Abstract
1. The effects of tetraethylammonium (TEA) on the membrane activity of the antral circular muscle of the guinea-pig stomach were investigated with micro-electrode and double sucrose gap methods.
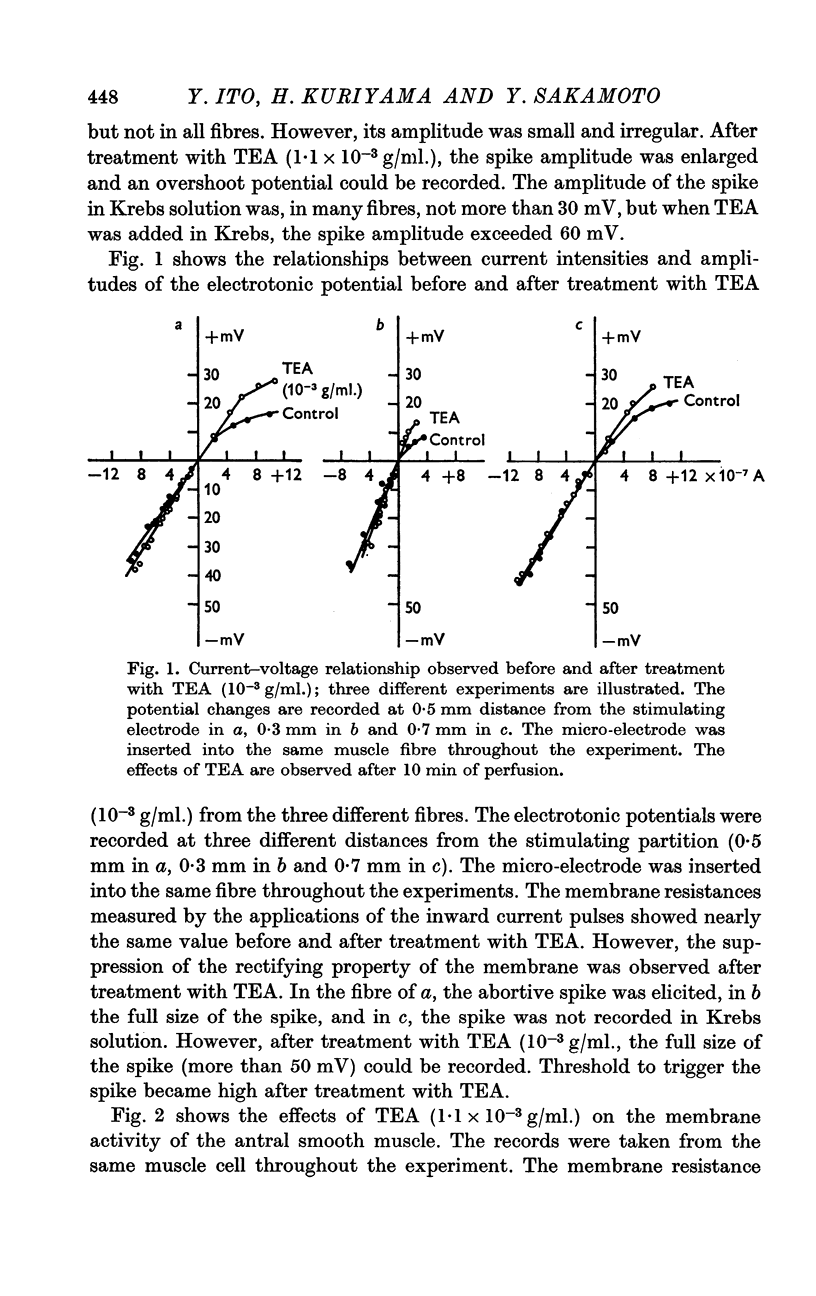
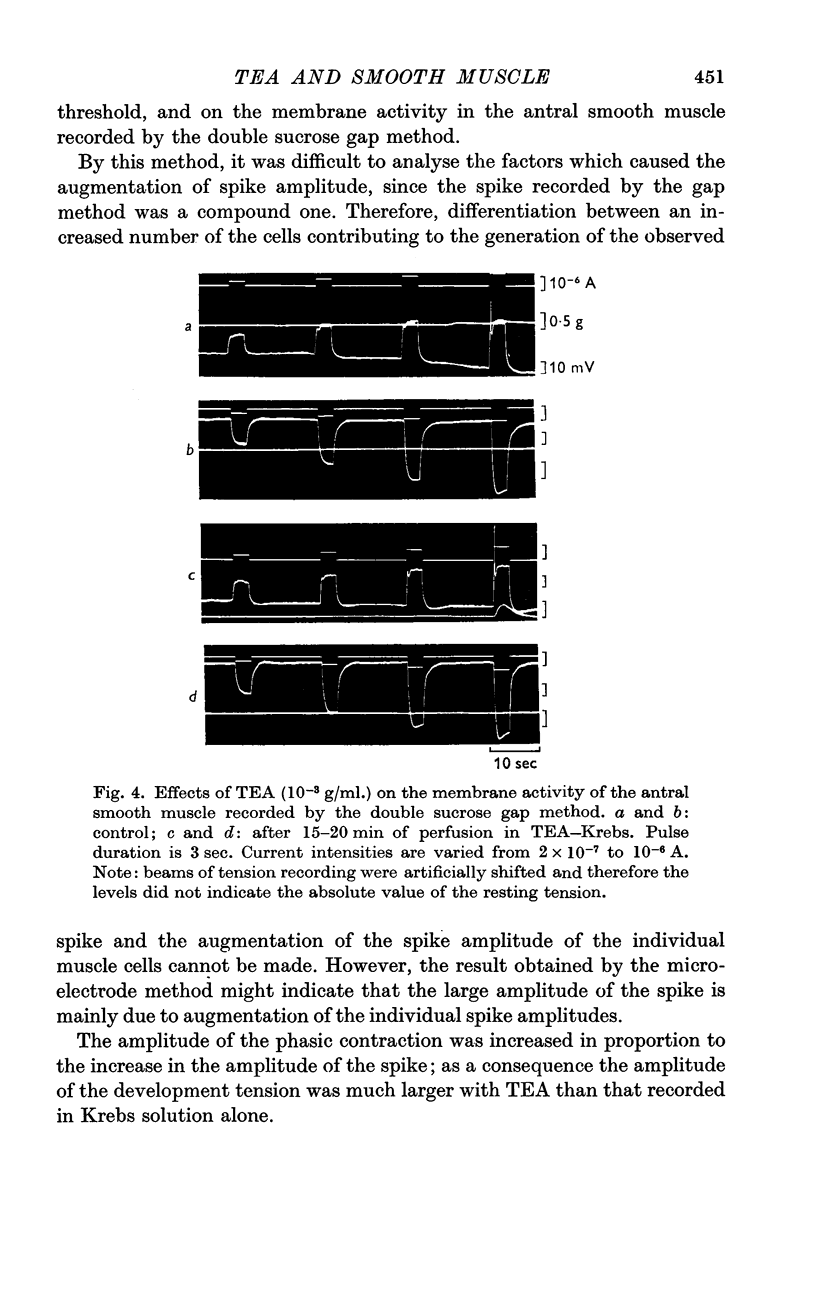
2. In a concentration of 1-1·5 × 10-3 g/ml. (3-5 mM), the membrane potential was not influenced; the membrane