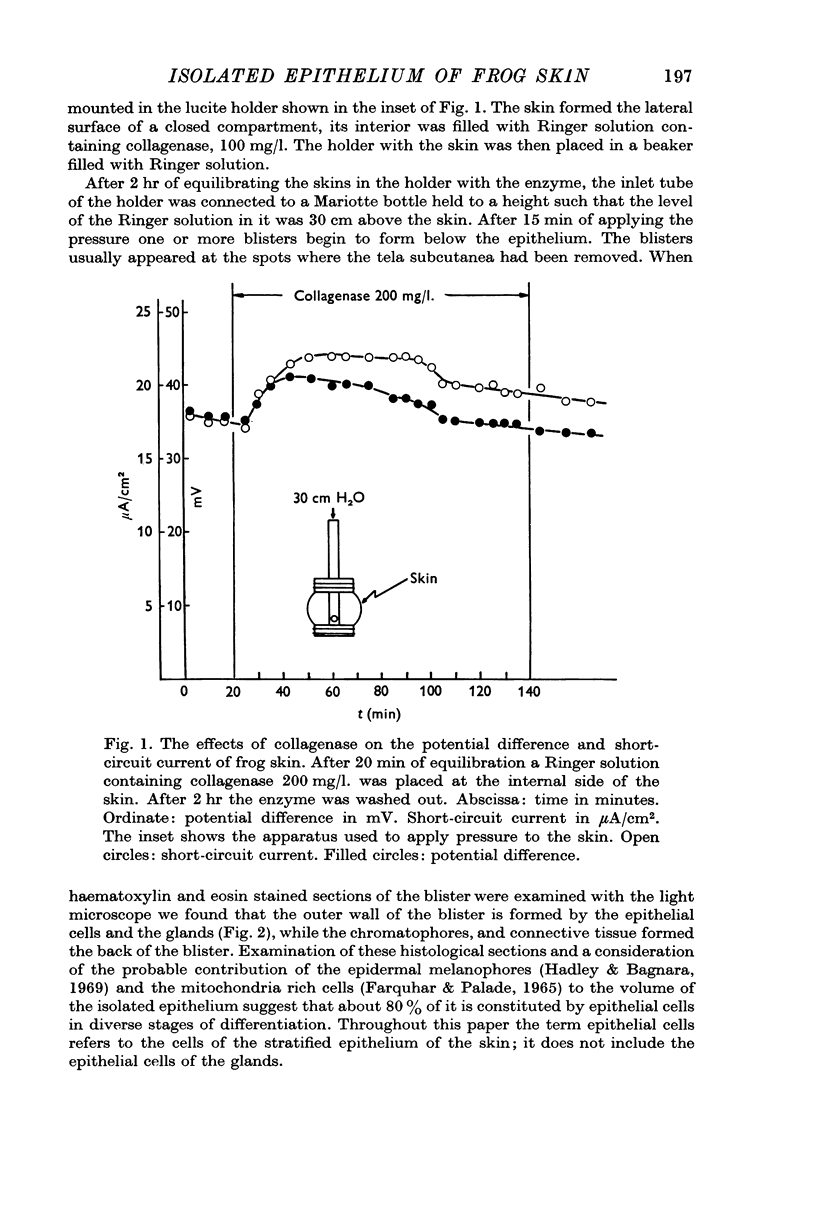
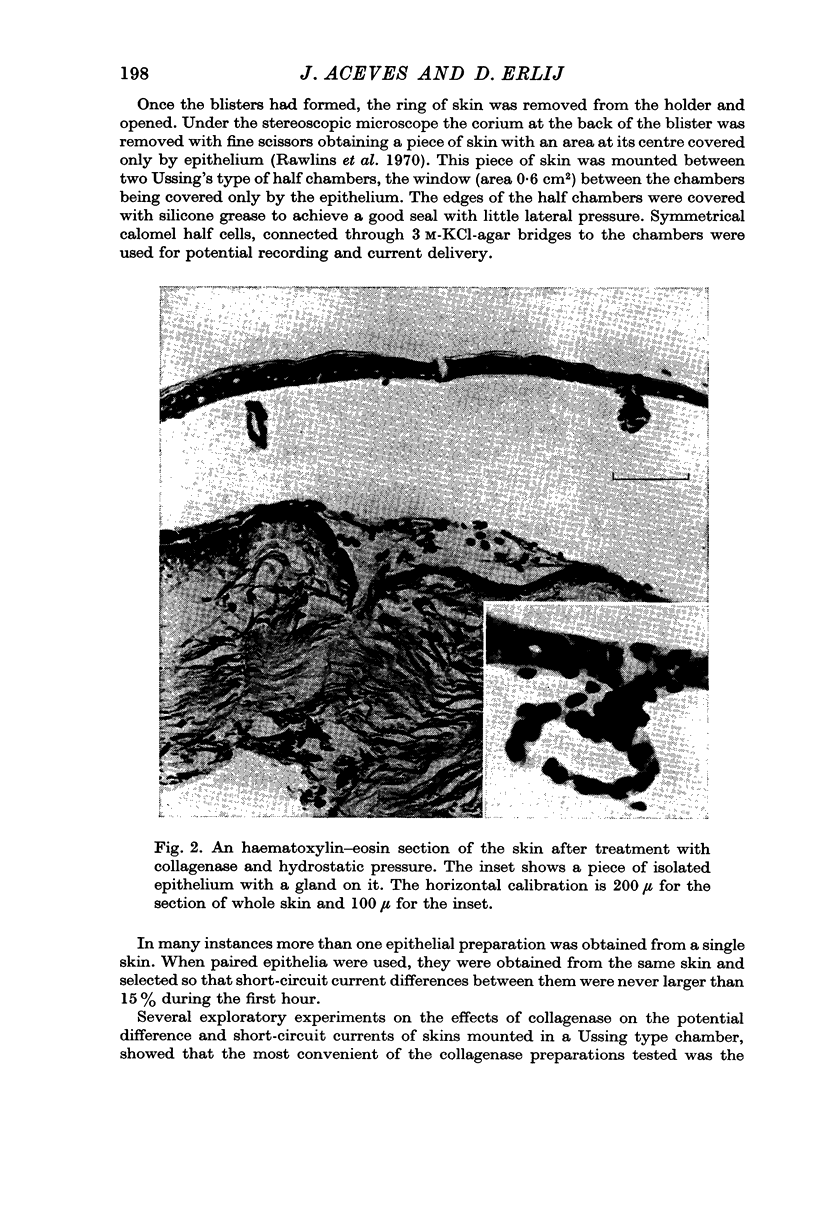
Abstract
1. A method to separate the epithelium from the underlying layers of the frog skin is described. The method is based on the combined use of collagenase and hydrostatic pressures.
