Abstract
Polar ether lipids of Thermoplasma acidophilum HO-62 were purified by high-performance liquid chromatography with an evaporative light-scattering detector. Structures of purified lipids were investigated by capillary gas chromatography, mass spectrometry, and nuclear magnetic resonance. Three types of ether lipids were found: phospholipids, glycolipids, and phosphoglycolipids. The two phospholipids had glycerophosphate as the phosphoester moiety. The seven glycolipids had different combinations of gulose, mannose, and glucose, which formed mono- or oligosaccharides. The eight phosphoglycolipids with two polar head groups contained glycerophosphate as the phosphoester moiety and gulose alone or gulose and mannose, which formed mono- or oligosaccharides, as the sugar moiety. Although gulose is an unusual sugar in nature, several glyco- and phosphoglycolipids contained gulose as one of the sugar moieties in Thermoplasma acidophilum. All the ether lipids had isopranoid chains of C40 or C20 with zero to three cyclopentane rings. The structures of these lipids including four new glycolipids and three new phosphoglycolipids were determined, and a glycosylation process for biosynthesis of these glycolipids was suggested.
Archaea have unique plasma membranes made of isopranoid glycerol ethers (10, 14). The isopranoid chains of the archaeal membrane lipids consist of C20, C25, and C40 hydrocarbons. The C20 and/or C25 chains are linked to glycerol to form a 2,3-di-O-alkyl-sn-glycerol diether (6, 15), and the C40 (biphytanyl) chains are connected to two glycerols to form dibiphytanyl diglyceryl tetraether (6, 14, 19). Although the basic structures of the core lipids of archaea are common, many types of polar head groups have been reported (for reviews, see references 10 and 32). Investigation of the structures of the archaeal lipids not only is useful for rapid identification of archaea as chemical markers (10) but may provide evolutionary insights and the knowledge of how they can adapt to the extreme environments (9, 13, 16, 17, 20).
Thermoplasma acidophilum is a facultative anaerobic, thermophilic, and acidophilic archaeon and grows optimally at pHs 1 to 2 and at 55 to 59°C. Tetraether-type glycolipids that have glucose and gulose (37) and lipopolysaccharide composed of mannose and glucose (31) have already been reported. Polar lipids are dominated by an MPL that occupies about half of the total lipids in Thermoplasma. The structures of MPL have been determined as β-L-gulopyranosyl-caldarchaetidyl-glycerol (35). Gulose is an unusual sugar component in nature. Some polar lipids have been resolved using thin-layer chromatography (19), and diglycosyl and triglycosyl variations of MPL-like lipids and phosphatidylglycerol diether lipid have been tentatively assigned based on the negative-ion fast atom bombardment-mass spectrometry spectrum of the total lipid extract (35); however, the detailed properties of the polar lipids remain ambiguous.
HPLC has been used to detect and separate ester lipids (8, 29). However, it is difficult to use the HPLC method for separating archaeal lipids since they do not have UV absorption, which is usually used for detection. Recently an ELSD (2) has been proven to be effective for detecting poor UV absorbers, such as most lipids (22, 33). We separated and detected polar lipids of T. acidophilum by the normal-phase HPLC method with an ELSD. Isolated peaks were investigated by capillary GC, MS, and NMR. The structures of four new glycolipids and three new phosphoglycolipids are proposed, and the glycosylation process and the possible role of the polar moiety of the lipids are discussed.
MATERIALS AND METHODS
Abbreviations.
MPL, main polar lipid; HPLC, high-performance liquid chromatography; ELSD, evaporative light-scattering detector; GC, gas chromatography; MS, mass spectrometry; HPTLC, high-performance thin layer chromatography; FID, flame ionization detector; PMAA, partially methylated alditol acetate; EI-MS, electron impact-mass spectrometry; liquid SIMS, liquid secondary ion mass spectrometry; ROESY, rotating frame nuclear Overhauser effect spectroscopy; TMS, trimethylsilyl.
Chemicals.
HPLC-grade chloroform and methanol were obtained from Nacalai Tesque, Inc. (Kyoto, Japan). NMR-grade benzene-D6 (99.96%, D), methanol-D4 (99.96%, D) and tetramethylsilane were purchased from Euriso-Top (Gif-sur-Yvette, France). α-Mannosidase from jack bean and β-mannosidase from Achatina fulica were purchased from Seikagaku Kogyo (Tokyo, Japan). Both mannosidases are exoglycosidases. l-gulose was purchased from Tokyo Chemical Industry Co., Ltd. All other chemicals were of analytical grade.
Organism and cultivation.
T. acidophilum HO-62 (40) was grown without shaking in 10 liters of the medium described by Yasuda et al. (40). Harvested cells from repeated cultures (five times) were stored at −80°C, combined (14.8 g of wet weight in total), and used for lipid extraction.
Extraction and fractionation of lipids.
Total lipid was extracted from lyophilized cells that were passed through a Sephadex G25 column (30) and was applied on a silica gel (Wakogel C200, 100-200 mesh; Wako, Osaka, Japan) column (30 by 250 mm) equilibrated with chloroform. After the elution of neutral and low-polarity lipids with chloroform and acetone, polar lipid fractions P1, P2, and P3 were eluted with 200 ml of chloroform-methanol (3:2, vol/vol), 300 ml of chloroform-methanol (3:2, vol/vol), and then 600 ml of methanol, respectively.
Separation of polar lipids by HPLC-ELSD and HPTLC.
The polar lipid fractions P1, P2, and P3 were applied to a Capcellpak silica UG80 column (4.6-mm inside diameter [i.d.] by 250 mm; Shiseido, Tokyo, Japan) attached to a Beckman System Gold HPLC system (Beckman Instruments, Fullerton, Calif.) at ambient temperature and were monitored by an Alltech model 500 ELSD (Deerfield, Ill.). Nebulization was done with compressed air with a flow rate of 3.0 liters/min. Each peak was collected by splitting the flow before the detector.
HPTLC was carried out on silica gel F254 plates (Merck, Darmstadt, Germany) and was developed with the solvent chloroform-methanol-water (65:25:4, vol/vol/vol). The spots were detected by spraying of an aqueous solution of 5% formaldehyde in 30% sulfuric acid followed by heating at 150°C for 15 min.