resistance measured by inward current pulses remained the same but the rectifying property of the membrane was suppressed.
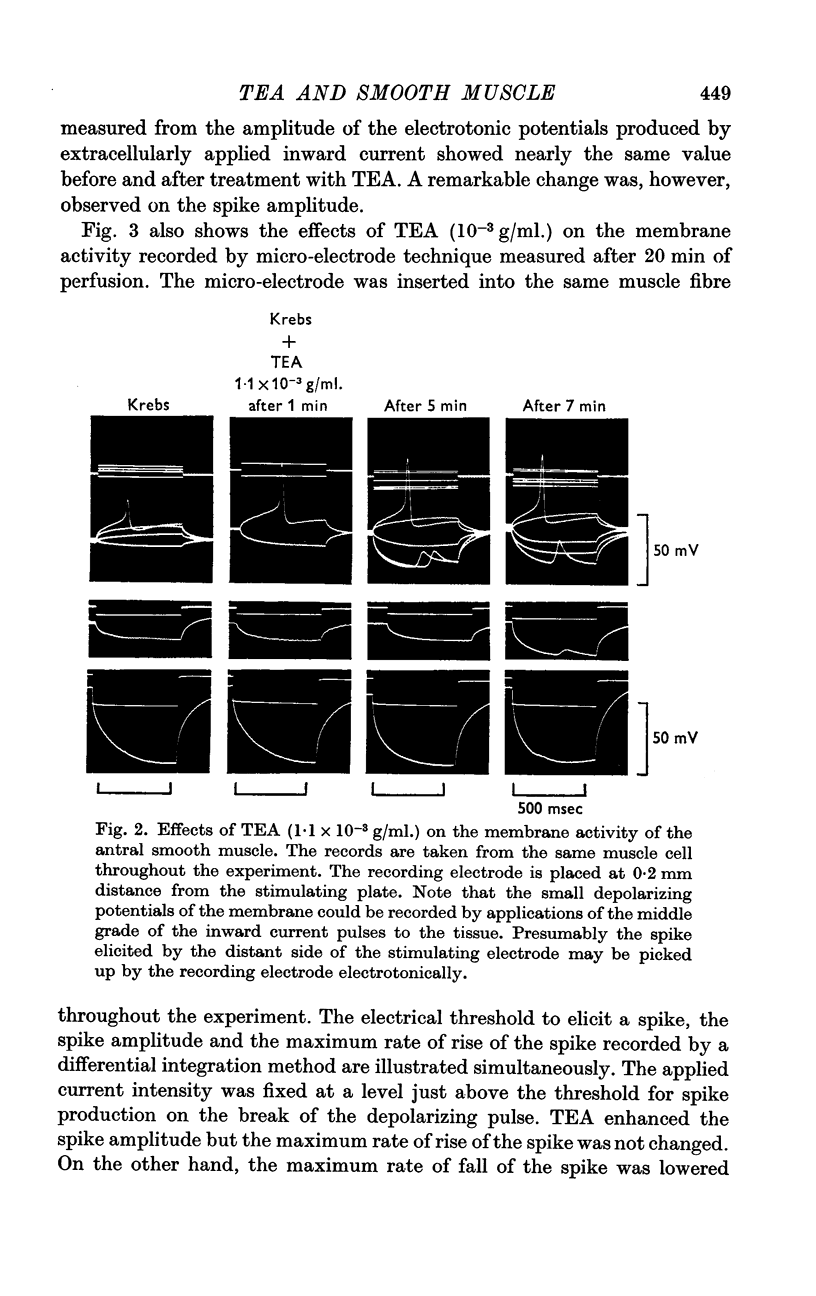
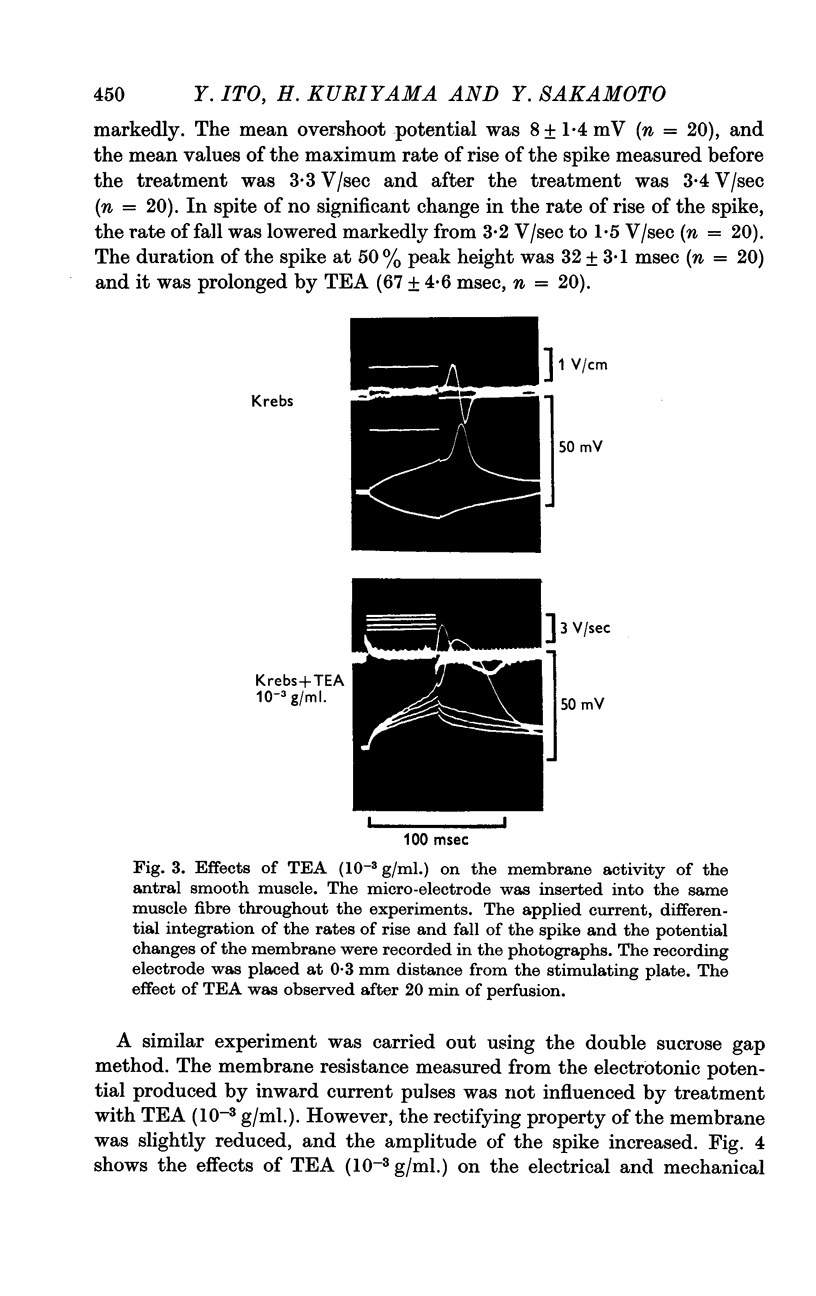
