Abstract
1. Observations have been made on the changes in optical retardation accompanying the passage of impulses along crab leg nerves and squid giant axons.
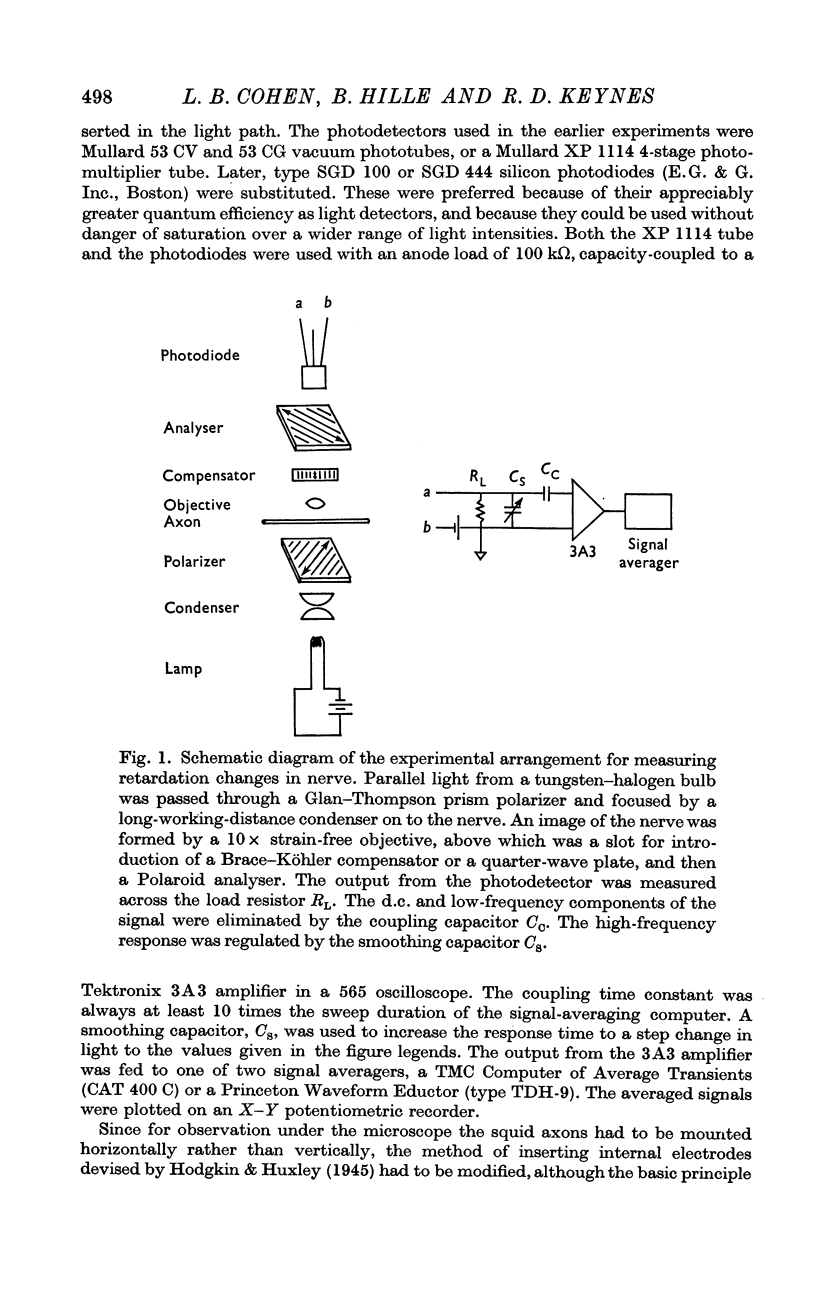
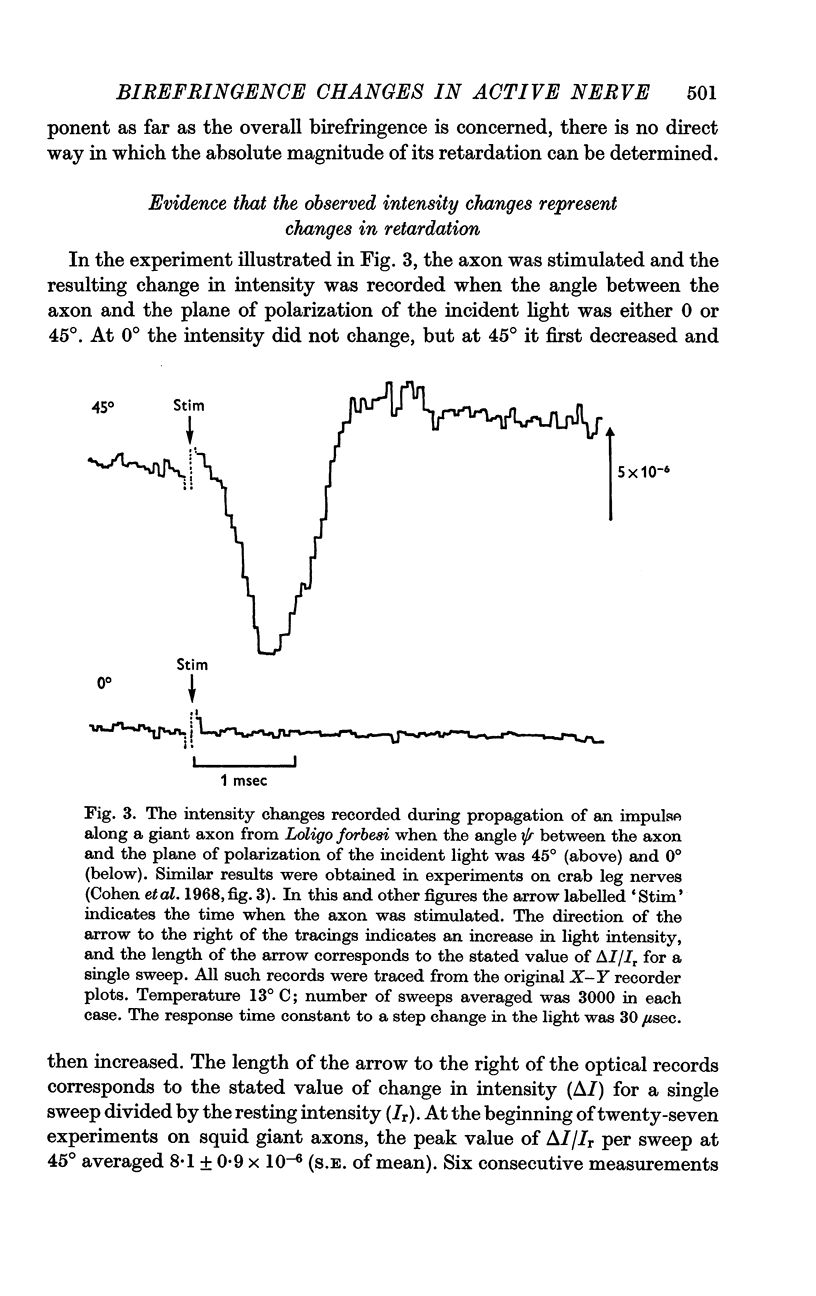
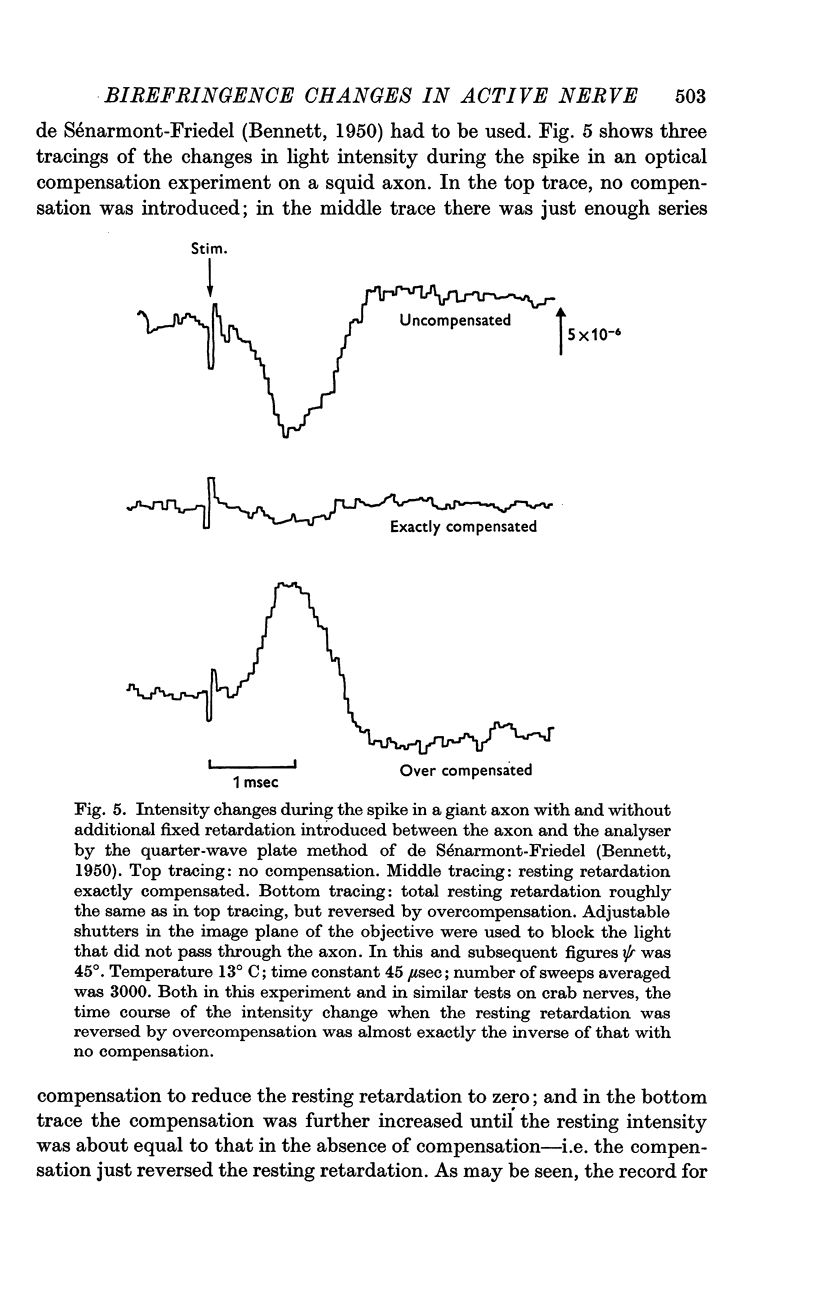
2. The nerves were mounted on the stage of a polarizing microscope, at 45° to the planes of polarization and analysis, brightly illuminated with white light. During the nerve impulse the intensity of the light passing the analyser decreased temporarily by 1 part in 103-106. Signal-averaging techniques were used to obtain an acceptable ratio of signal to noise.
