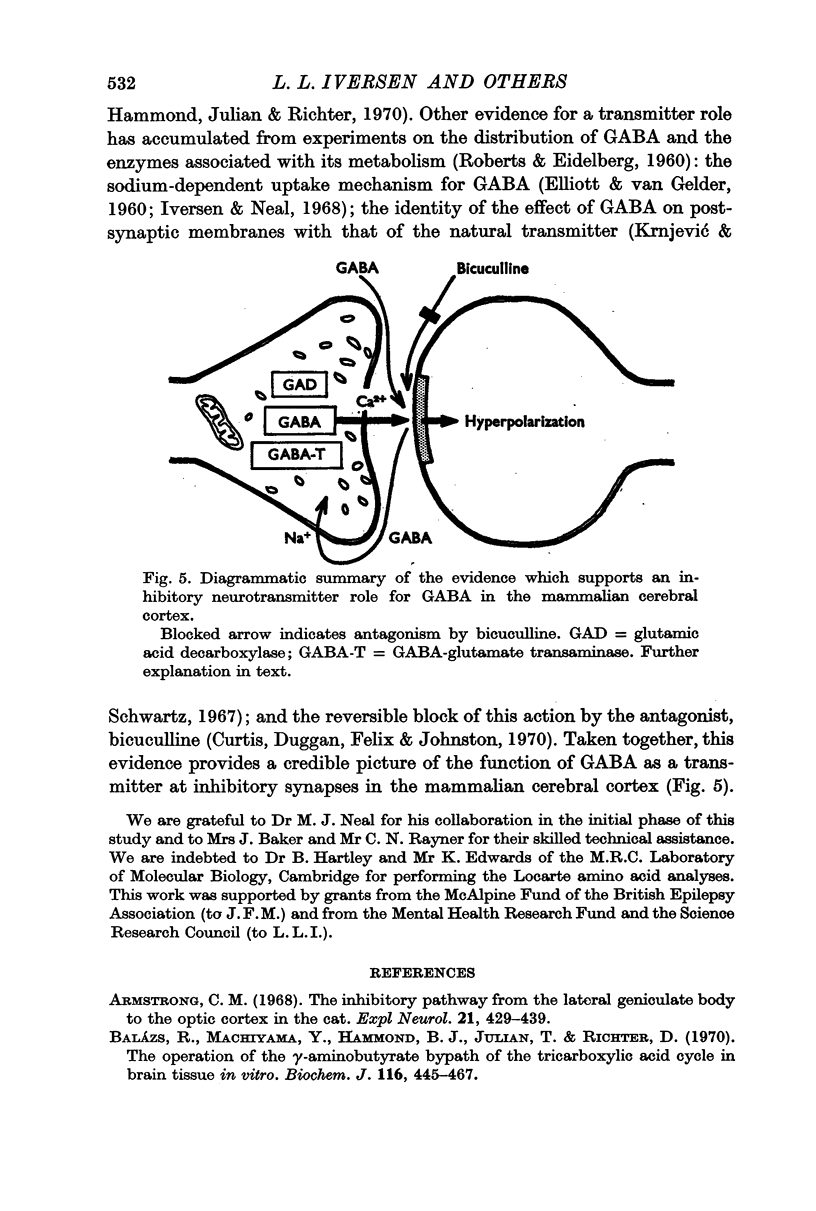
6. It is suggested that since the release of GABA observed during inhibitory stimulation of the cortex is calcium-dependent and specific, it may originate from inhibitory nerve terminals in the cortex. The present findings support the view that GABA is a central inhibitory neurotransmitter.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. The inhibitory path from the lateral geniculate body to the optic cortex in the cat. Exp Neurol. 1968 Aug;21(4):429–439. doi: 10.1016/0014-4886(68)90063-0. [DOI] [PubMed] [Google Scholar]
- BASEMORE A. W., ELLIOT K. A., FLOREY E. Isolation of factor I. J Neurochem. 1957;1(4):334–339. doi: 10.1111/j.1471-4159.1957.tb12090.x. [DOI] [PubMed] [Google Scholar]
- BAXTER C. F., ROBERTS E. Elevation of gamma-aminobutyric acid in brain: selective inhibition of gamma-aminobutyric-alpha-ketoglutaric acid transaminase. J Biol Chem. 1961 Dec;236:3287–3294. [PubMed] [Google Scholar]
- Balázs R., Machiyama Y., Hammond B. J., Julian T., Richter D. The operation of the gamma-aminobutyrate bypath of the tricarboxylic acid cycle in brain tissue in vitro. Biochem J. 1970 Feb;116(3):445–461. doi: 10.1042/bj1160445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F. Metabolic response of synaptosomes to electrical stimulation: release of amino acids. Brain Res. 1970 Apr 14;19(2):239–247. doi: 10.1016/0006-8993(70)90437-3. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PERRIN D. D., WATKINS J. C. The excitation of spinal neurones by the ionophoretic application of agents which chelate calcium. J Neurochem. 1960 Aug;6:1–20. doi: 10.1111/j.1471-4159.1960.tb13443.x. [DOI] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- ELLIOTT K. A., VAN GELDER N. M. The state of factor I in rat brain: the effects of metabolic conditions and drugs. J Physiol. 1960 Oct;153:423–432. doi: 10.1113/jphysiol.1960.sp006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOREY E., MCLENNAN H. The release of an inhibitory substance from mammalian brain, and its effect on peripheral synaptic transmission. J Physiol. 1955 Aug 29;129(2):384–392. doi: 10.1113/jphysiol.1955.sp005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsworth B. A., Mitchell J. F. The characteristics of acetylcholine release mechanisms in the auditory cortex. Br J Pharmacol. 1969 May;36(1):161–170. doi: 10.1111/j.1476-5381.1969.tb08313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- JASPER H. H., KHAN R. T., ELLIOTT K. A. AMINO ACIDS RELEASED FROM THE CEREBRAL CORTEX IN RELATION TO ITS STATE OF ACTIVATION. Science. 1965 Mar 19;147(3664):1448–1449. doi: 10.1126/science.147.3664.1448. [DOI] [PubMed] [Google Scholar]
- Jasper H. H., Koyama I. Rate of release of amino acids from the cerebral cortex in the cat as affected by brainstem and thalamic stimulation. Can J Physiol Pharmacol. 1969 Oct;47(10):889–905. doi: 10.1139/y69-146. [DOI] [PubMed] [Google Scholar]
