Abstract
Electrical stimulation of the trigeminal nerve evokes a `short latency depolarization' (SLD) in the first order sensory neurones of the mesencephalic nucleus (MSN) of the Vth nerve in the rat. A series of experiments suggesting `electrotonic coupling' as the mechanism for this SLD is provided.
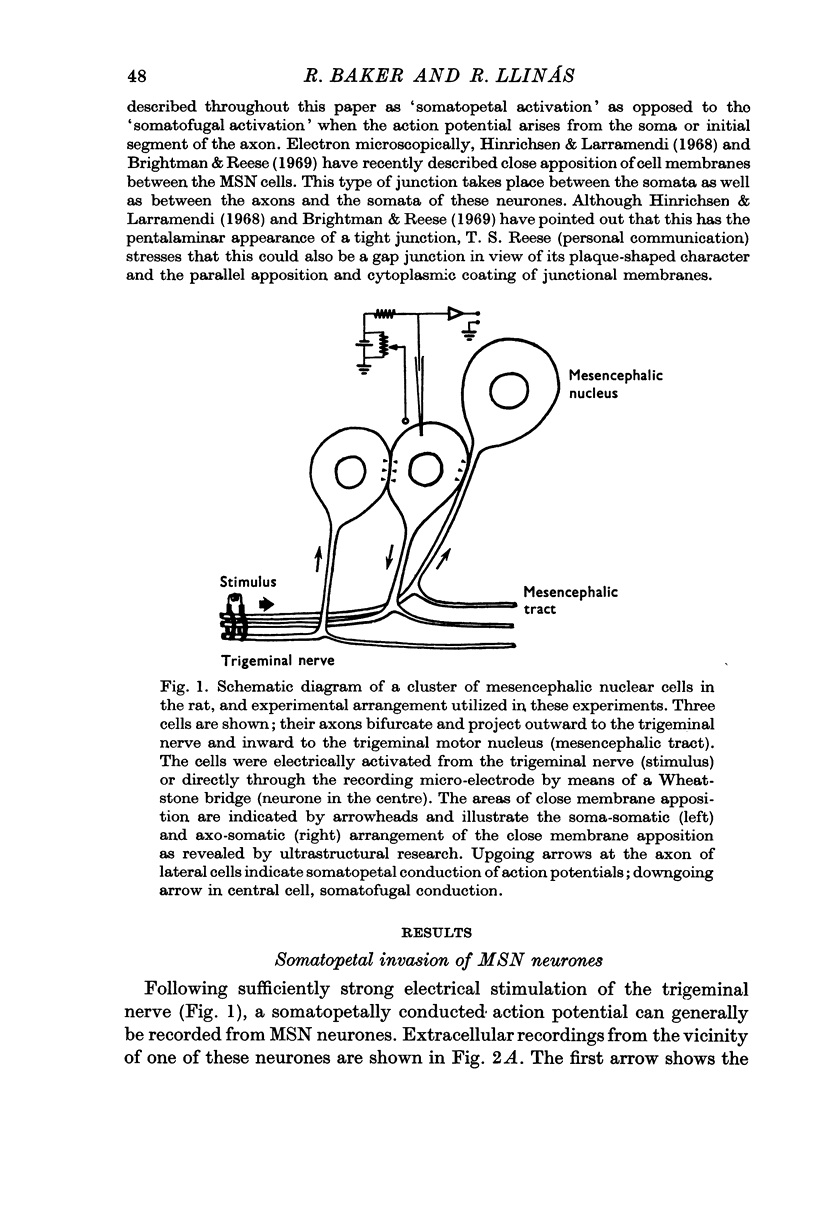
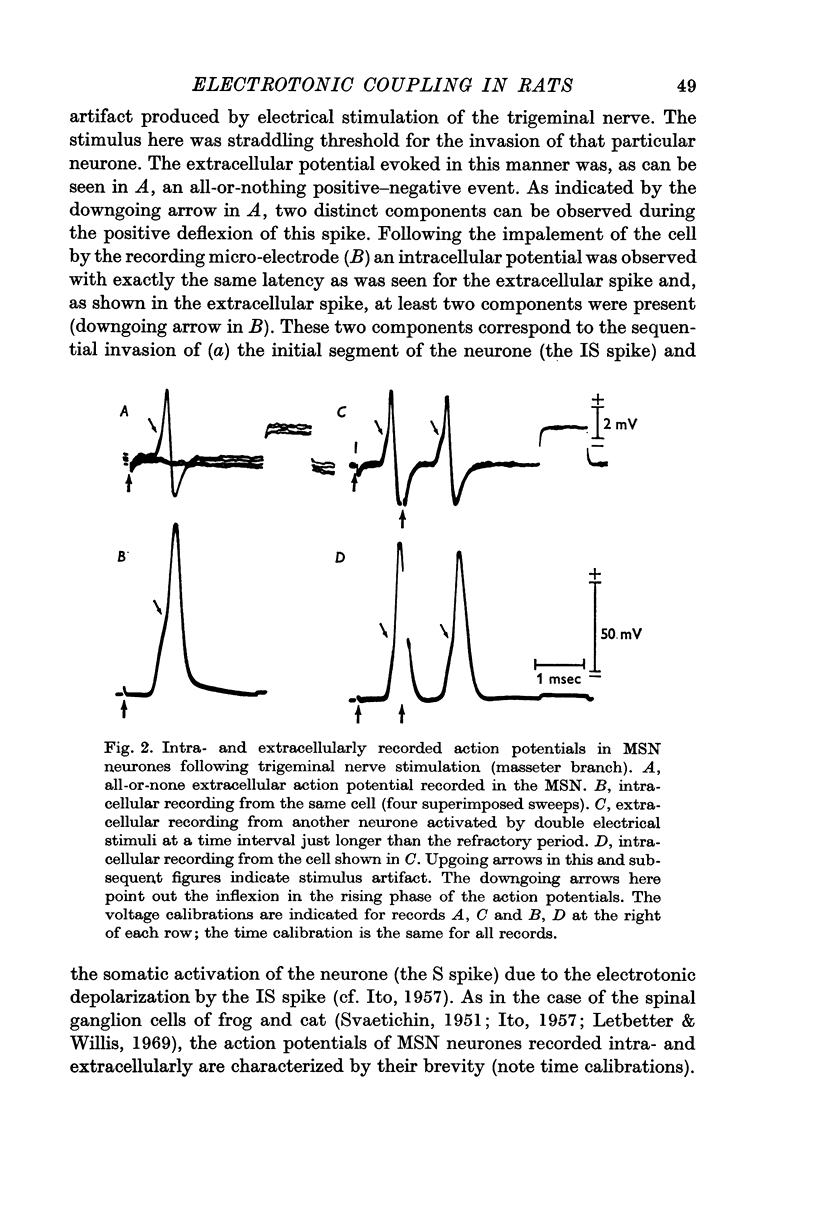
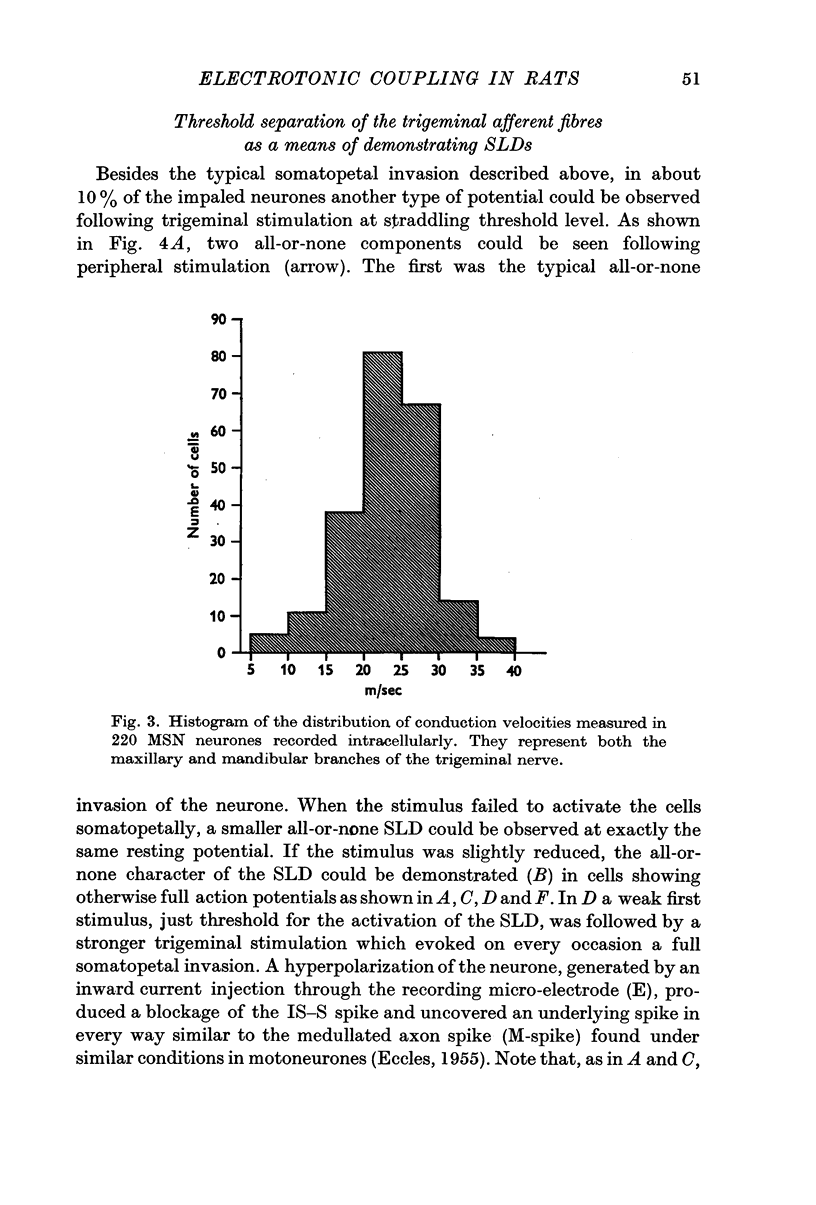
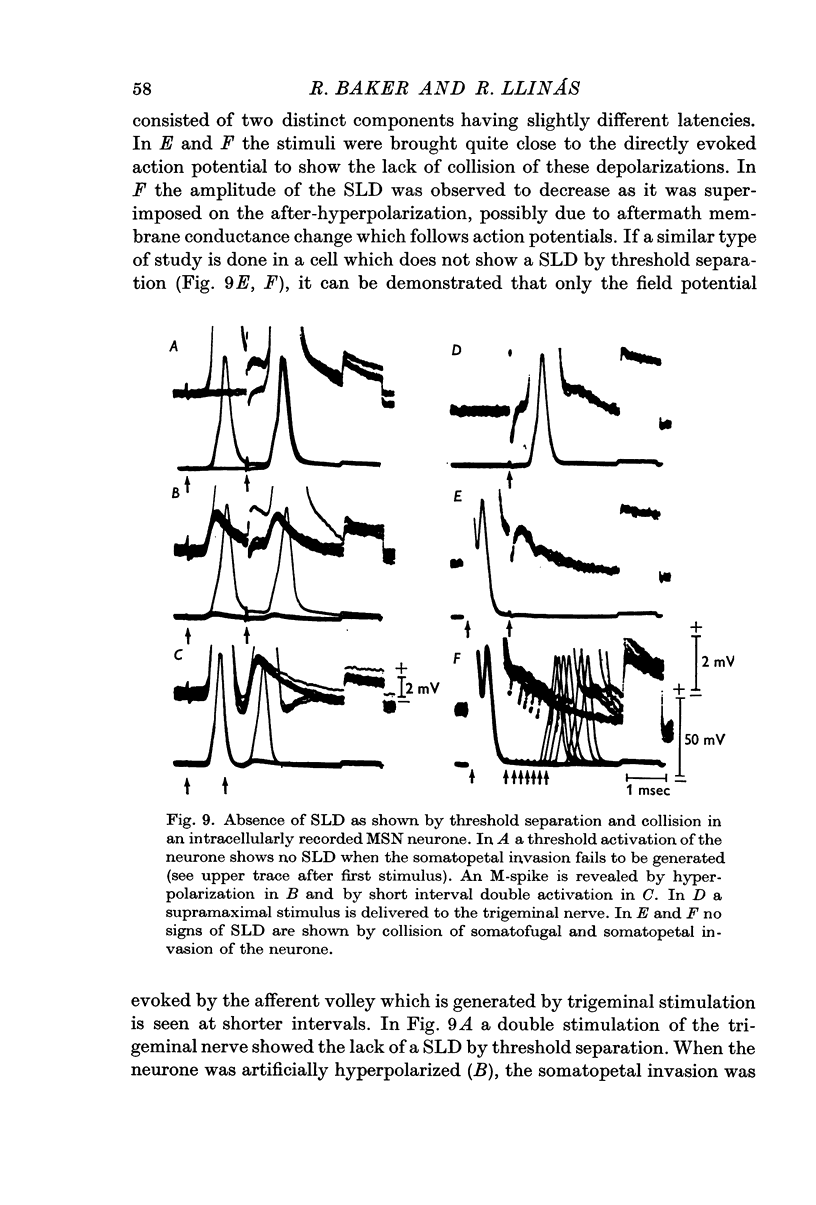
1. Electrical activity of MSN neurones was recorded intracellularly as action potentials were conducted from the periphery (somatopetally) to the masticatory nucleus. Typical sequential invasion of the initial segment and somatic region (IS—S) of the neurones was seen. The somatopetal activation of MSN neurones was characterized by the brevity, short refractoriness, high safety factor (IS—S), and short after-hyperpolarization of the spike potential.