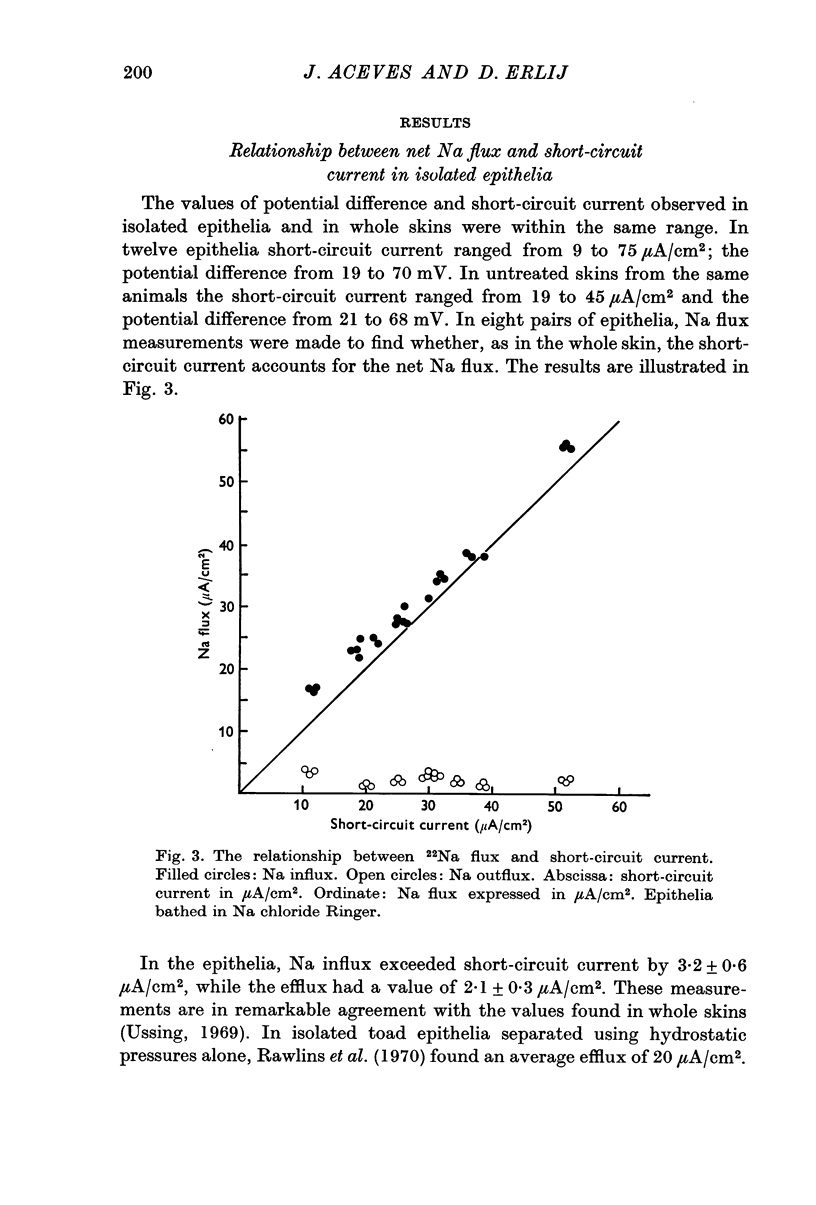
2. The potential difference and the short-circuit current values of isolated epithelia and whole skins are similar. Na net flux and short-circuit current are equivalent.
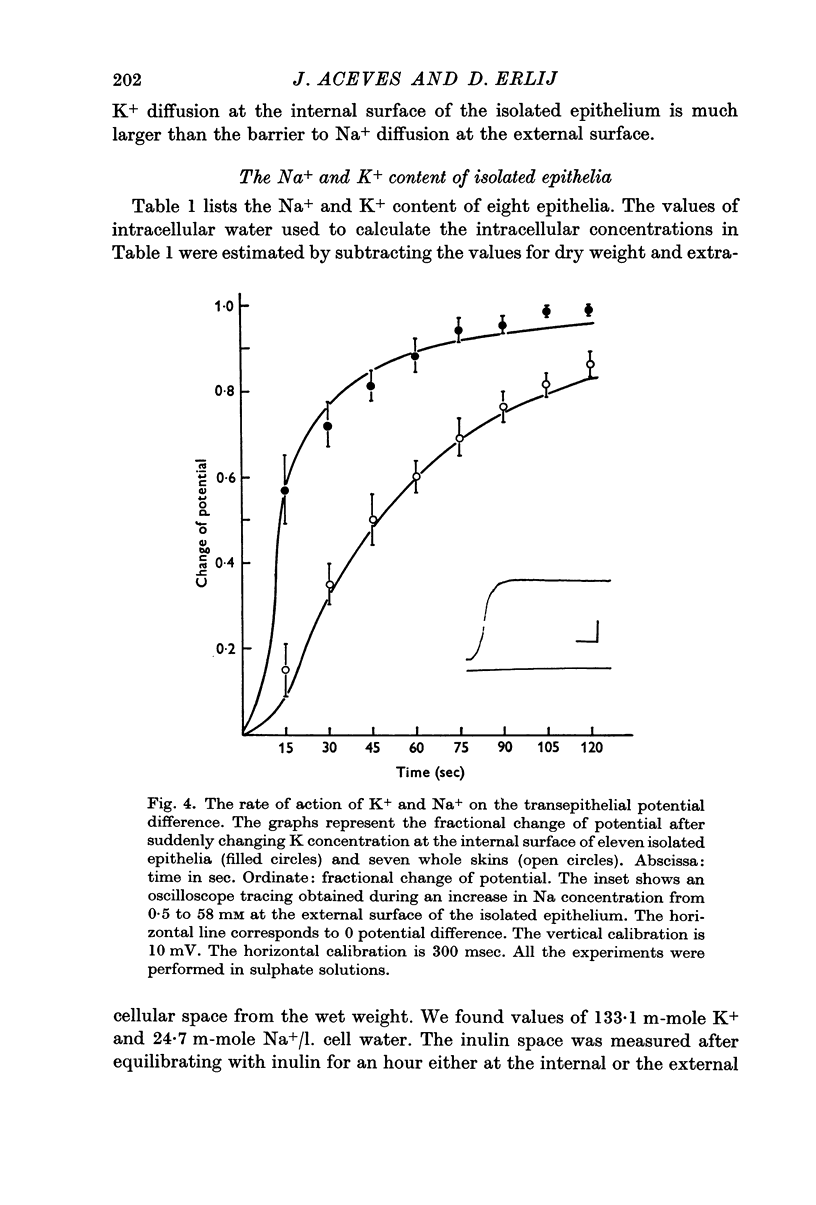
3. The time course of changes in potential following rapid changes in composition of the bathing solutions shows that the barrier to K diffusion at the internal surface of the isolated epithelium is larger than the barrier to Na diffusion at the external surface.