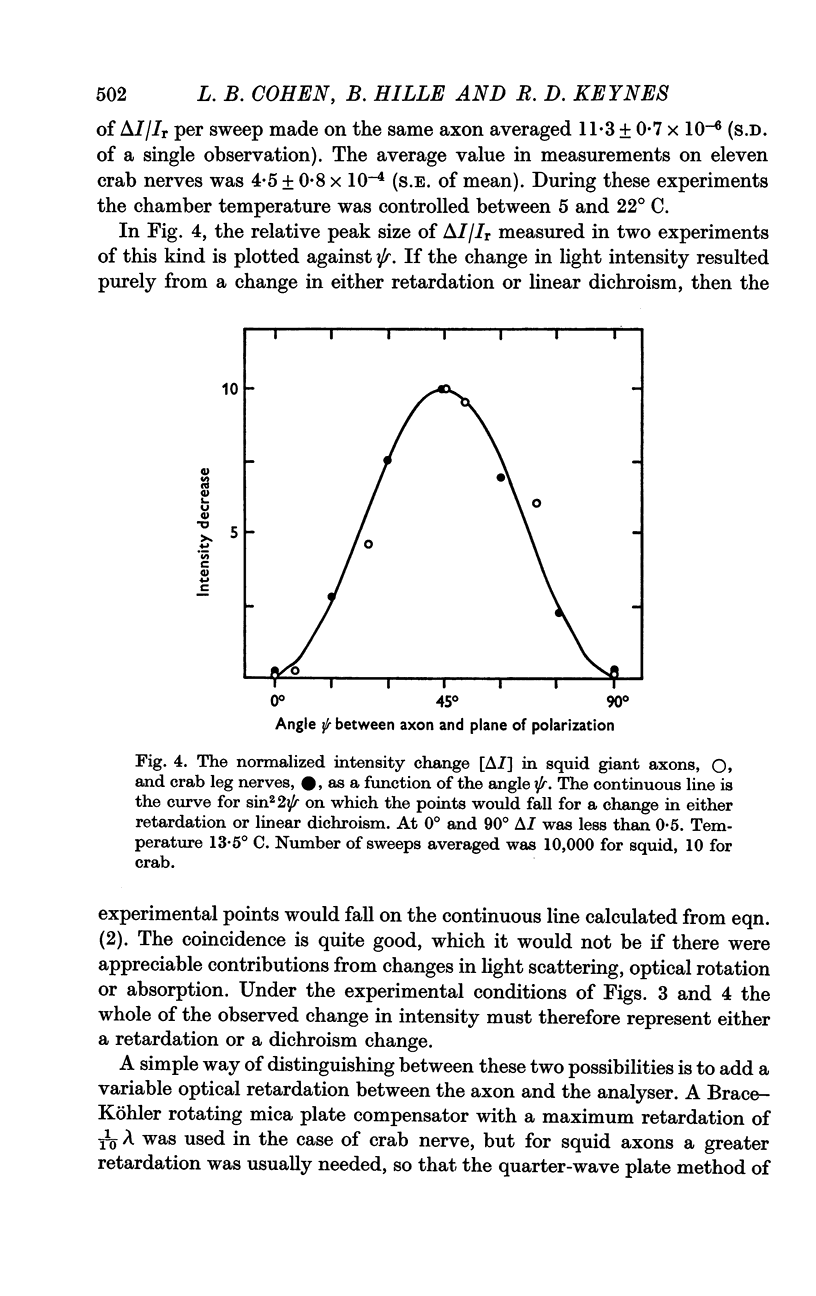
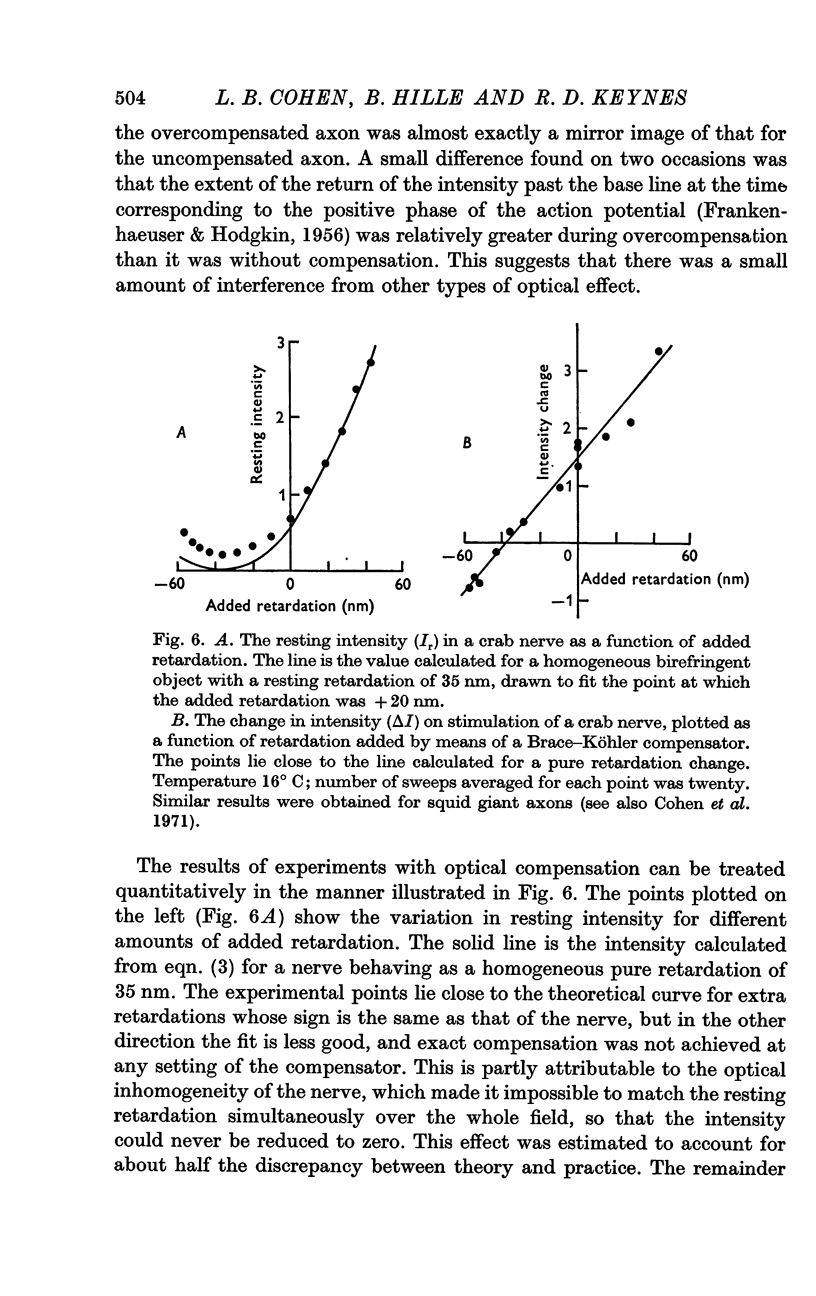
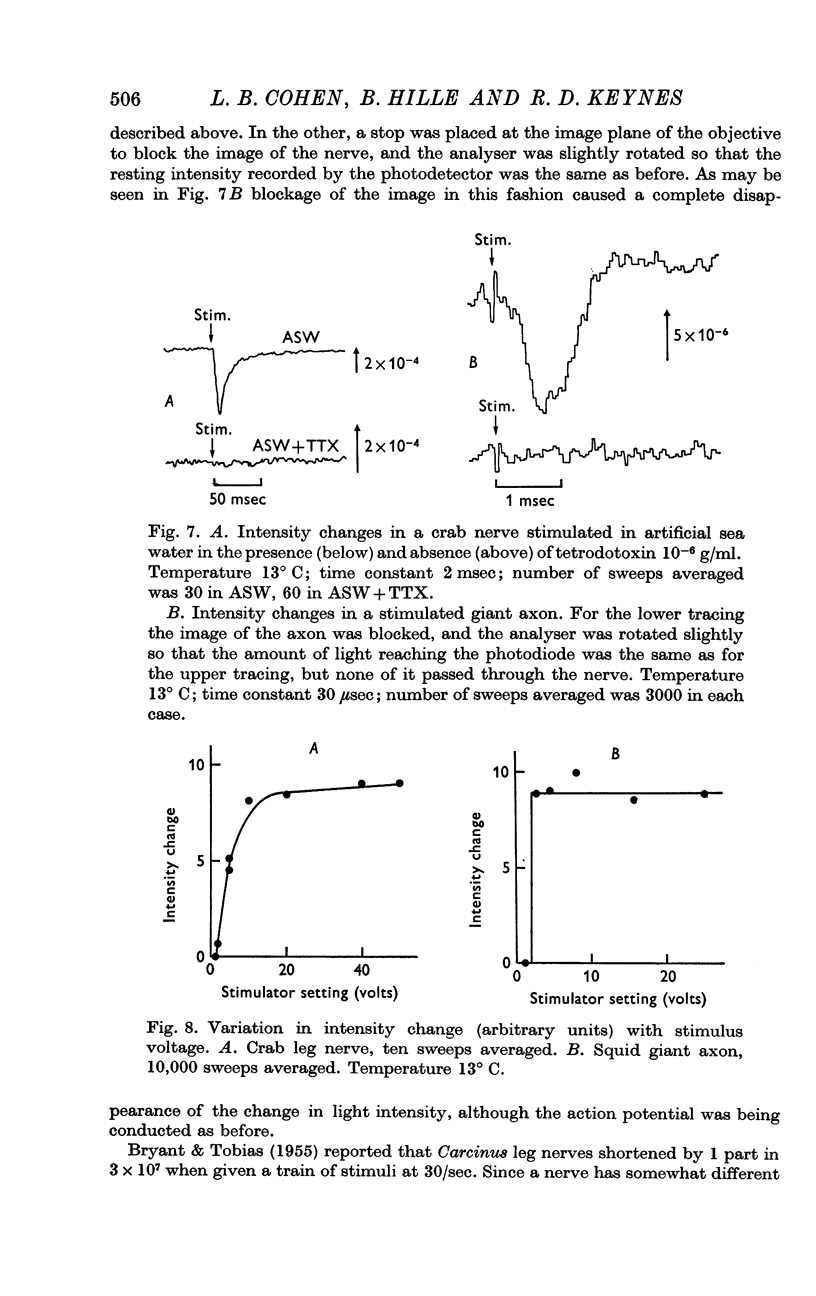
3. The changes in light