Analysis of sugar moieties of polar lipids.
Methanolysis of lipids and trimethylsilylation of methylglycosides were done according to the methods of Yang and Haug (39) and Martinez-Castro et al. (24), respectively. The methylglycosides were trimethylsilylated by incubation with pyridine-trimethylsilylimidazole (2:1, vol/vol) at 70°C for 30 min. TMS-methylglycosides was analyzed with a DB-1701, 30-m by 0.25-mm i.d. (thickness of film [df] = 0.25 μm) capillary column (J&W Scientific Inc., Folsom, Calif.), attached to a Hewlett-Packard (Avondale, Pa.) model HP5890 gas chromatograph equipped with FID. TMS-methylglycosides were analyzed with a temperature gradient from 140 to 250°C with an increment of 5 degrees/min.
In order to distinguish 1,3-linkage and 1,4-linkage, the sugar moieties were reduced by sodium borodeuteride. The 1-deuterated PMAA derivatives of the sugar moieties were prepared essentially by the method of Lebery and Hakomori (21), with the modification of methylation by the method of Ciucanu and Kerek (4). The sample solution of PMAAs was analyzed by gas chromatography (GC)-MS. A DB-5, 30-m by 0.30-mm i.d. (df = 0.1 μm) capillary column (J&W) was used with a temperature gradient from 50 to 300°C with an increment of 10 degrees/min. The GC peaks were identified by EI-MS, using an Autospec E mass spectrometer (Micromass Inc., Manchester, England) equipped with an EI-MS probe operated at 70-eV ionization voltage, instead of the FID.
Anomeric configurations of mannose moieties were investigated by enzymatic digestion of lipids using exoglycosidase-type α- and β-mannosidases (23, 34). The reaction mixture contained glycolipid fractions, 0.1 U of α- or β-mannosidase, and 1-mg/ml taurodeoxycholic acid sodium salt in 100 μl of 50 mM citrate buffer (pH 4.5) and was incubated at 37°C for 15 h. Five hundred microliters of chloroform-methanol (2:1, vol/vol) was added to the reaction mixture to stop the enzymatic reaction. The chloroform layer was recovered and evaporated. Each sample was analyzed by HPTLC as described in the previous paragraph to identify the enzymatic hydrolysis products.
To investigate the similarities of the sugar moieties between the glycolipids and phosphoglycolipids, the phosphoglycolipid fractions were hydrolyzed under strongly alkaline condition (18) and analyzed by HPTLC.
MS of polar lipids.
Liquid SIMS was performed at an acceleration voltage of 10 eV on an Autospec E mass spectrometer equipped with a SIMS probe. Triethanolamine-2-nitrobenzylalcohol (2:1, vol/vol) was used as the matrix.
NMR analysis of polar lipids.
Proton NMR experiments were carried out on a Bruker DPX-400 spectrometer operated at proton frequencies of 400 MHz. Each sample was dissolved in 0.55 ml of deuterobenzene-deuteromethanol (3:1, vol/vol). Tetramethylsilane was used as an internal standard. All NMR spectra were measured using Bruker standard methods at ambient temperature. Two-dimensional ROESY spectra were recorded with a mixing time of 250 ms and a spectral width of 4,000 by 4,000 Hz. The spectra composed of 512 points in the F1 and F2 dimensions were accumulated for 320 to 400 scans.
Analysis of core lipids.
Caldarchaeol in the hexane layer after methanolysis was converted to biphytanyl iodides and then to their alcohol derivatives via their acetates according to the literature (15). The trimethylsilylation of alcohol derivatives and GC analysis were done as described in the previous paragraph with a DB-5, 30-m by 0.30-mm i.d. (df = 0.1 μm) capillary column (J&W) with a temperature gradient from 200 to 350°C with an increment of 10 degrees/min. The number of cyclopentane rings of each GC peak was estimated by analyzing the MS with an EI-MS (39). The average cyclization of isopranoid chains in each HPLC fraction was calculated from the GC peak area detected by FID (7) using the equation in footnote b of Table 1.
TABLE 1.
Profiles of high-polarity lipids in T. acidophilum HO-62
| Fraction
|
Core lipid
|
Sugars
|
Classificationf | r.t. (min)g | Reporth | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Open column | HPLC | Skelton | No. of ringsa | Avg. no. of ringsb | No.c | Compositiond | Minore | |||
| Fraction P1 | A1-1 | Ci | 0 | 0.3 | 0 | PL | 11.6 | 36 | ||
| A1-2 | Aj | 0 | NDm | 0 | glc | PL | 11.6 | 35, 36 | ||
| A2 | C | 4 | 1.7 | 2 | 1β gulp | GL | 18.2 | 37 | ||
| A3 | C | 0 | 0.4 | 1 | 1β gulp | PGL (MPL) | 20.0 | 35 | ||
| A4-1 | C | 3 | 1.4 | 1 | 1β gulp | PGL (MPL) | 24.7 | 35 | ||
| A4-2 | C | 2 | 1.2 | 2 | 1β gulp 3-1 α manp | glc | GL | 24.7 | ||
| A5 | C | ND | 1.5 | 3 | 1β gulp 3-1 α manp, (1 β gulp) | glc | GL | 32.7 | ||
| A6 | C | 0 | 1.2 | 3 | 1β gulp 3-1 α manp 3-1 α manp | GL | 40.0 | |||
| A7 | C | 1 | 0.9 | 4 | man, glc (3:1)k | gul | GL | 44.8 | ||
| A8 | ND | ND | ND | ND | gul, man, glcl | GL | 49.8 | |||
| A9 | C | 1 | 0.8 | 4 | gul, manl | glc | GL | 52.1 | ||
| Fraction P2 | C | 0 | 1.0 | 1 | 1β gulp | man | PGL (MPL) | 19.4 | 35 | |
| Fraction P3 | C1 | C | 0 | 1.2 | 1 | 1β gulp | PGL (MPL) | 20.0 | 35 | |
| C2 | C | 3 | 1.5 | 2 | 1β gulp 3-1 α manp | PGL | 35.6 | |||
| C3 | C | 2 | 1.6 | 3 | 1β gulp 3-1 α manp 3-1 α manp | PGL | 50.4 | |||
| C5 | C | 2 | 1.2 | 4 | 1β gulp 3-(1 α manp 3)2-1 α manp | glc | PGL | 57.7 | ||
Number of cyclopentane rings per molecule estimated from liquid SIMS spectra.