3. TEA (1-1·5 × 10-3 g/ml.) enhanced the spike amplitude markedly even from fibres which generated graded responses.
4. TEA (1-1·5 × 10-3 g/ml.) did not increase the maximum rate of rise of the spike but decreased the maximum rate of fall of the spike markedly.
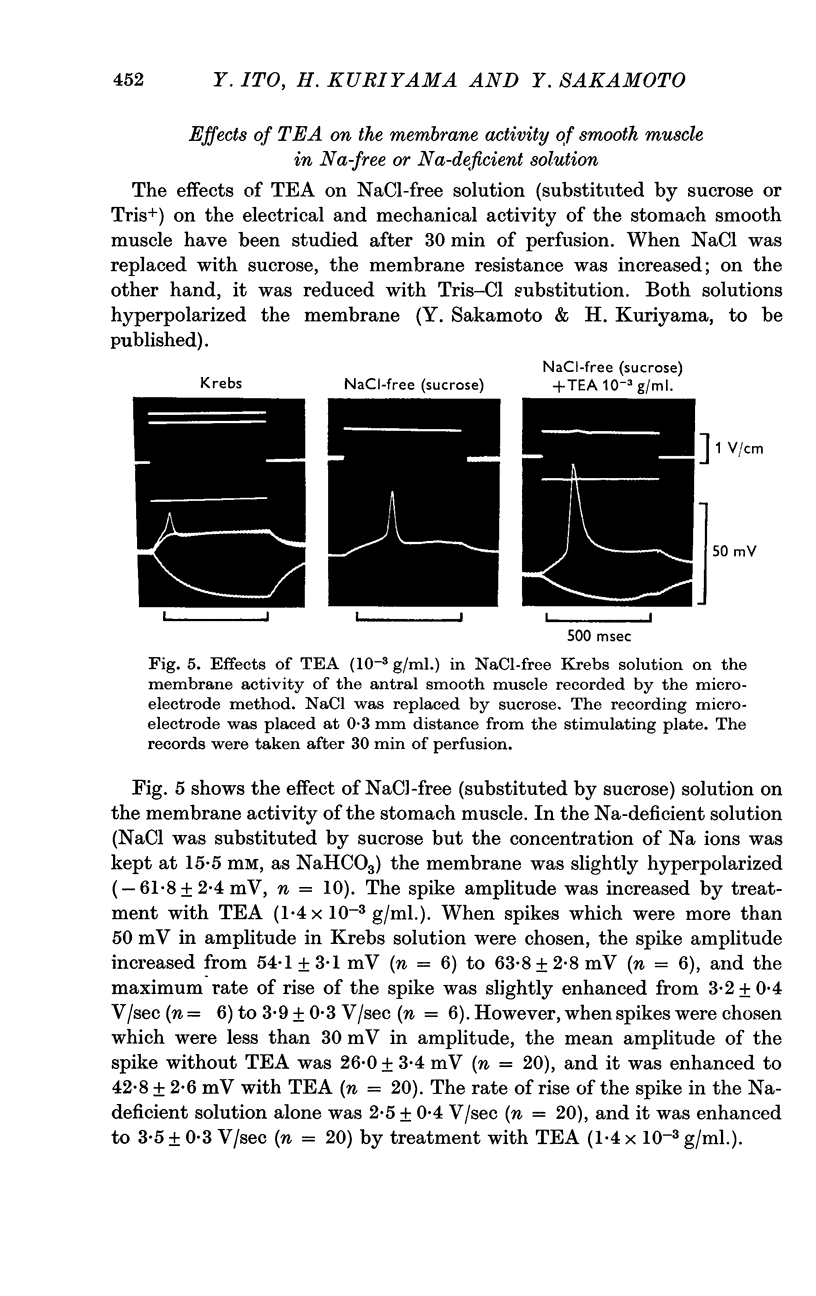
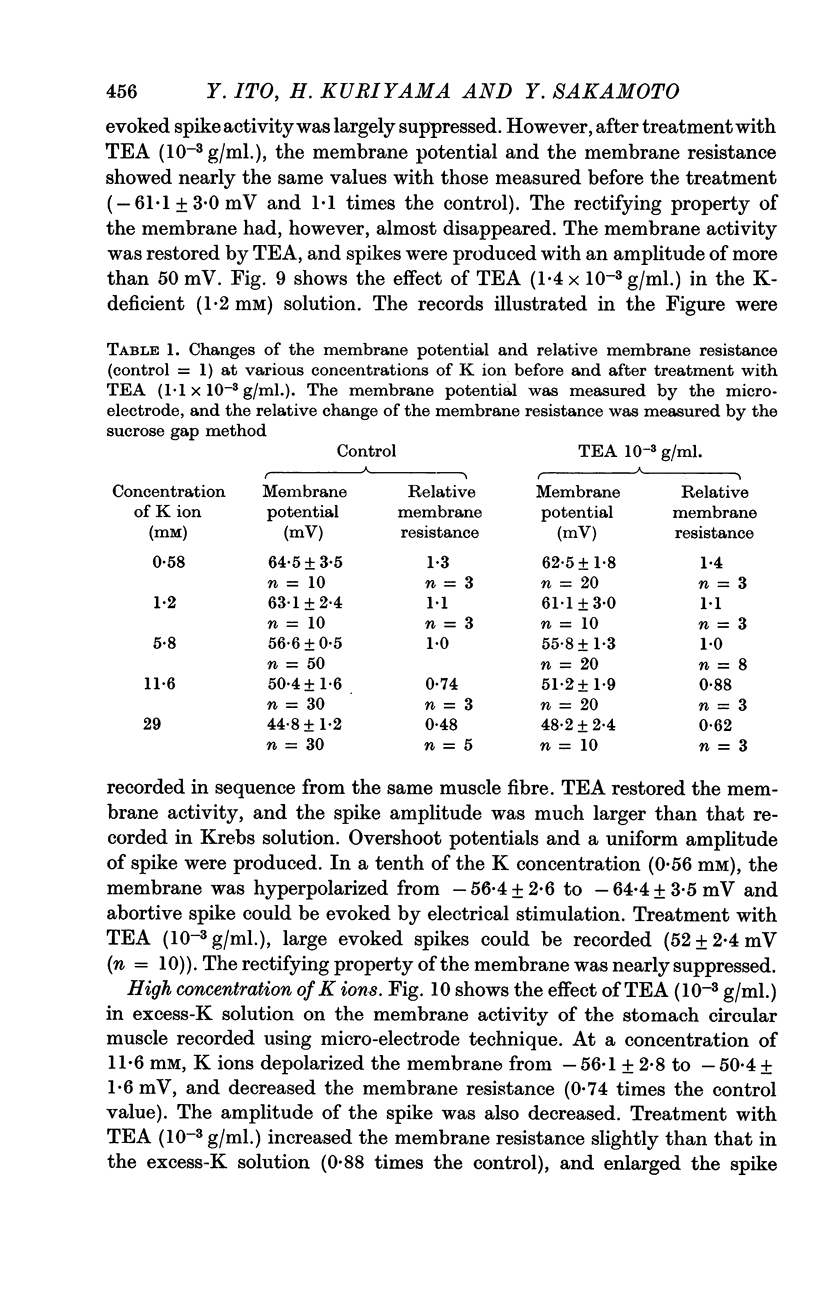
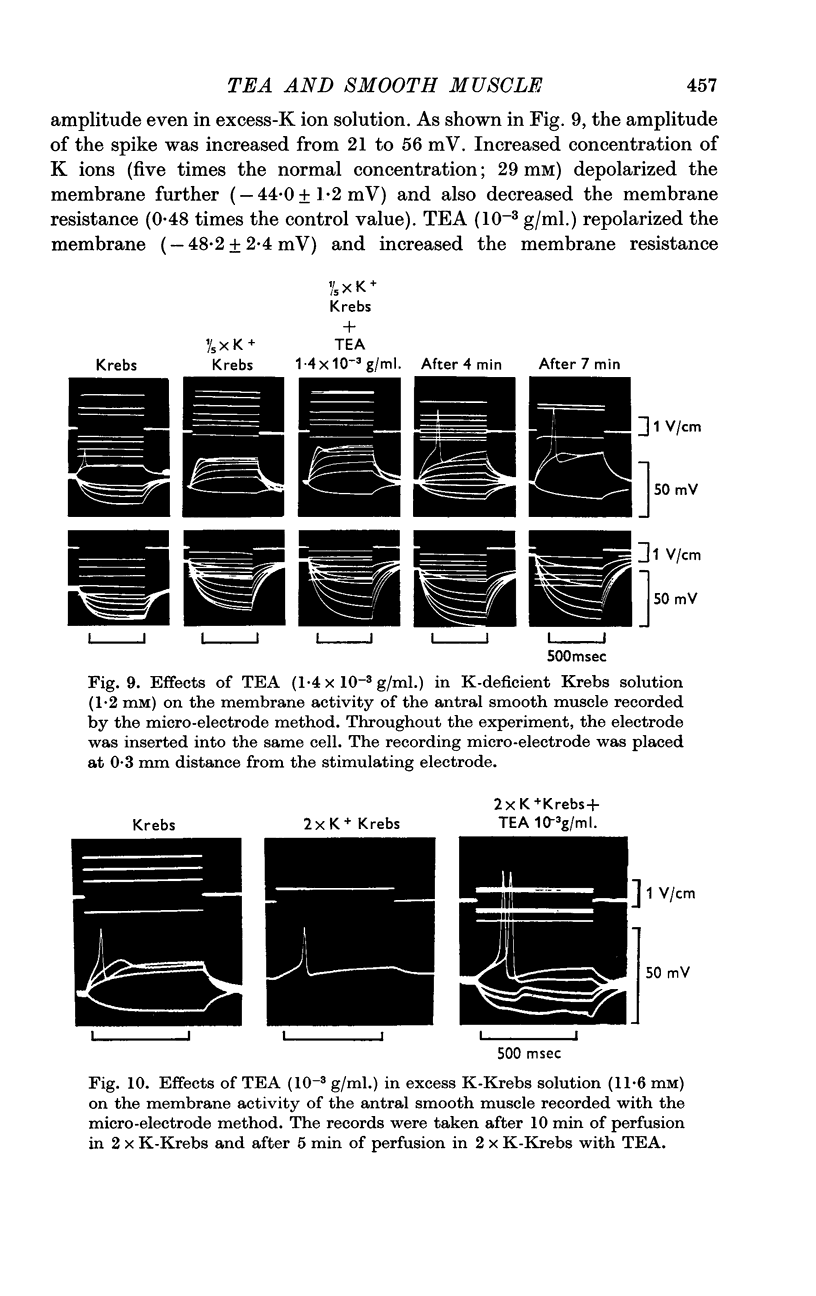
5. In Na-free (Tris or sucrose) solution, in K-deficient and excess-K solutions, TEA (1-1·5 × 10-3 g/ml.) suppressed the rectifying property of the membrane and enhanced the spike amplitude.