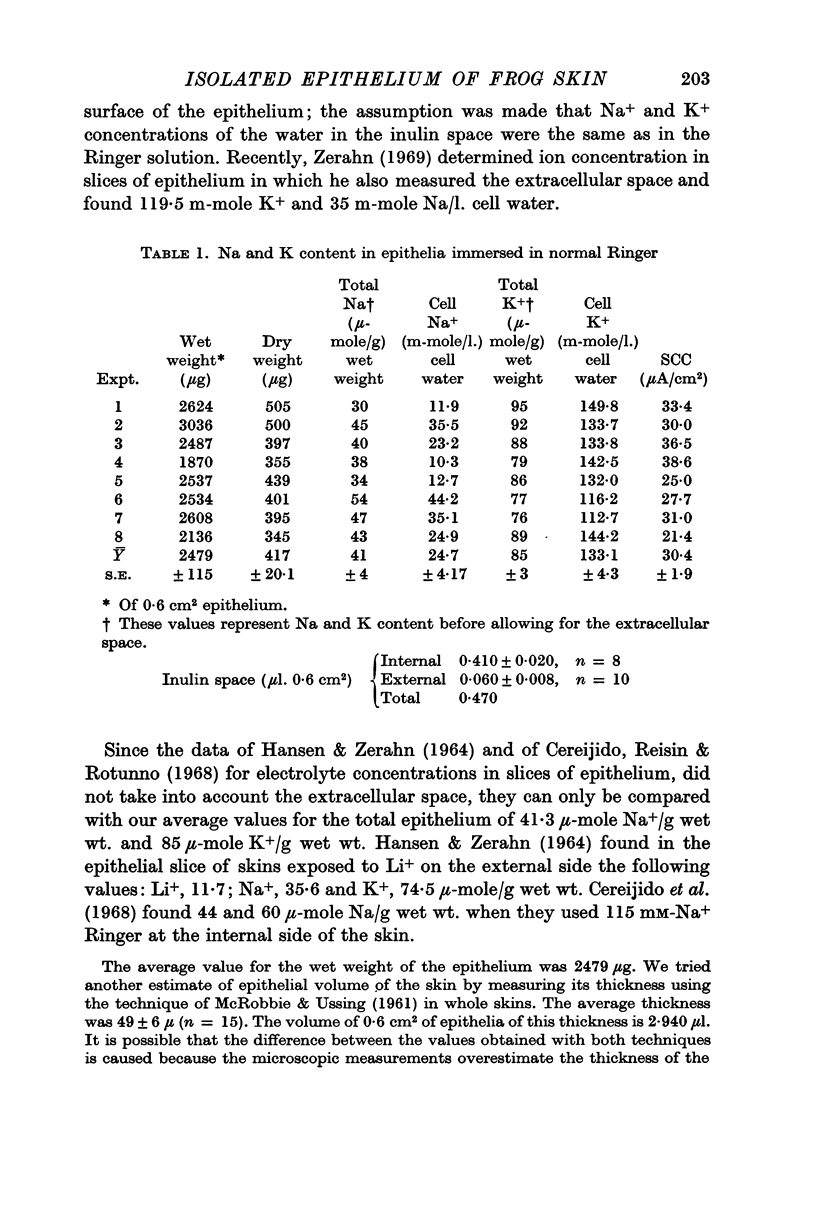
4. In the isolated epithelium there are 133 m-mole K+ and 24·7 m-mole Na/l. cellular water. The amount of extracellular water was considered to be equal to the inulin space.