intensity recorded under these conditions were shown to arise almost entirely from alterations in retardation, with little or no interference from scattering, absorption, linear dichroism or optical rotation effects; the occurrence of stimulus and coupling artifacts was also ruled out.
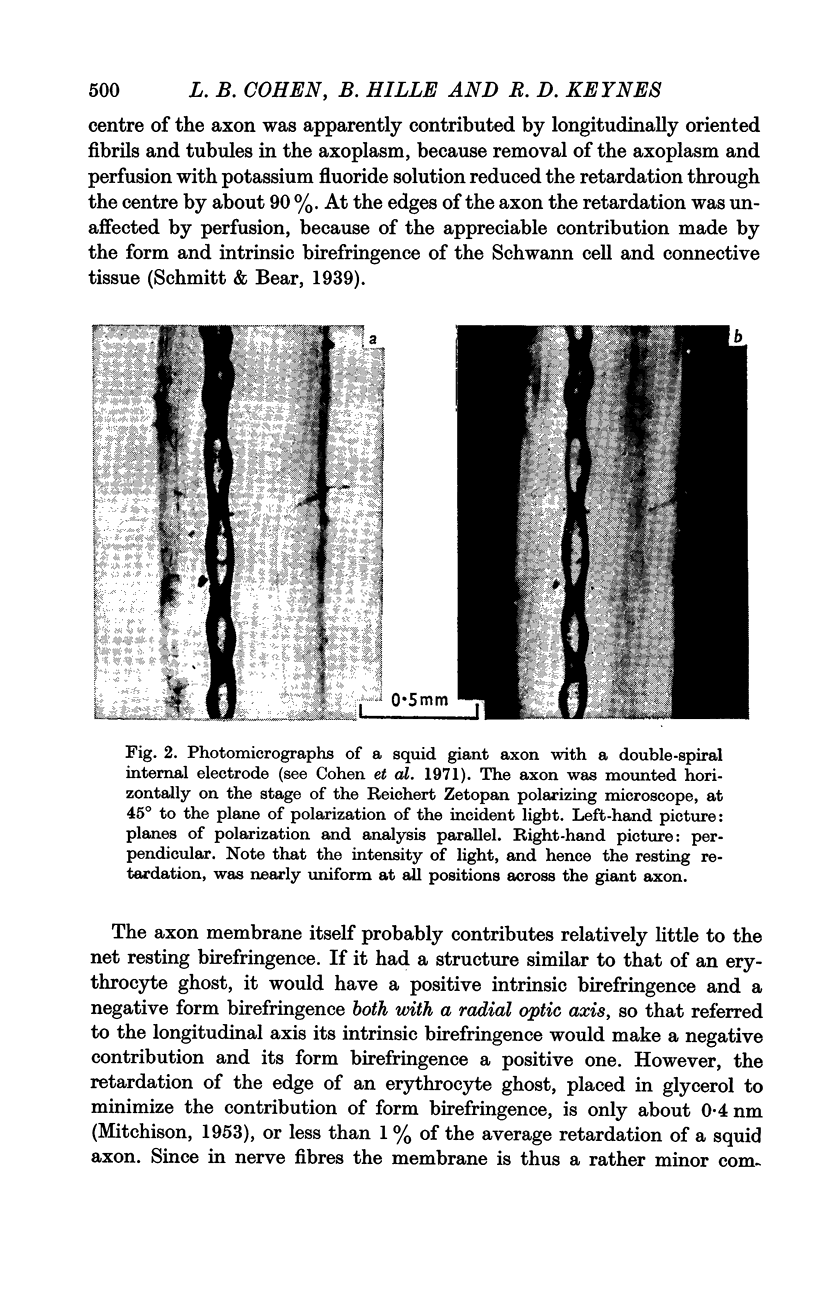
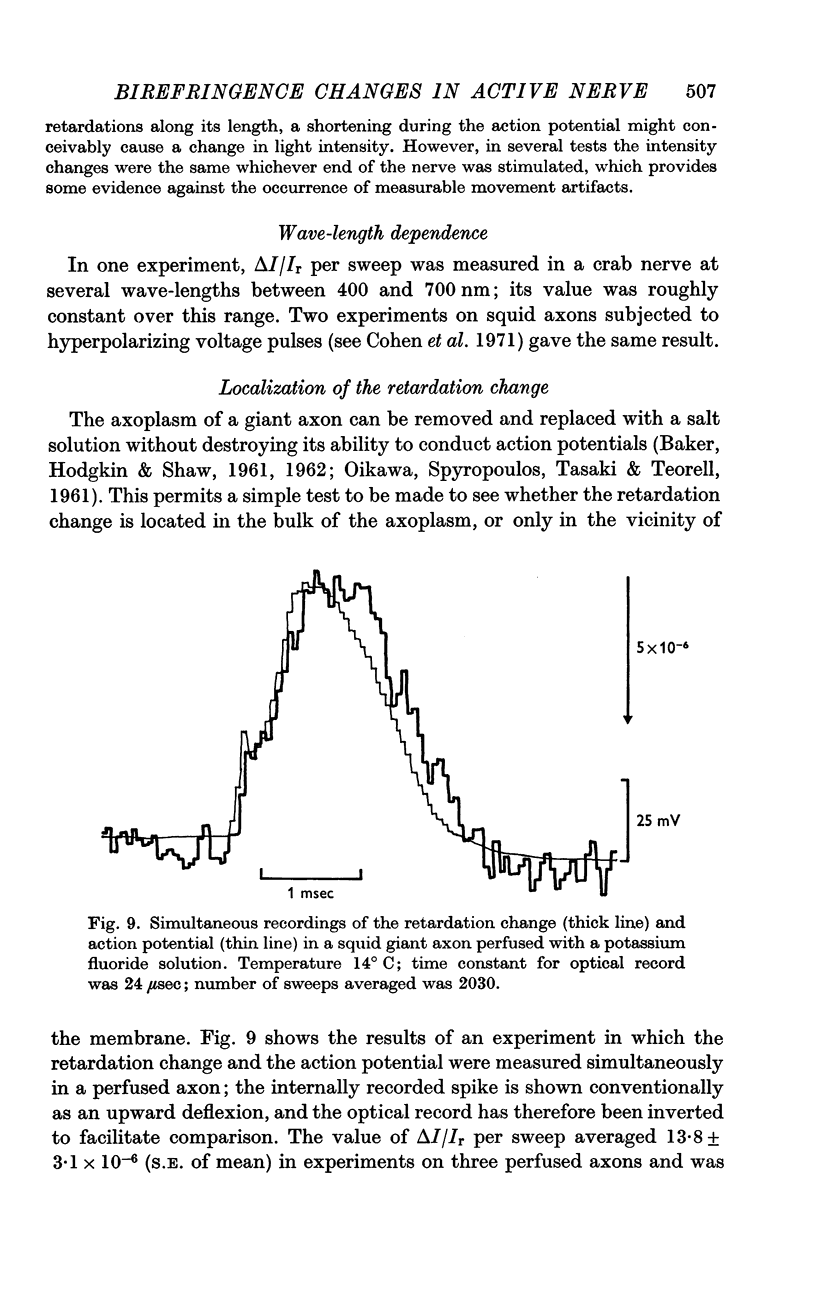
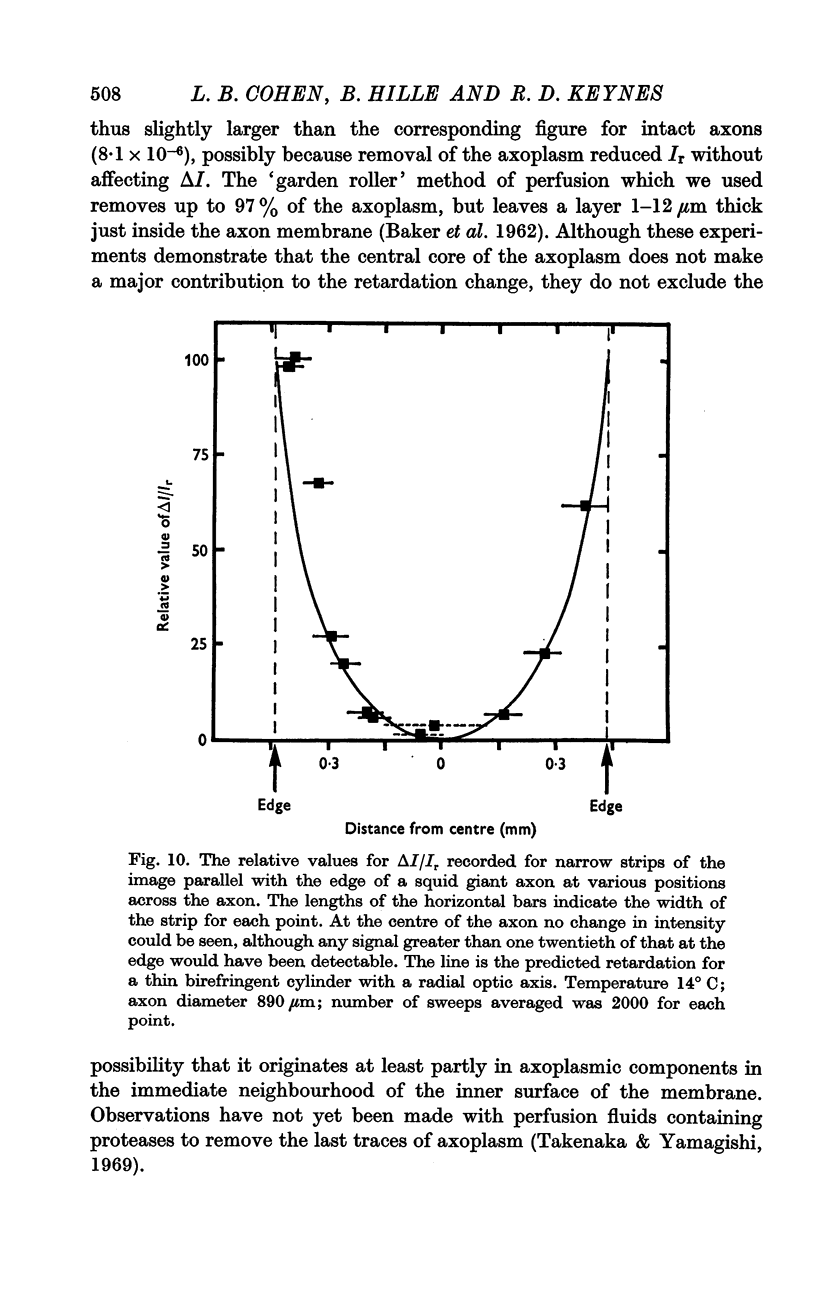
4. In the squid giant axon, the retardation change was shown to be located in a thin cylinder immediately surrounding the axoplasm, and to have a radially oriented optic axis.