6. Atropine (10-6 g/ml.) had no effect on the enhancement of the spike amplitude produced by TEA.
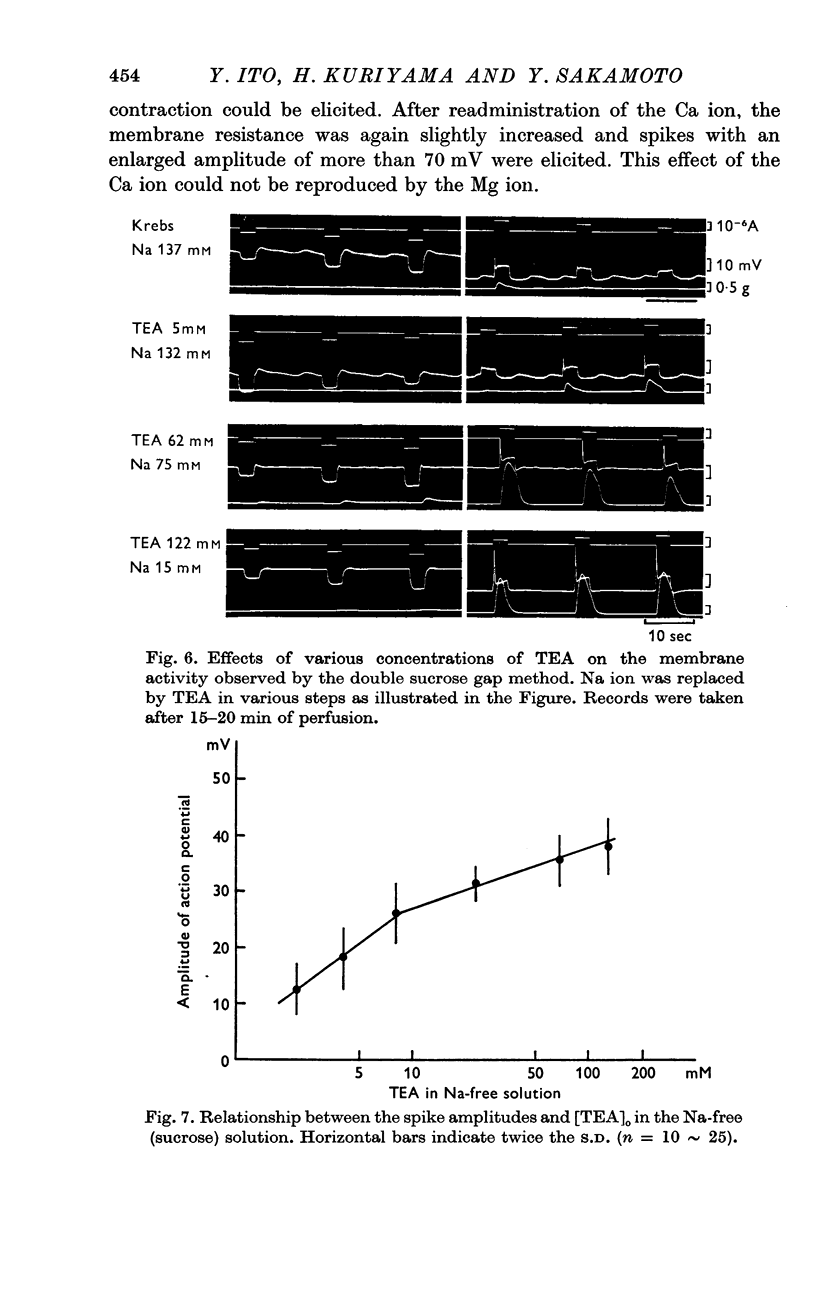
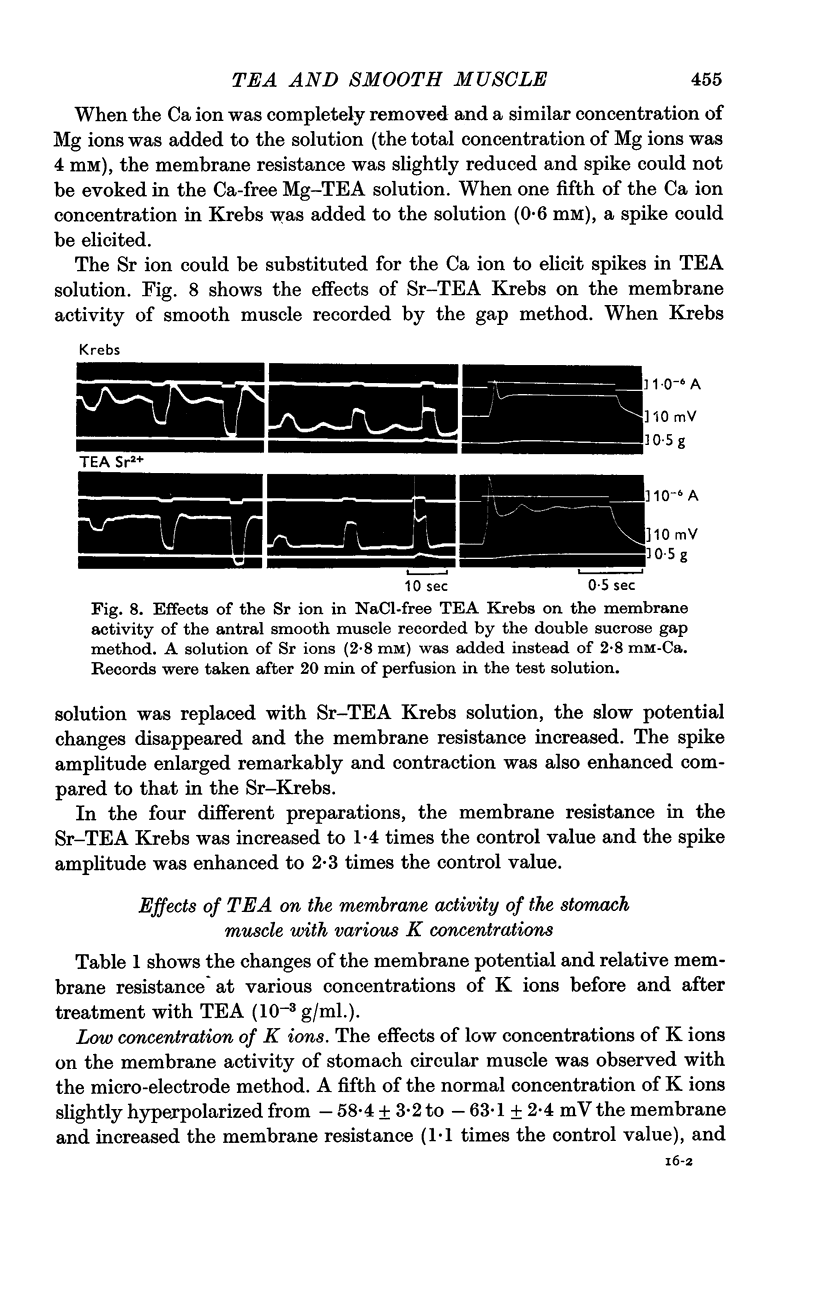
7. The minimum concentration of Ca ions required for the effect of TEA on the spike amplitude was one fifth of the normal concentration. TEA also enhanced the spike amplitude in Sr—Krebs.
8. The possible role of TEA on the membrane activity is considered to be due to suppression of the K conductance when the membrane is depolarized. Alternative possible roles of TEA on the spike amplitude are also discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B. Tetraethylammonium-induced contractions of frog's skeletal muscle. 3. Mechanism of action by calcium release. Can J Physiol Pharmacol. 1967 Sep;45(5):845–855. doi: 10.1139/y67-099. [DOI] [PubMed] [Google Scholar]
- Bergmann C., Nonner W., Stämpfli R. Sustained spontaneous activity of Ranvier nodes induced by the combined actions of TEA and lack of calcium. Pflugers Arch. 1968;302(1):24–37. doi: 10.1007/BF00586780. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effect of calcium, barium and manganese on the action of adrenaline in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):121–136. doi: 10.1098/rspb.1969.0015. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Voltage-current relations in nerve cell membrane of Onchidium verruculatum. J Physiol. 1959 Oct;148:161–179. doi: 10.1113/jphysiol.1959.sp006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. The effect of tetraethylammonium chloride on the muscle membrane examined with an intracellular microelectrode. J Physiol. 