Average number of cyclopentane rings of a biphytanyl chain calculated as [% monocyclic + (2 × % bicyclic) + (3 × % tricyclic)] × 10−2. The percentages of monocyclic, bicyclic, and tricyclic rings were obtained from GC-FID analysis of TMS derivatives of biphytanyl alcohols.
Number of sugars estimated from liquid SIMS spectra.
The component is assigned by GC analysis of TMS-methyl and PMAA derivatives, enzymatic digestion, and 1H-NMR analysis of the sugar moieties. gul, gulose; glc, glucose; man, mannose.
Minor component.
Classification was determined by GC analysis. PL, phospholipid; GL, glycolipid; PGL, phosphoglycolipid.
Retention time of HPLC obtained from Fig. 1A and B.
Previous publications reporting the presence of the compound in T. acidophilum. Reference numbers are given.
C, caldarchaeol moiety.
A, C20-C20-archaeol moiety.
Molar ratios of sugar moieties estimated from NMR spectra.
Molar ratio was not determined.
ND, not determined.
RESULTS
Separation of lipids using HPLC-ELSD.
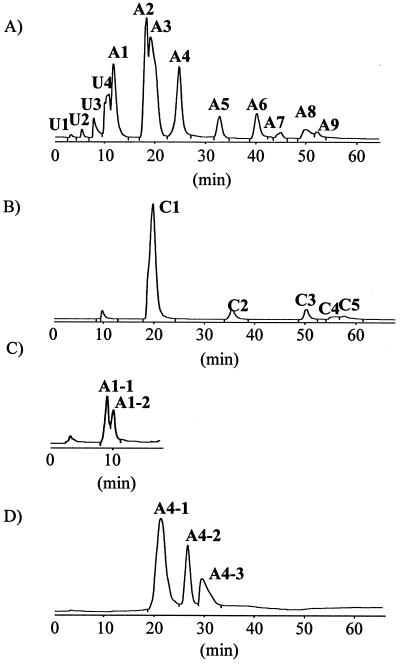
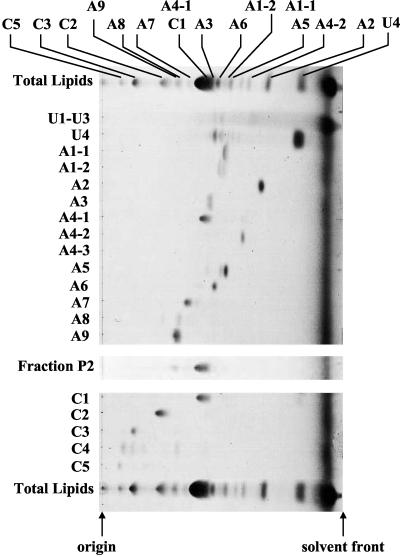
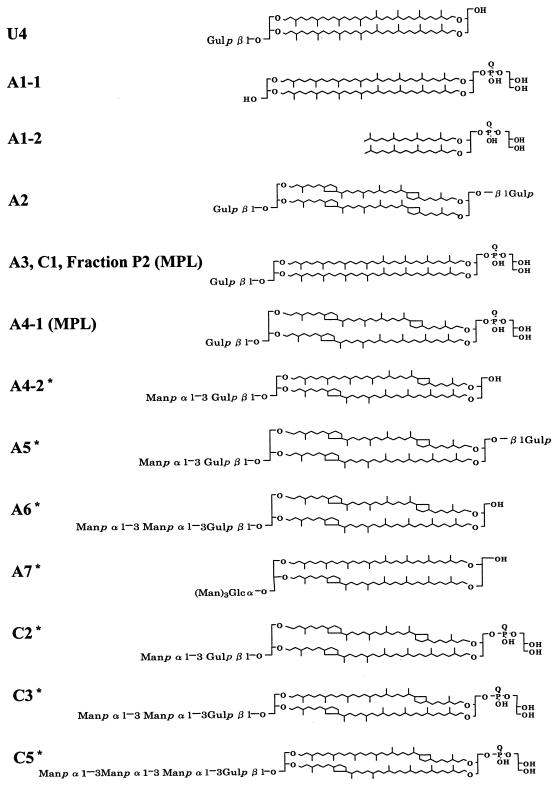
Total lipid extract from T. acidophilum was fractionated into polar lipid fractions P1, P2, and P3 with a silica gel column after the elution of neutral and low-polarity lipids. The fractions P1 and P3 were applied to HPLC. HPLC chromatograms of the polar lipid fractions P1 and P3 are shown in Fig. 1A) and B), respectively. Each HPLC peak was recovered and analyzed by HPTLC (Fig. 2). Because A1 and A4 contained more than one component (data not shown), they were subjected to HPLC under different conditions as shown in Fig. 1C and D). Subfractions A1-1, A1-2, A4-1, A4-2, and A4-3 were recovered and analyzed by HPTLC (Fig. 2). Although most fractions showed one spot, A1-2 was still contaminated with the component from A1-1, and A5 was contaminated with a small amount of unique components. Because the components in U1-U3 migrated to the top of the HPTLC plate, they were expected to have low polarity and were not analyzed for this report. Fraction C4 was found to be still crude, and the amount of A4-3 was too small to be detected; thus, these peaks were not analyzed further. Silica gel fraction P2 showed one spot with a slight contamination of A9. In this study, U4, A1-1, A1-2, A2, A3, A4-1, A4-2, A5, A6, A7, A8, A9, C1, C2, C3, C5, and silica gel fraction P2 were used for the following analyses. The proposed structures of the polar lipids based on the results obtained in this report are shown in Fig. 3.
FIG. 1.