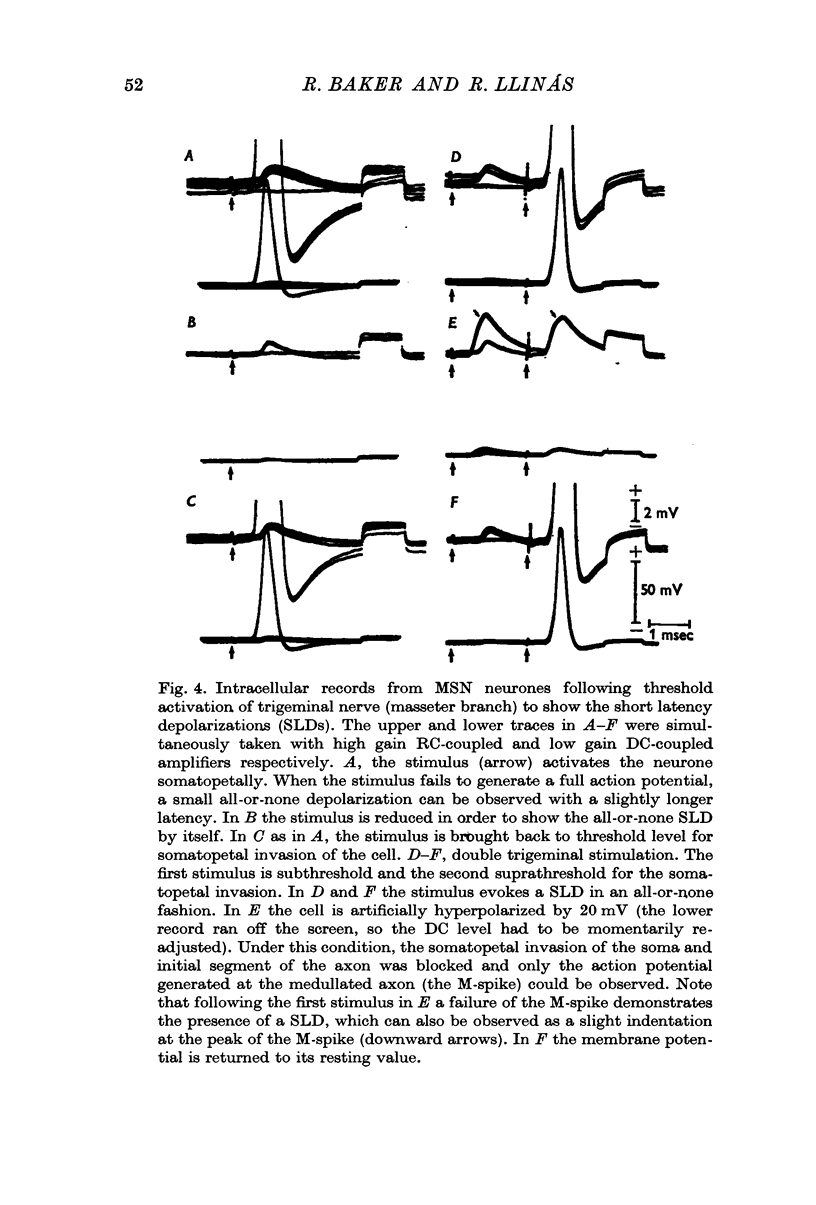
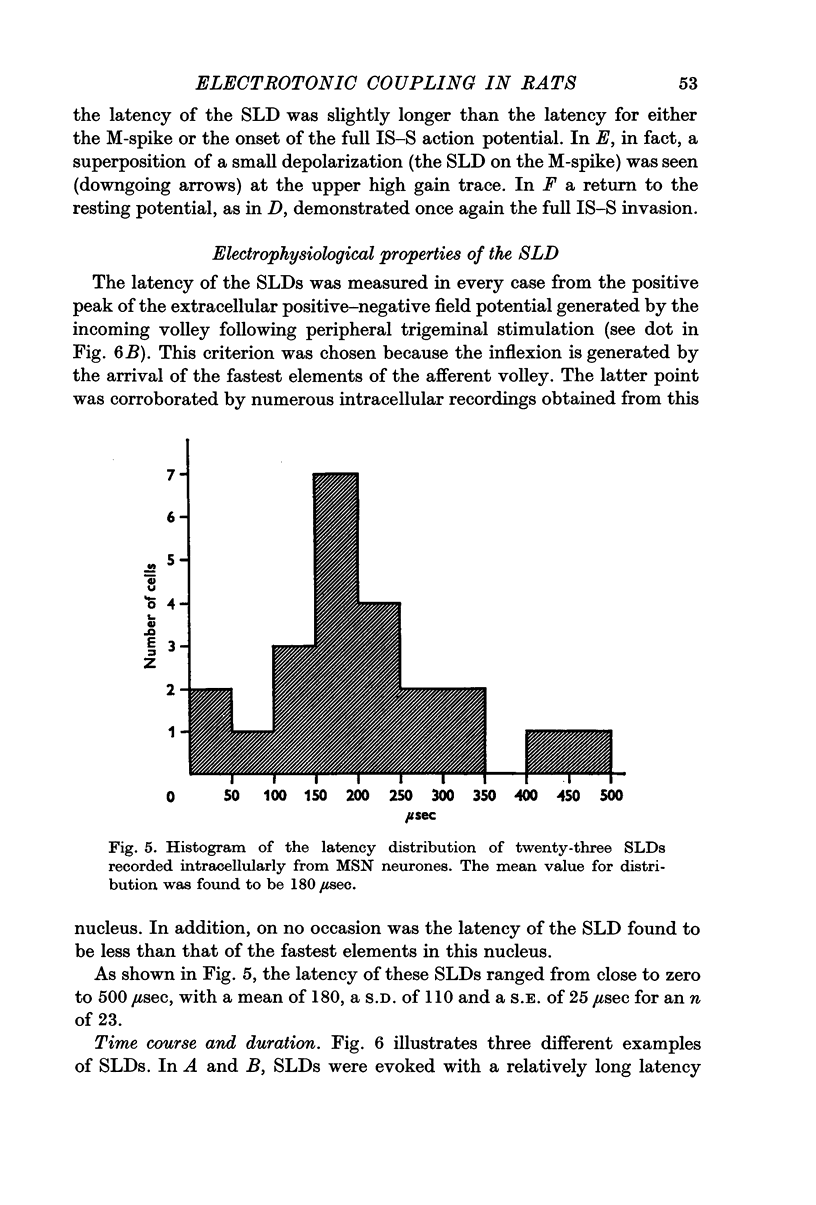
2. In twenty-three (10·5%) of the penetrated neurones, stimulation at levels subthreshold for somatopetal activation uncovered a SLD with a mean latency of 180 μsec.
3. The SLDs were found to be all-or-none in nature, and to have constant amplitude and latency for a given cell, plus a short half decay time.