1955 Sep 28;129(3):513–527. doi: 10.1113/jphysiol.1955.sp005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Tetraethylammonium ions: effect of presynaptic injection on synaptic transmission. Science. 1967 Mar 10;155(3767):1257–1259. doi: 10.1126/science.155.3767.1257. [DOI] [PubMed] [Google Scholar]
- Liu J., Prosser C. L., Job D. D. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969 Nov;217(5):1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966 Mar;49(4):629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. II. The action potential and excitation. Pharmacol Rev. 1958 Jun;10(2):165–273. [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., INOMATA H. Effect of tetraethyl ammonium ion on the electrical activity of smooth muscle cell. Nature. 1963 Mar 2;197:908–909. doi: 10.1038/197908a0. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Stämpfli R. Die Wirkung von Tetraäthylammoniumchlorid auf den einzelnen Ranvierschen Schnürring. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(4):311–325. [PubMed] [Google Scholar]
- TASAKI I., HAGIWARA S. Capacity of muscle fiber membrane. Am J Physiol. 1957 Mar;188(3):423–429. doi: 10.1152/ajplegacy.1957.188.3.423. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHIZU Y. The effect of TEA on the electrical activities of spinal motoneurons. Jpn J Physiol. 1959 Sep 15;9:311–321. doi: 10.2170/jjphysiol.9.311. [DOI] [PubMed] [Google Scholar]