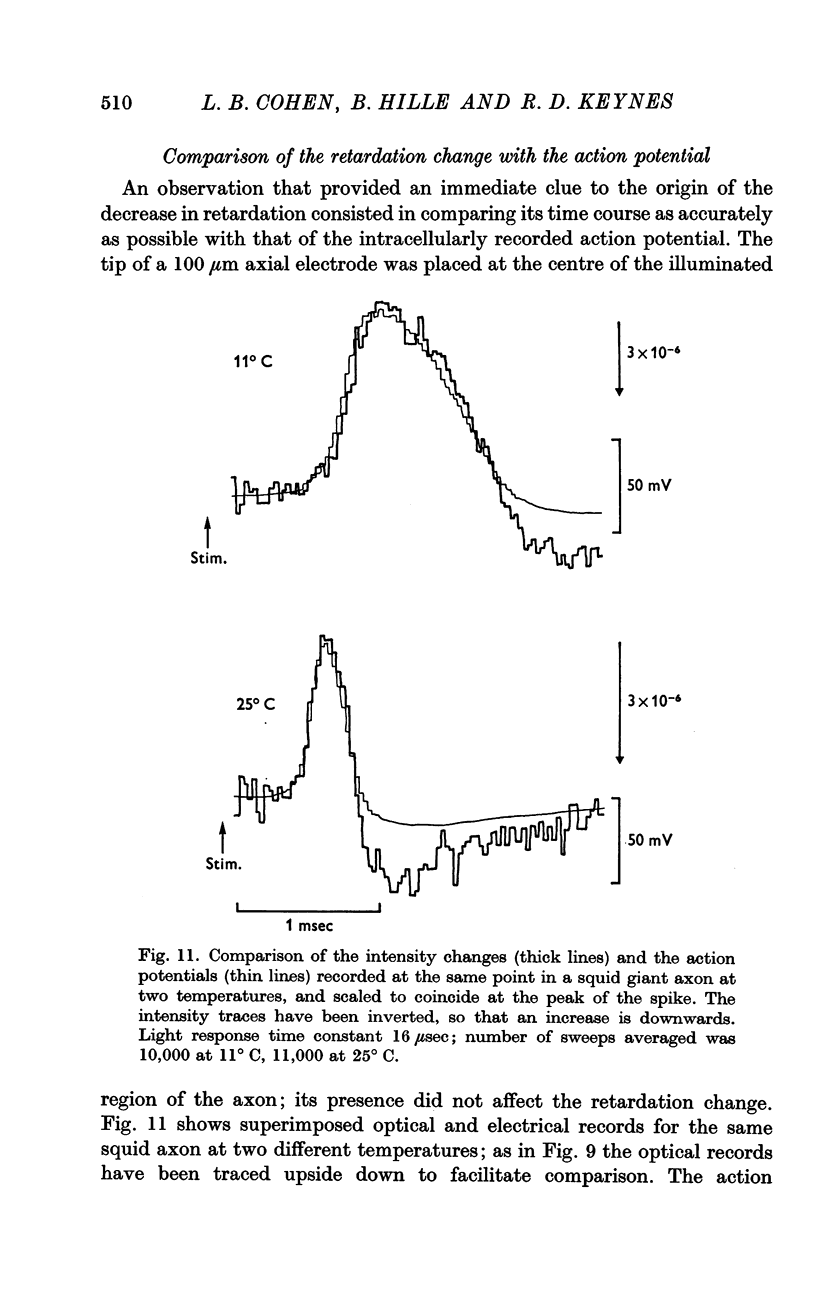
5. The time course of the decrease in optical retardation was very similar to that of the action potential recorded with an intracellular electrode, suggesting that the retardation closely followed the electrical potential across the membrane.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- BRYANT S. H., TOBIAS J. M. Optical and mechanical concomitants of activity in Carcinus nerve. I. Effect of sodium azide on the optical response. II. Shortening of the nerve with activity. J Cell Physiol. 1955 Aug;46(1):71–95. doi: 10.1002/jcp.1030460105. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berestovskii G. N., Liberman E. A., Lunevskii V. Z., Frank G. M. Opticheskie issledovaniia izmeneniia struktury membrany nerve pri prokhozhdenii nervnogo impul'sa. Biofizika. 1970 Jan-Feb;15(1):62–68. [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Light scattering and birefringence changes during activity in the electric organ of electrophorus electricus. J Physiol. 1969 Aug;203(2):489–509. doi: 10.1113/jphysiol.1969.sp008876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- FINEAN J. B., BURGE R. E. THE DETERMINATION OF THE FOURIER TRANSFORM OF THE MYELIN LAYER FROM A STUDY OF SWELLING PHENOMENA. J Mol Biol. 1963 Dec;7:672–682. doi: 10.1016/s0022-2836(63)80115-1. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa K. The depolarization of crustacean nerve by stimulation or oxygen want. J Physiol. 1929 Jul 25;67(4):325–342. doi: 10.1113/jphysiol.1929.sp002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the opacity of a crustacean nerve trunk and its relation to fibre diameter. J Physiol. 1950 Oct 16;111(3-4):283–303. doi: 10.1113/jphysiol.1950.sp004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The volume change resulting from stimulation of a giant nerve fibre. J Physiol. 1950 Oct 16;111(3-4):304–327. doi: 10.1113/jphysiol.1950.sp004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K., Keynes R. D. Opacity changes in stimulated nerve. J Physiol. 1949 May 15;108(3):278–281. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Huxley A. F. Resting and action potentials in single nerve fibres. J Physiol. 1945 Oct 15;104(2):176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIKAWA T., SPYROPOULOS C. S., TASAKI I., TEORELL T. Methods for perfusing the giant axon of Loligo pealii. Acta Physiol Scand. 1961 Jun;52:195–196. doi: 10.1111/j.1748-1716.1961.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O., Schmitt O. H. Partial excitation and variable conduction in the squid giant axon. J Physiol. 1940 Mar 14;98(1):26–46. doi: 10.1113/jphysiol.1940.sp003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka T., Yamagishi S. Morphology and electrophysiological properties of squid giant axons perfused intracellularly with protease solution. J Gen Physiol. 1969 Jan;53(1):81–96. doi: 10.1085/jgp.53.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]