HPLC chromatograms of phospholipid fractions of T. acidophilum HO-62. HPLC analysis was achieved on a Capcellpak silica UG80 column (4.6 by 250 mm) and monitored with an ELSD. The following eluents were used: H1, chloroform; H2, chloroform-methanol-trifluoroacetic acid (50:50:1, vol/vol/vol); H3, chloroform-methanol-trifluoroacetic acid (50:50:5, vol/vol/vol); H4, chloroform-methanol-water (82:16:2, vol/vol/vol). The silica gel fractions P1 (A) and P3 (B) were separated with eluents H1 and H2 gradient with a program of H2 percentages (min) of 0 (0) to 10 (2) to 50 (42) to 100 (62), at a flow rate of 1 ml/min. (C) The sample of fraction A1 recovered from the HPLC of panel A was separated with eluent H4 at a flow rate of 1 ml/min. (D) The sample of fraction A4 recovered from the HPLC of panel A was separated with eluents H1 and H3 gradient with a program of H3 percentages (min) of 0 (0) to 10 (2) to 50 (42) to 100 (62), at a flow rate of 1 ml/min.
FIG. 2.
HPTLC of the fractions represented by HPLC peaks of Fig. 1. HPTLC was carried out on silica gel F254 plates of 10 by 10 cm and 0.1-mm thickness and was developed with chloroform-methanol-water (65:25:4, vol/vol/vol).
FIG. 3.
Proposed structures of high-polarity lipids found in T. acidophilum HO-62. U4, gulopyranosyl-(β1-1)-caldarchaeol; A1-1, caldarchaetidylglycerol; A1-2, archaetidylglycerol; A2, gulopyranosyl-(β1-1)-[gulopyranosyl-(β1prime;-1prime;)]-caldarchaeol; A3, gulopyranosyl-(β1-1)-caldarchaetidylglycerol; A4-1, gulopyranosyl-(β1-1)-caldarchaetidylglycerol; A4-2, mannopyranosyl-(α1-3)-gulopyranosyl-(β1-1)-caldarchaeol; A5, mannopyranosyl-(α1-3)-gulopyranosyl-(β1-1)-[gulopyranosyl-(β1prime;-1prime;)]-caldarchaeol; A6, mannopyranosyl-(α1-3)-mannopyranosyl-(α1-3)-gulopyranosyl-(β1-1)-caldarchaeol; A7, trimannopyranosylglucosylcaldarchaeol; C2, mannopyranosyl-(α1-3)-gulopyranosyl-(β1-1)-caldarchaetidylglycerol; C3, mannopyranosyl-(α1-3)-mannopyranosyl-(α1-3)-gulopyranosyl-(β1-1)-caldarchaetidylglycerol; C5, mannopyranosyl-(α1-3)-(mannopyranosyl)2-(α1-3)-gulopyranosyl-(β1-1)-caldarchaetidylglycerol. The naming of the lipids followed the semisystematic naming of archaeal lipids proposed by Koga et al. (25). The number of cyclopentane rings of each structure is estimated from the main peaks of liquid SIMS. Each polar lipid is expected to consist of molecules with different numbers of cyclopentane rings. The average number of cyclopentane rings of a biphytanyl chain is listed in Table 1. The positions of rings are indicated according to the report of Yang et. al. (39). Asterisks indicate new compounds.
Analysis of sugar moieties of polar lipids.
Sugar moieties in the lipids were converted into TMS-methylglycosides by methanolysis followed by trimethylsilylation and were analyzed by GC-MS. GC peaks were assigned by comparison with the TMS-methyl derivatives of standard sugars. Two to four GC peaks were observed for each sugar, which corresponded to their tautomers: α and β configurations of pyranose and furanose rings (24). TMS-methyl derivatives of α-glycerophosphoric acid were also detected in the same GC conditions. The results of GC analysis are listed in Table 1. Fractions of most of the HPLC peaks contained a small amount of glucose that is probably derived from minor compounds in respective HPLC fractions.
To investigate the position of the glycosidic linkages of A4-2, A5, A6, C1, and C2, sugar moieties of the lipids in HPLC fractions were converted to their PMAA derivatives after the reduction with sodium borodeuteride. The primary ion fragments observed in the GC-MS spectra were assigned based on the report of Jansson et al. (11). 1,3-Linked hexose can be distinguished from 1,4-linked hexose by the existence of m/z 161 in the former. The results are listed in Table 2.
TABLE 2.
Primary ion fragments and relative retention times of 1-deuterated partially methylated alditol acetates obtained from HPLC fractions
| Fraction | Sugar | Pa | Primary ion fragment (m/z) | Tb | |||
|---|---|---|---|---|---|---|---|
| A4-2 | gul | 1,3 | 45 | 118 | 161 | 234 | 1.070 |
| man | 1 | 45 | 118 | 161 | 205 | 1.000 | |
| A5 | gul | 1 | 45 | 118 | 161 | 205 | 0.991 |
| gul | 1,3 | 45 | 118 | 161 | 234 | 1.070 | |
| man | 1 | 45 | 118 | 161 | 205 | 1.000 | |
| A6 | gul | 1,3 | 45 | 118 | 161 | 234 | 1.072 |
| man | 1,3 | 45 | 118 | 161 | 234 | 1.046 | |
| man | 1 | 45 | 118 | 161 | 205 | 1.000 | |
| C1 | gul | 1 | 45 | 118 | 161 | 205 | 0.993 |
| C2 | gul | 1,3 | 45 | 118 | 161 | 234 | 1.069 |
| man | 1 | 45 | 118 | 161 | 205 | 1.000 | |
Positions of glycosidic linkages.
Retention times relative to that of 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl-d-mannitol (= 1.000).
The phosphoester linkage of phosphoglycolipid was hydrolyzed under strongly alkaline conditions, and the product was analyzed by HPTLC. U4, A4-2, A6, and A9 were derived from C1, C2, C3, and C5, respectively, under such conditions (data not shown). C1, C2, C3, and C5 are thus phosphoester forms of U4, A4-2, A6, and A9, respectively (see Fig. 3).
Liquid SIMS spectra.
Relative masses of the deprotonated main molecular ions ([M-H]−) of HPLC fractions were analyzed by liquid SIMS (negative ion mode) and are listed in Table 3. The liquid SIMS spectrum of each fraction showed several [M-H]− peaks due to different numbers of cyclopentane rings. Most fractions appeared to be mixtures of lipids with zero to six cyclopentane rings. However, the proportion of these components could not be determined due to the presence of overlapping 13C isotope peaks. Numbers of cyclopentane rings and hexoses were determined only for the main molecular ions by this method.