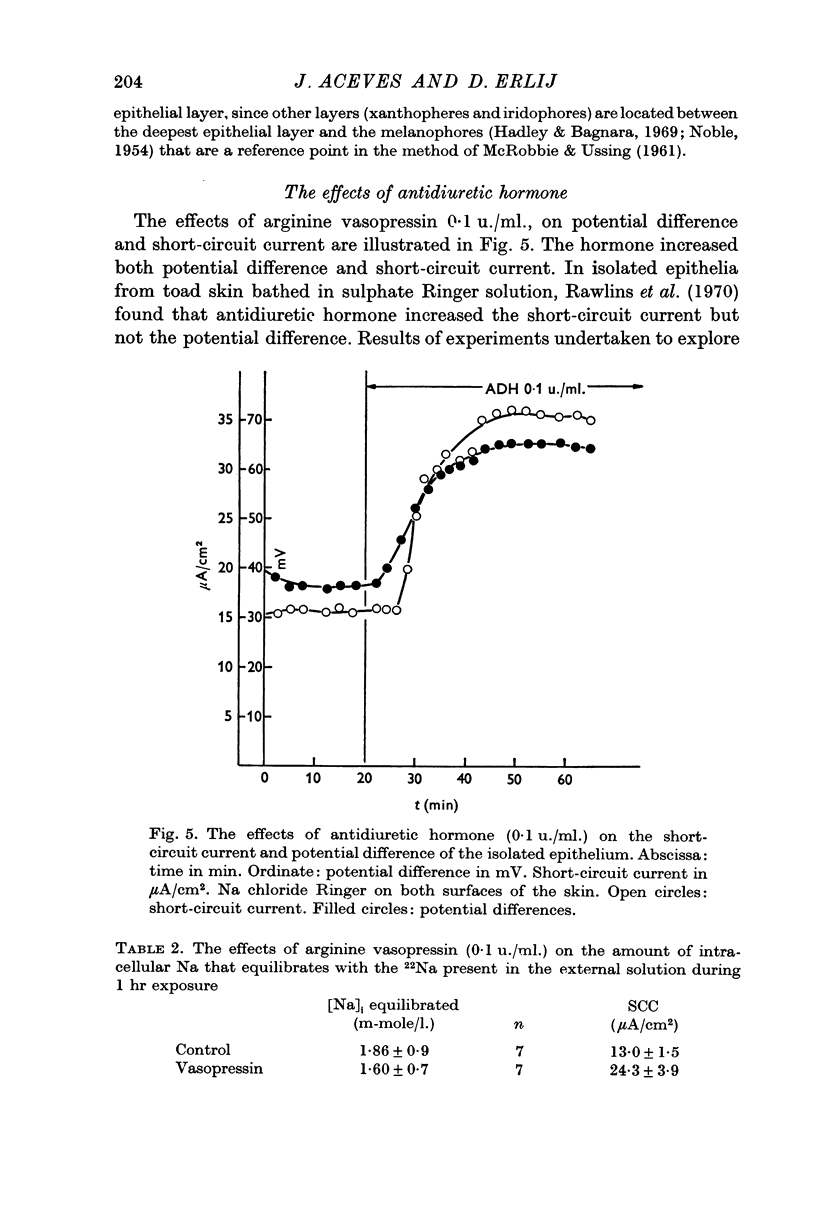
5. Arginine vasopressin (0·1 u./ml.) markedly increased short-circuit current and potential difference in isolated epithelia. The amount of Na in the epithelium that equilibrated with Na in the external solution was not increased by the hormone.