4. Hyperpolarization of a MSN neurone through the recording electrode produced a blockage of the IS—S spike and revealed M-spikes and SLDs which could be clearly separated, in every instance, as distinct all-or-none components. The amplitude of the SLD was found to be insensitive to the level of membrane potential within the ranges tested.
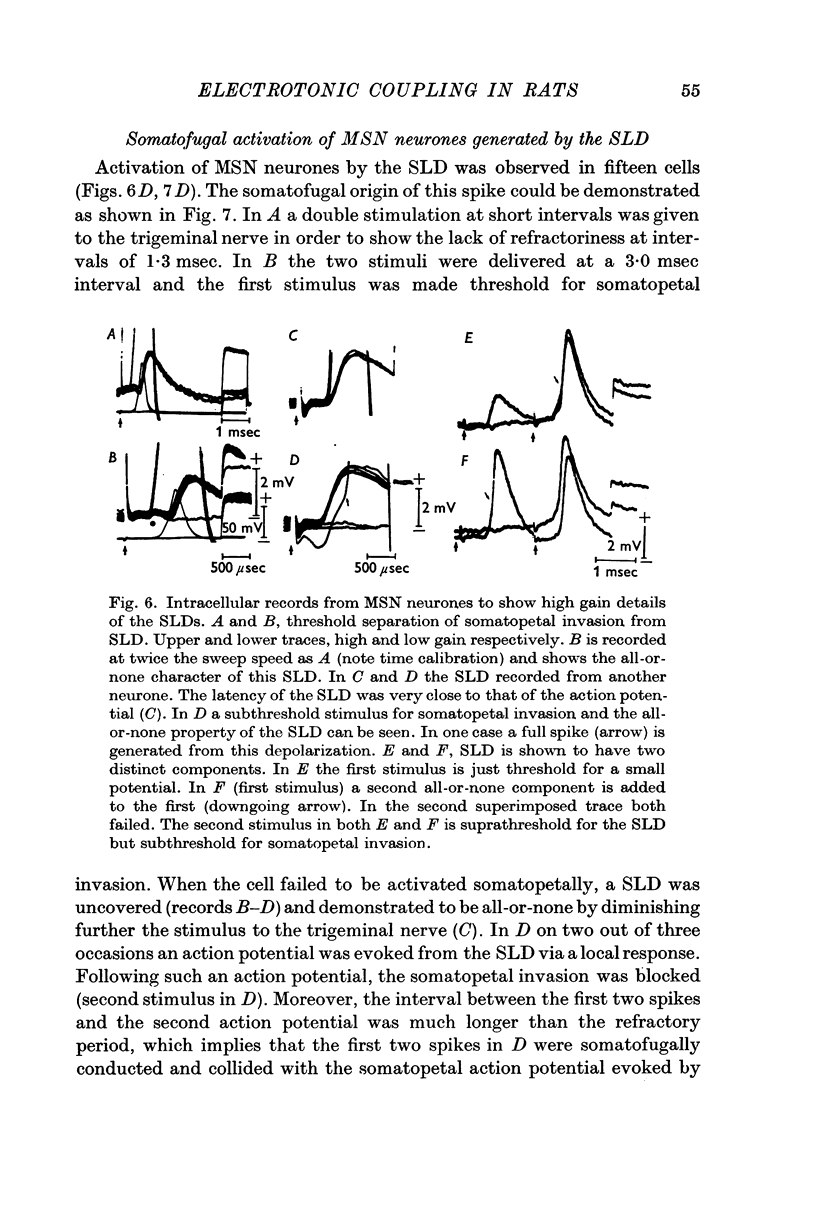
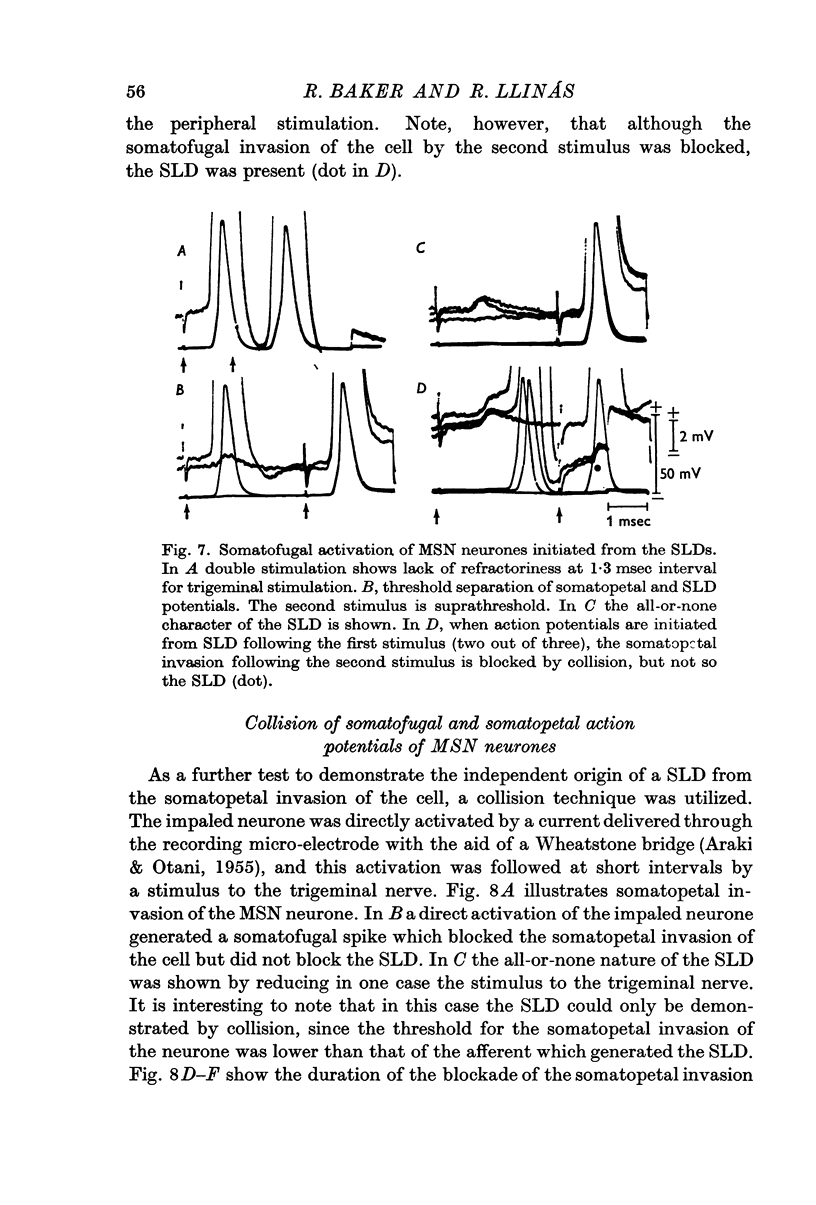
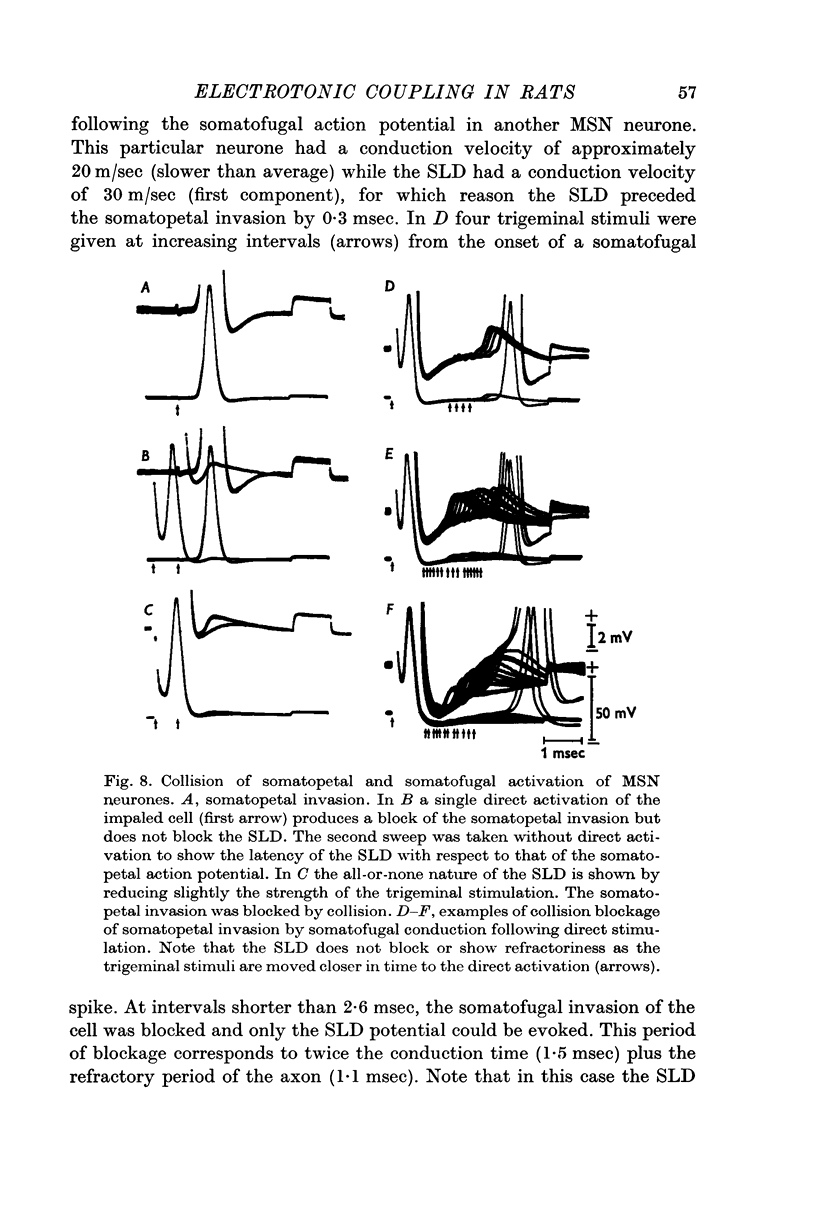
5. In fifteen neurones the SLD generated action potentials which were conducted somatofugally as shown by their collision with somatopetally conducted action potentials in the same cell. The lack of collision between the SLD and somatopetal spikes demonstrated the independent origin of these two potentials.
6. The independence of the SLD from the somatopetal invasion of the cell was also demonstrated by collision of a somatofugal action potential following direct stimulation through the recording micro-electrode and a somatopetal spike following trigeminal stimulation.