TABLE 3.
Experimental and calculated relative masses of deprotonated molecular ions ([M-H]−) of HPLC fractions
| Fraction | [M-H]− (m/z)
|
No. of ringsc | No. of hexosesd | |
|---|---|---|---|---|
| Experimentala | Calculatedb | |||
| A1-1 | 1,454.8,* 805.9 | 1,454.3 | 0 | 0 |
| A1-2 | 805.6 | 805.7 | 0 | 0 |
| A2 | 1,616.9 | 1,616.4 | 4 | 2 |
| A3 | 1,616.9 | 1,616.4 | 0 | 1 |
| A4-1 | 1,609.9 | 1,610.3 | 3 | 1 |
| A4-2 | 1,620.7 | 1,620.4 | 2 | 2 |
| A5 | ND | |||
| A6 | 1,786.0 | 1,786.5 | 0 | 3 |
| A7 | 1,946.3 | 1,946.5 | 1 | 4 |
| A8 | ND | |||
| A9 | 1,947.0,* 2,098.9 | 1,946.5 | 1 | 4 |
| P2 | 1,616.2 | 1,616.4 | 0 | 1 |
| C1 | 1,616.8 | 1,616.4 | 0 | 1 |
| C2 | 1,771.3 | 1,772.4 | 3 | 3 |
| C3 | 1,933.7* | 1,932.4 | 2 | 3 |
| C5 | 2,098.9,* 1,934.9 | 2,098.5 | 2 | 4 |
[M-H]− was measured by liquid SIMS analysis. Asterisk denotes the main peak in the spectra. ND, not determined.
Calculated deprotonated molecular mass expected from the numbers of cyclopentane rings and hexoses estimated.
Number of cyclopentane rings per molecule estimated from experimental [M-H]−.
Number of hexoses estimated from experimental [M-H]−.
A1-1 showed a peak at m/z 1,454.8 and agreed well with the calculated molecular mass of caldarchaetidyl glycerol (1,454.3 Da) (Fig. 3). A1-2 was found to contain no cyclopentane ring, since A1-2 showed a strong peak at m/z 805.6, in good agreement with the calculated molecular mass of C20-C20-archaetidyl glycerol with no cyclopentane ring (805.7) (Fig. 3).
For the other fractions, glycolipids (A2, A4-2, A6, A7, A9) and phosphoglycolipids (A3, A4-1, C1, C2, C3, C5), the number of hexoses of each fraction was estimated from each [M-H]− value (Table 3). A3, A4-1, C1, and silica gel fraction P2 were MPL, since they have one gulose and glycerophosphate, based on GC analysis. It is known that MPL occupies about half of the total lipids (19). Although the polar lipids were fractionated into the three polar lipid fractions P1, P2 and P3 by silica gel column chromatography, MPL was not completely separated due to their large amounts in the total lipids. Because U4 was derived from C1 by strong alkaline hydrolysis, U4 was thought to be a tetraether lipid that contains only one gulose as the polar moiety, as reported by Uda et al. (37).
NMR spectra.
To investigate in detail structures of sugar moieties, 1H NMR spectra were measured. GC analysis of TMS-methyl derivatives of sugar moieties suggested that A2, A3, A4-1, and C1 contained only gulose. The anomeric protons of these components showed the doublet with the same chemical shift (δ = 4.85 ppm) and vicinal coupling constant 3JHH (8.3 Hz), indicating that the dihedral angle between the anomeric proton and the proton of the C-2 position of gulose is nearly 180 degrees. Accordingly, gulose is a β-anomer in A2, A3, A4-1, and C1. Though A2 is expected to have two gulose moieties from the liquid SIMS spectrum, only one signal is observed at δ = 4.85 ppm. Thus, the two molecules of gulose of A2 are expected to be attached symmetrically to both sides of the caldarchaeol moiety (37).
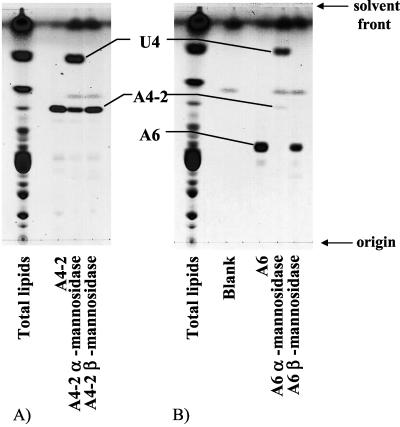
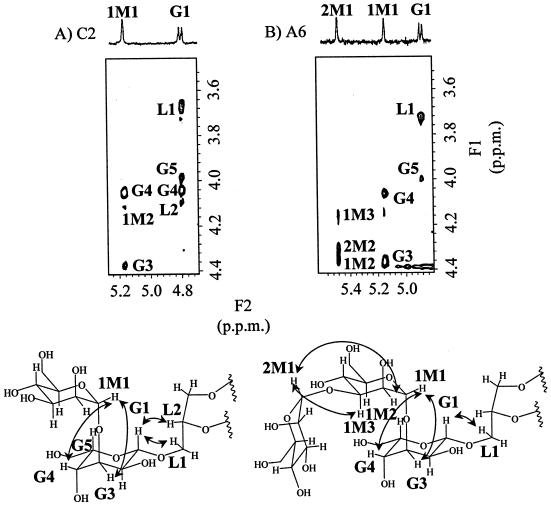
Both A4-2 and C2 contained gulose and mannose as their sugar moieties. The anomeric protons of these components showed signals similar to those of β-gulose at around 4.80 ppm (doublet, 8.3 Hz) and signals of mannose at 5.15 to 5.18 ppm (singlet). The ratio of integrated signal intensities of gulose and mannose was 1:1. Because the proton of the C-2 position of mannose is equatorial, the configuration of mannosidic linkages could not be determined. Exoglycosidase-type α- and β-mannosidases were used to determine the anomeric configuration of the mannosidic linkages. A4-2 was degraded only with α-mannosidase (Fig. 4A). Accordingly, mannose in A4-2 has an α configuration. Since C2 was converted to A4-2 by strong alkaline hydrolysis, C2 is expected to be the phosphoglycerol ester of A4-2. Thus, mannose in C2 is also expected to have an α configuration. These results suggest that the β-gulose-α-mannose moiety is attached to one side of caldarchaeol in C2 and A4-2. The ROESY spectrum of C2 and the structures of the sugar moieties are shown in Fig. 5A. Correlation signals between the anomeric proton of β-gulose (G1) and the glycerol protons of core lipid (L1 and L2) were observed (Fig. 5A). Accordingly, the gulose is expected to be attached directly to caldarchaeol in C2 and in A4-2.