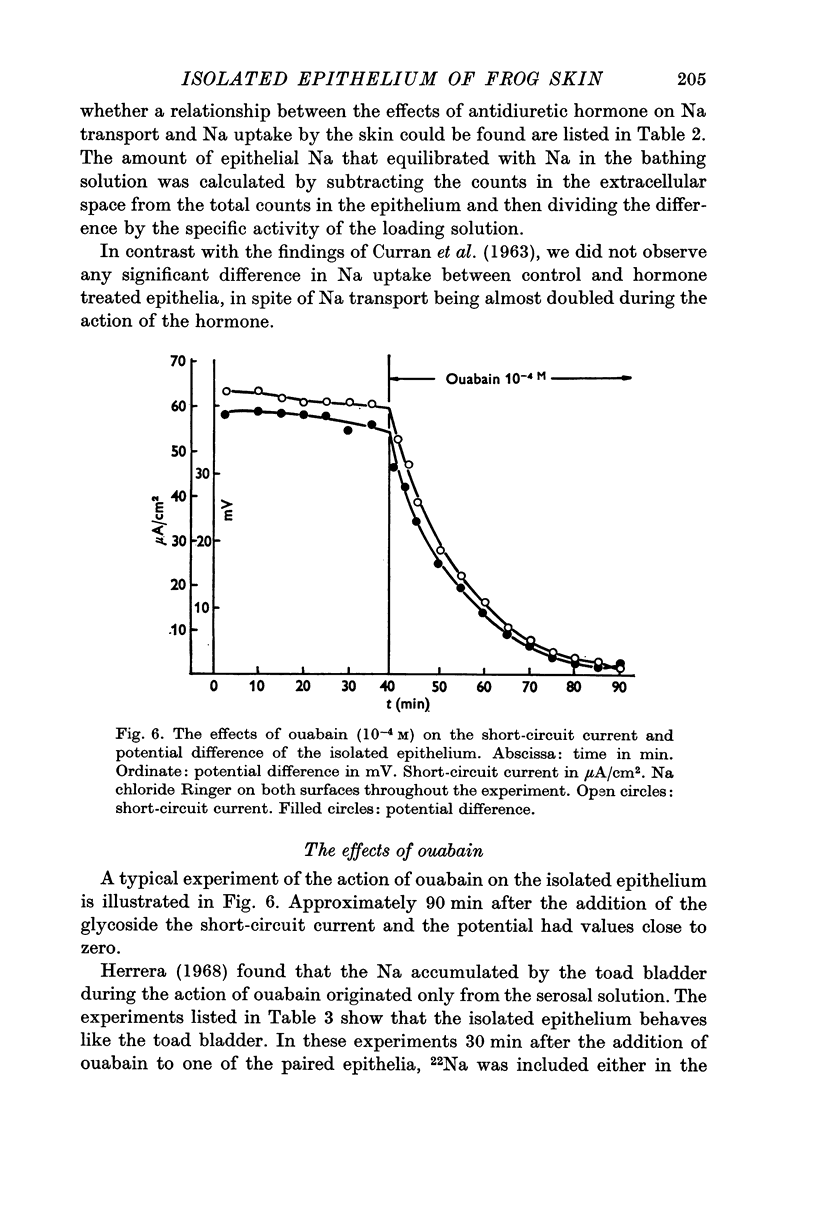
6. Ouabain (10-4 M) reduced short circuit current and potential difference to values close to zero. The ouabain treated epithelia contained an increased amount of Na originating in the internal solution. On the other hand the amount of Na that originated from the external solution was not increased.