7. Two possible mechanisms are considered for the genesis of the SLD: chemical synaptic transmission and electrotonic coupling between neighbouring cells. The conclusion is drawn that SLDs must be generated by the latter mechanism.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- BENNETT M. V., ALJURE E., NAKAJIMA Y., PAPPAS G. D. Electrotonic junctions between teleost spinal neurons: electrophysiology and ultrastructure. Science. 1963 Jul 19;141(3577):262–264. doi: 10.1126/science.141.3577.262. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Nakajima Y., Pappas G. D. Physiology and ultrastructure of electrotonic junctions. 3. Giant electromotor neurons of Malapterurus electricus. J Neurophysiol. 1967 Mar;30(2):209–235. doi: 10.1152/jn.1967.30.2.209. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Nakajima Y., Pappas G. D. Physiology and ultrastructure of electrotonic junctions. I. Supramedullary neurons. J Neurophysiol. 1967 Mar;30(2):161–179. doi: 10.1152/jn.1967.30.2.161. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Pappas G. D., Aljure E., Nakajima Y. Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. J Neurophysiol. 1967 Mar;30(2):180–208. doi: 10.1152/jn.1967.30.2.180. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Pappas G. D., Giménez M., Nakajima Y. Physiology and ultrastructure of electrotonic junctions. IV. Medullary electromotor nuclei in gymnotid fish. J Neurophysiol. 1967 Mar;30(2):236–300. doi: 10.1152/jn.1967.30.2.236. [DOI] [PubMed] [Google Scholar]
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch. 1955;260(5):385–415. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- FADIGA E., BROOKHART J. M. Monosynaptic activation of different portions of the motor neuron membrane. Am J Physiol. 1960 Apr;198:693–703. doi: 10.1152/ajplegacy.1960.198.4.693. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. "ELECTRICAL TRANSMISSION" AT AN EXCITATORY SYNAPSE IN A VERTEBRATE BRAIN. Science. 1964 May 15;144(3620):878–880. doi: 10.1126/science.144.3620.878. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D. A study of the interaction between motoneurones in the frog spinal cord. J Physiol. 1966 Feb;182(3):612–648. doi: 10.1113/jphysiol.1966.sp007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., MORITA H. Electrotonic transmission between two nerve cells in leech ganglion. J Neurophysiol. 1962 Nov;25:721–731. doi: 10.1152/jn.1962.25.6.721. [DOI] [PubMed] [Google Scholar]
- Hinrichsen C. F., Larramendi L. M. Features of trigeminal mesencephalic nucleus structure and organization. I. Light microscopy. Am J Anat. 1969 Dec;126(4):497–505. doi: 10.1002/aja.1001260408. [DOI] [PubMed] [Google Scholar]
- Hinrichsen C. F., Larramendi L. M. Synapses and cluster formation of the mouse mesencephalic fifth nucleus. Brain Res. 1968 Feb;7(2):296–299. doi: 10.1016/0006-8993(68)90105-4. [DOI] [PubMed] [Google Scholar]
- ITO M., SAIGA M. The mode of impulse conduction through the spinal ganglion. Jpn J Physiol. 1959 Mar 25;9(1):33–42. doi: 10.2170/jjphysiol.9.33. [DOI] [PubMed] [Google Scholar]
- ITO M. The electrical activity of spinal ganglion cells investigated with intracellular microelectrodes. Jpn J Physiol. 1957 Dec 20;7(4):297–323. doi: 10.2170/jjphysiol.7.297. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G. Interaction between spinal motoneurons of the cat. J Neurophysiol. 1966 Mar;29(2):275–287. doi: 10.1152/jn.1966.29.2.275. [DOI] [PubMed] [Google Scholar]
- Pappas G. D., Bennett M. V. Specialized junctions involved in electrical transmission between neurons. Ann N Y Acad Sci. 1966 Jul 14;137(2):495–508. doi: 10.1111/j.1749-6632.1966.tb50177.x. [DOI] [PubMed] [Google Scholar]
- Payton B. W., Bennett M. V., Pappas G. D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969 Dec 26;166(3913):1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. THE OCCURRENCE OF A SUBUNIT PATTERN IN THE UNIT MEMBRANES OF CLUB ENDINGS IN MAUTHNER CELL SYNAPSES IN GOLDFISH BRAINS. J Cell Biol. 1963 Oct;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. Ultrastructure of excitable membranes and the crayfish median-giant synapse. Ann N Y Acad Sci. 1961 Sep 6;94:339–389. doi: 10.1111/j.1749-6632.1961.tb35552.x. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Wuerker R. B., Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967 Sep;30(5):1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Marking single neurons by staining with intracellular recording microelectrodes. Science. 1966 Mar 25;151(3717):1538–1539. doi: 10.1126/science.151.3717.1538. [DOI] [PubMed] [Google Scholar]
- WATANABE A., BULLOCK T. H. Modulation of activity of one neuron by subthreshold slow potentials in another in lobster cardiac ganglion. J Gen Physiol. 1960 Jul;43:1031–1045. doi: 10.1085/jgp.43.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE A., GRUNDFEST H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961 Nov;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]