FIG. 4.
HPTLC of HPLC fractions after treatment with α- or β-mannosidase. HPTLC was carried out on silica gel F254 plates of 10 by 10 cm at 0.1-mm thickness and was developed with chloroform-methanol-water (65:25:4, vol/vol/vol). (A) A4-2, (B) A6. The “Blank” lane was treated with the solution containing only taurodeoxycholic acid sodium salt in citrate buffer (pH 4.5).
FIG. 5.
ROESY spectra and nuclear Overhauser effect (NOE) correlation of C2 (A) and A6 (B). F1 and F2 axes show proton chemical shifts. ROESY spectra were obtained with data size of 512 by 512 points, and mixing time was 250 ms. Signals of C2 and A6 were accumulated at 320 and 400 scans, respectively. 1H NMR assignments were done using standard methods of correlated spectroscopy and totally correlated spectroscopy (data not shown). The assigned cross-peaks in ROESY spectra are indicated in the structural formulas. NOE correlation involved in conjugation between units is also indicated in the structural formulas by arrows.
The anomeric protons of A6, which contained gulose and two mannoses, and those of C3, which contained the same sugar moieties, are expected to be the same because C3 was altered to A6 by strong alkaline hydrolysis. Both showed three anomeric protons, represented by a doublet at 4.88 to 4.89 ppm (8.3 Hz) (β-gulose) and singlets at 5.14 to 5.16 ppm (α-mannose) and 5.48 to 5.89 ppm (α-mannose). The ratio of integrated intensities of these signals was 1:1:1. A6 was altered to U4 by enzymatic digestion using α-mannosidase (Fig. 4B). The ROESY spectrum of A6 and the structures of the sugar moieties are shown in Fig. 5B. Correlation signals between the anomeric proton of β-gulose (G1) and the glycerol proton of core lipid (L1) were observed (Fig. 5B), showing that the gulose is attached directly to caldarchaeol.
A5 contained two β-gulose moieties with proton signals at 4.80 and 4.84 ppm, respectively, and a mannose with a signal at 5.18 ppm. The ratio of the integrated intensities of these signals was 1:1:1. Furthermore, A5 was altered to A2 when it was digested by α-mannosidase (data not shown). Since caldarchaeol has two OH groups in one molecule, there are two sites for polar residues. A5 contained 1-gulopyranoside, 1,3-gulopyranoside, and 1-mannopyranoside according to GC-MS analysis. Accordingly, A5 is expected to be composed of β-gulose attached to one side of caldarchaeol and α-mannosyl-β-gulose attached to the other side (Fig. 3).
A7 contained one glucose (4.94 ppm) and three mannose residues (5.40, 5.44, and 5.53 ppm). The ratio of integrated intensities of these signals was 1:1:1:1. The glucose of A7 was an α-anomer, since the coupling constant was 3.9 Hz (37). The configuration of mannose was not determined in this study.
C5 was found to contain four sugar moieties by liquid SIMS and GC analysis. Three signals of anomeric protons were observed in the 1H NMR spectrum: a doublet at 4.89 ppm (8.3 Hz) (β-gulose) and singlets at 5.16 (α-mannose) and 5.46 ppm (α-mannose). The ratio of integrated intensities of these signals was 1:1:2. The presence of three α-mannose moieties per molecule of lipid is expected. Although glycosidic linkages in C5 were not determined by GC-MS analysis, they were expected to be 1,3-linkages based on the proton chemical shifts. The amounts of compounds in A1-1, A1-2, A8, and A9 were too little to be analyzed by NMR. The analytical results of HPLC, GC, MS, and NMR are summarized in Table 1. The proposed structures of the main components of several peaks are shown in Fig. 3.
Analysis of core lipids.
The number of cyclopentane rings estimated by liquid SIMS is that estimated for the dominant species of the core lipid. Thus, we investigated the average number of cyclopentane rings in an isopranoid chain by GC analysis (7, 39). First, TMS derivatives of biphytanyl alcohols in fraction P2 were analyzed by GC-MS. GC peaks with M+ values of 665.5 and 738.6 were assigned as the derivatives of biphytanyl alcohol with no cyclization, since both peaks were accompanied by the fragment ions at m/z values of 558.5 and 633.5, which are unique fragment ions of the noncyclized component (39). Peak pairs with M+ values of 663.5 and 736.6 and of 662.0 and 734.6 were assigned as the derivatives of biphytanyl alcohol with one cyclopentane ring and two cyclopentane rings, respectively. The peak with an M+ value of 732.5 was assigned as the derivative of biphytanyl alcohol with three cyclopentane rings.
TMS derivatives of biphytanyl alcohols of the other HPLC fractions were analyzed by GC equipped with FID. GC peaks detected by FID were assigned according to their retention times, estimated in the above-described experiment. The average number of cyclization rings in a biphytanyl chain was calculated from the peak areas in the GC-FID chart of each fraction and is listed in Table 1. Almost every caldarchaeol lipid except A1-1 and A3 contained isopranyl chains with one to three cyclopentane rings, and the average number ranged from 0.3 to 1.7 (Table 1).