- KRAVITZ E. A., POTTER D. D. A FURTHER STUDY OF THE DISTRIBUTION OF GAMMA-AMINOBUTYRIC ACID BETWEEN EXCITATORY AND INHIBITORY AXONS OF THE LOBSTER. J Neurochem. 1965 Apr;12:323–328. doi: 10.1111/j.1471-4159.1965.tb06768.x. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. An inhibitory process in the cerebral cortex. J Physiol. 1966 May;184(1):16–48. doi: 10.1113/jphysiol.1966.sp007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Mitchell J. F., Srinivasan V. The release of [3H] gamma-aminobutyric acid from the surface of the brain by electrical stimuli which produce synaptic inhibition. J Physiol. 1969 Sep;204(1):23P–24P. [PubMed] [Google Scholar]
- Mitchell J. F. The spontaneous and evoked release of acetylcholine from the cerebral cortex. J Physiol. 1963 Jan;165(1):98–116. doi: 10.1113/jphysiol.1963.sp007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Subcellular distribution of endogenous and (3H) gamma-aminobutyric acid in rat cerebral cortex. J Neurochem. 1969 Aug;16(8):1245–1252. doi: 10.1111/j.1471-4159.1969.tb05972.x. [DOI] [PubMed] [Google Scholar]
- Obata K., Takeda K. Release of gamma-aminobutyric acid into the fourth ventricle induced by stimulation of the cat's cerebellum. J Neurochem. 1969 Jul;16(7):1043–1047. doi: 10.1111/j.1471-4159.1969.tb05948.x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Iversen L. L., Hall Z. W., Kravitz E. A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS E., EIDELBERG E. Metabolic and neurophysiological roles of gamma-aminobutyric acid. Int Rev Neurobiol. 1960;2:279–332. doi: 10.1016/s0074-7742(08)60125-7. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The role of calcium in neurohumoral and neurohormonal extrusion processes. J Pharm Pharmacol. 1968 Dec;20(12):889–910. doi: 10.1111/j.2042-7158.1968.tb09672.x. [DOI] [PubMed] [Google Scholar]
- Van Gelder N. M. A possible enzyme barrier for gamma-aminobutyric acid in the central nervous system. Prog Brain Res. 1968;29:259–271. doi: 10.1016/S0079-6123(08)64161-8. [DOI] [PubMed] [Google Scholar]
- Vogt M. Release from brain tissue of compounds with possible transmitter function: interaction of drugs with these substances. Br J Pharmacol. 1969 Oct;37(2):325–337. doi: 10.1111/j.1476-5381.1969.tb10570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACH D. P. Studies on the GABA pathway. I. The inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase in vitro and in vivo by U-7524 (amino-oxyacetic acid). Biochem Pharmacol. 1961 Feb;5:323–331. doi: 10.1016/0006-2952(61)90023-5. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Konishi M., Creutzfeldt O. D. Postsynaptic potentials in the cat's visual cortex following electrical stimulation of afferent pathways. Exp Brain Res. 1966;1(3):272–283. doi: 10.1007/BF00234347. [DOI] [PubMed] [Google Scholar]