7. The amount of epithelial Na that equilibrated with Na in the external solution was 0·009 μ-equiv/cm2. This figure is about ten times smaller than the values found in whole skins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B., USSING H. H. Solvent drag on non-electrolytes during osmotic flow through isolated toad skin and its response to antidiuretic hormone. Acta Physiol Scand. 1957 Jun 8;39(2-3):228–239. doi: 10.1111/j.1748-1716.1957.tb01425.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN B., ZERAHN K. METHOD FOR NON-DESTRUCTIVE DETERMINATION OF THE SODIUM TRANSPORT POOL IN FROG SKIN WITH RADIOSODIUM. Acta Physiol Scand. 1963 Dec;59:319–329. doi: 10.1111/j.1748-1716.1963.tb02747.x. [DOI] [PubMed] [Google Scholar]
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. P., Aiyawar R. M., Berry E. R., Huf E. G. Electrolytes in frog skin secretions. Comp Biochem Physiol. 1967 Oct;23(1):213–223. doi: 10.1016/0010-406x(67)90489-6. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Reisin I., Rotunno C. A. The effect of sodium concentration on the content and distribution of sodium in the frog skin. J Physiol. 1968 May;196(1):237–253. doi: 10.1113/jphysiol.1968.sp008504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. Transport and distribution of sodium across frog skin. J Physiol. 1967 Jun;190(3):481–497. doi: 10.1113/jphysiol.1967.sp008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Painter E. Independent action of antidiuretic hormone, theophylline and cyclic 3',5'-adenosine monophosphate on cell membrane permeability in frog skin. J Physiol. 1968 Dec;199(3):593–612. doi: 10.1113/jphysiol.1968.sp008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty J., House C. R. Unstirred layers in frog skin. J Physiol. 1966 Jan;182(1):66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. Calcium effects in the electrical excitability of "split" frog skin. Biochim Biophys Acta. 1968 Apr 29;150(3):482–487. doi: 10.1016/0005-2736(68)90148-x. [DOI] [PubMed] [Google Scholar]
- HANSEN H. H., ZERAHN K. CONCENTRATION OF LITHIUM, SODIUM AND POTASSIUM IN EPITHELIAL CELLS OF THE ISOLATED FROG SKIN DURING ACTIVE TRANSPORT OF LITHIUM. Acta Physiol Scand. 1964 Jan-Feb;60:189–196. doi: 10.1111/j.1748-1716.1964.tb02882.x. [DOI] [PubMed] [Google Scholar]
- HOSHIKO T., LINDLEY B. D., EDWARDS C. DIFFUSION DELAY IN FROG SKIN CONNECTIVE TISSUE: A SOURCE OF ERROR IN TRACER INVESTIGATIONS. Nature. 1964 Feb 29;201:932–933. doi: 10.1038/201932a0. [DOI] [PubMed] [Google Scholar]
- HOSHIKO T., USSING H. H. The kinetics of Na24 flux across amphibian skin and bladder. Acta Physiol Scand. 1960 May 25;49:74–81. doi: 10.1111/j.1748-1716.1960.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Hadley M. E., Bagnara J. T. Integrated nature of chromatophore responses in the in vitro frog skin bioassay. Endocrinology. 1969 Jan;84(1):69–82. doi: 10.1210/endo-84-1-69. [DOI] [PubMed] [Google Scholar]
- Herrera F. C. Action of ouabain on bioelectric properties and ion content in toad urinary bladder. Am J Physiol. 1968 Jul;215(1):183–189. doi: 10.1152/ajplegacy.1968.215.1.183. [DOI] [PubMed] [Google Scholar]
- Imamura A., Takeda H., Sasaki N. The accumulation of sodium and calcium in a specific layer of frog skin. J Cell Physiol. 1965 Oct;66(2):221–225. doi: 10.1002/jcp.1030660208. [DOI] [PubMed] [Google Scholar]
- KIDDER G. W., 3rd, CEREIJIDO M., CURRAN P. F. TRANSIENT CHANGES IN ELECTRICAL POTENTIAL DIFFERENCES ACROSS FROG SKIN. Am J Physiol. 1964 Oct;207:935–940. doi: 10.1152/ajplegacy.1964.207.4.935. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Myhill J. Investigation of the effect of data error in the analysis of biological tracer data. Biophys J. 2008 Dec 31;7(6):903–911. doi: 10.1016/S0006-3495(67)86628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins F., Mateu L., Fragachan F., Whittembury G. Isolated toad skin epithelium: transport characteristics. Pflugers Arch. 1970;316(1):64–80. doi: 10.1007/BF00587897. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Voûte C. L., Ussing H. H. Some morphological aspects of active sodium transport. The epithelium of the frog skin. J Cell Biol. 1968 Mar;36(3):625–638. doi: 10.1083/jcb.36.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTEMBURY G. Action of antidiuretic hormone on the equivalent pore radius at both surfaces of the epithelium of the isolated toad skin. J Gen Physiol. 1962 Sep;46:117–130. doi: 10.1085/jgp.46.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerahn K. Nature and localization of the sodium pool during active transport in the isolated frog skin. Acta Physiol Scand. 1969 Nov;77(3):272–281. doi: 10.1111/j.1748-1716.1969.tb04572.x. [DOI] [PubMed] [Google Scholar]