DISCUSSION
Most archaeal lipids do not absorb UV and thus cannot be detected by UV spectrophotometric detectors. Since no appropriate detectors have been available so far, it has been difficult to analyze them using HPLC. In this study, we used ELSD to detect archaeal lipids. Twenty peaks were resolved from the polar lipids extracted from T. acidophilum HO-62, and 15 of them were analyzed by GC, liquid SIMS, and NMR. The structures of the main components in these HPLC peaks were proposed and are shown in Fig. 3. High-polarity lipids in T. acidophilum mainly consisted of caldarchaeol as the hydrophobic core and of glycerophosphate, gulose, mannose, or glucose as the hydrophilic part. A4-2, A5, A6, A7, C2, C3, and C5 are new compounds.
Mannose moieties in A4-2, A5, A6, C2, C3, and C5 were attached to gulose, the core sugar, which is in turn attached directly to the glycerol moiety of caldarchaeol (Fig. 5). The lipids with more sugar groups tended to have more mannose. The glyco- and phosphoglycolipids in T. acidophilum HO-62 may be synthesized from the core structure in which gulose is attached to the caldarchaeol moiety, and mannose may be added one by one.
The presence of lipopolysaccharide in T. acidophilum has been reported, and the structure has been reported to be [manp-(α1-2)-manp-(α1-4)-manp-(α1-3)]8-glcp-(α1-1) diglycerol tetraether (31). The mannose moieties of this lipid are attached to glucose, the core sugar. Although lipopolysaccharide contains manp-(α1-2), manp-(α1-3), and manp-(α1-4), oligosaccharide observed in this study contained only α1-3 conjugation. The structures of the oligosaccharides are not compatible with that of lipopolysaccharide. Thus, the polar lipids containing gulose do not appear to be precursors of the lipopolysaccharide in Thermoplasma.
On the other hand, A7 contained mannose moieties attached to glucose, instead of gulose. Several HPLC fractions, A4-2, A5, A9, and C5, contained minor lipids with glucose moiety. Though the position of the mannosidic linkage in these minor lipids could not be determined in this study, A7 and minor lipids having a glucose moiety may be precursors of the lipopolysaccharide.
The relationships between the varieties of polar moieties and taxonomic implications have been found in halophiles and methanogens (12, 14, 16). In thermophilic archaea, glucose, galactose, nonitol, and inositol phosphate have been reported as the polar moiety (14, 32). The polar lipids in T. acidophilum contained mannose and/or glycerophosphate. Though they have not been found in the other thermophilic archaea, they have been found in many halophiles. The whole-genome analysis of T. acidophilum has revealed the chimeric nature of the genome: while a part of the genome is related to euryarchaea, the other part is related to Sulfolobus (28). Though the enzymes or genes involved in the biosynthesis of the polar part of the lipids in T. acidophilum have not been reported, those may have euryarchaeal origin.
The presence of isopranyl units in the hydrophobic core is a common characteristic in archaeal lipids. However, there are several structural variations in the isopranyl chains. Halophiles have no tetraether lipids but have diether lipids, such as C20-C20-, C20-C25- and/or C25-C25-archaeol, while thermophilic archaea have tetraether lipids with cyclopentane rings (20, 32). Methanogens have both diether and tetraether lipids with no cyclopentane ring (17). Most of the lipids analyzed in this study were tetraether lipids with cyclopentane rings. In this context, Thermoplasma is related to other thermophilic archaea. When the heat stabilities of liposomes which consisted of a different ratio of diether to tetraether were compared, the liposomes that consisted of tetraether lipids were more stable than those of diether lipids (3). Lipids in T. acidophilum are compatible with the thermophilic nature of the organism.
The membrane fluidity of bacterial and eukaryotic lipids is regulated by double bond formation in the fatty acid chain (38). In the case of archaeal membranes, it is established by alteration of the number of cyclopentane rings in the isopranoid chains (7, 39). It is interesting that less-polar lipids (phospholipids) tend to have fewer rings. The situation may be related to the cyclization process and may suggest that the process occurs at the later stage of the phosphoglycolipid biosynthesis.
In contrast to the core lipid, the function of polar head groups in the archaeal membrane is less clear. Some reports, though they are not on archaea, have discussed the function of glycolipids. They are thought to stabilize the membrane against environmental stress such as osmotic stress and temperature alteration through hydrogen bonding via glycosyl head groups (5). The major lipid of Thermus thermophilus, a thermophilic eubacterium, is glycolipid (26). In the cases of Thermus aquaticus and Cyanidium caldarium, the amount of glycolipids was increased at elevated growth temperature (1, 27). Thus, glycolipids in T. acidophilum may also be responsible for the stability of the membrane at high temperature.
Though β-gulose is an unusual sugar in nature, it was found attached to the caldarchaeol part of MPL in T. acidophilum by Swain et al. (35). The present study revealed that there are many lipids containing β-gulose in the polar part. Since gulose was not contained in the growth medium, it was obviously synthesized by T. acidophilum. While most other organisms including archaea do not have gulose, the reason why gulose exists only in Thermoplasma spp. as the sugar moiety in many of their lipid components remains unclear. It may be related to its stability under low pH at high temperature. Analysis of the stability of these polar components of the lipids is on progress. The ELSD-HPLC method that simplified the analysis of polar lipids of Thermoplasma will be useful for further investigation of the functions and biosynthesis of polar lipids.
Acknowledgments
We thank H. Kumata and T. Kaise for helpful discussions and technical advice on GC analysis.
REFERENCES
- 1.Adams, B. L., V. McMahon, and J. Seckbach. 1971. Fatty acids in the thermophilic alga, Cyanidium Caldarium. Biochem. Biophys. Res. Commun. 42:359–365. [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth, J. M. 1978. Evaporative analyzer as a mass detector for liquid chromatography. Anal. Chem. 50:1414–1420. [Google Scholar]
- 3.Choquet, C. G., G. B. Patel, and G. D. Sprott. 1996. Heat sterilization of archaeal liposomes. Can. J. Microbiol. 42:183–186. [Google Scholar]
- 4.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209–217. [Google Scholar]
- 5.Curatolo, W. 1987. Glycolipid function. Biochim. Biophys. Acta 906:137–160. [DOI] [PubMed] [Google Scholar]
- 6.de Rosa, M., and A. Gambacorta. 1986. Lipid biogenesis in archaebacteria. Syst. Appl. Microbiol. 7:278–285. [Google Scholar]
- 7.de Rosa, M., E. Esposito, A. Gambacorta, B. Nicolaus, and J. D. bu’Lock. 1980. Effects of temperature on ether lipid composition of Caldariella acidophila. Phytochemistry 19:827–831. [Google Scholar]
- 8.Dugan, L. L., P. Demediuk, C. E. Pendley II, and L. A. Horrocks. 1986. Separation of phospholipids by high-performance liquid chromatography: all major classes, including ethanolamine and choline plasmalogenes, and most minor classes, including lysophosphatidylethanolamine. J. Chromatogr. 378:317–327. [DOI] [PubMed] [Google Scholar]
- 9.Gambacorta, A., A. Gliozzi, and M. de Rosa. 1995. Archaeal lipids and their biotechnological applications. World J. Microbiol. Biotechnol. 11:115–131. [DOI] [PubMed] [Google Scholar]
- 10.Gambacorta, A., A. Trincone, B. Nicolaus, L. Lama, and M. de Rosa. 1994. Unique features of lipids of archaea. Syst. Appl. Microbiol. 16:518–527. [Google Scholar]
- 11.Jansson, P. E., L. Kenne, H. Liedgren, B. Lindberg, and J. Lonngren. 1976. A practical guide to the methylation analysis of carbohydrates. Chem. Commun. Univ. Stockholm 8:1–75. [Google Scholar]
- 12.Kamekura, M., and M. Kates. 1999. Structural diversity of membrane lipids in members of halobacteriaceae. Biosci. Biotechnol. Biochem. 63:969–972. [DOI] [PubMed] [Google Scholar]
- 13.Kates, M. 1993. Biology of halophilic bacteria, part 2. Membrane lipids of extreme halophiles: biosynthesis, function and evolutionary significance. Experientia 49:1027–1936. [DOI] [PubMed] [Google Scholar]
- 14.Kates, M. 1993. Membrane lipids of Archaea, p.261–295. In M. Kates, D. J. Kushner, and A. T. Matheson (ed.), The biochemistry of archaea, vol. 26. Elsevier, New York, N.Y.
- 15.Kates, M., L. S. Yengoyan, and P. S. Sastry. 1965. A diether analog of phosphatidyl glycerophosphate in Halobacterium cutirubrum. Biochim. Biophys. Acta 98:252–268. [DOI] [PubMed] [Google Scholar]
- 16.Koga, Y., M. Akagawa-Matsushita, M. Ohga, and M. Nishihara. 1993. Taxonomic significance of the distribution of component parts of ether lipids in Methanogens. Syst. Appl. Microbiol. 16:342–351. [Google Scholar]
- 17.Koga, Y., M. Nishihara, H. Morii, and M. Akagawa-Matsushita. 1993. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol. Rev. 57:164–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushwaha, S. C., M. Kates, G. D. Sprott, and I. C. P. Smith. 1981. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim. Biophys. Acta 664:156–173. [DOI] [PubMed] [Google Scholar]
- 19.Langworthy, T. A., P. F. Smith, and W. R. Mayberry. 1972. Lipids of Thermoplasma acidophilum. J. Bacteriol. 112:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langworthy, T. A., and J. L. Pond. 1986. Archaebacterial ether lipids and chemotaxonomy. Syst. Appl. Microbiol. 7:253–257. [Google Scholar]
- 21.Lebery, S. B., and S. Hakomori. 1987. Microscale methylation analysis of glycolipids using capillary gas chromatography-chemical ionization mass fragmentography with selected ion monitoring, p.13–25. In V. Ginsburg (ed.), Methods in enzymology, vol. 138. Academic Press Inc., New York, N.Y. [DOI] [PubMed]
- 22.Letter, W. S. 1992. A rapid method for phospholipid class separation by HPLC using an evaporative light-scattering detector. J. Liq. Chromatogr. 15:253–266. [Google Scholar]
- 23.Li, Y.-T., and S.-C. Li. 1978. α-Mannosidase, β-N-acetylhexosaminidase, and β-galactosidase from Jack bean meal, p.702–713. In V. Ginsburg (ed.), Methods in enzymology, vol. 28. Academic Press, Inc., New York, N.Y.
- 24.Martinez-Castro, I., M. I. Paez, J. Sanz, and A. Garcia-Raso. 1989. Gas chromatographic behavior of carbohydrate trimethylsilyl ethers. J. Chromatogr. 462:49–60. [DOI] [PubMed] [Google Scholar]
- 25.Nishihara, M., H. Morii, and Y. Koga. 1987. Structure determination of a quartet of novel polar lipids from Methanobacterium thermoautotrophicum. J. Biochem. 101:1007–1015. [DOI] [PubMed] [Google Scholar]
- 26.Oshima, M., and T. Yamakawa. 1972. Isolation and partial characterization of a novel glycolipid from an extremely thermophilic bacterium. Biochem. Biophys. Res. Commun. 49:185–191. [DOI] [PubMed] [Google Scholar]
- 27.Ray, P. H., D. C. White, and T. D. Brock. 1971. Effect of growth temperature on lipid composition on Thermus aquaticus. J. Bacteriol. 108:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruepp, A., W. Graml, M. L. Santos-Martinez, K. K. Koretke, C. Volker, H. W. Mewes, D. Frishman, S. Stocker, A. N. Lupas, and W. Baumeister. 2000. The genome sequence of the thermoacidophilic scavenger Thermoplasma acidophilum. Nature 407:508–513. [DOI] [PubMed] [Google Scholar]
- 29.Shafiq-ur-Rehman. 1991. Rapid isocratic method for the separation and quantification of major phospholipid classes by high-performance liquid chromatography. J. Chromatogr. 567:29–37. [DOI] [PubMed] [Google Scholar]
- 30.Shimada, H., Y. Shida, N. Nemoto, T. Oshima, and A. Yamagishi. 2001. Quinone profiles of Thermoplasma acidophilum HO-62. J. Bacteriol. 183:1462–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, P. F. 1980. Sequence and glycosidic bond arrangement of sugars in lipopolysaccharide from Thermoplasma acidophilum. Biochim. Biophys. Acta 619:367–373. [DOI] [PubMed] [Google Scholar]
- 32.Sprott, G. D. 1992. Structures of archaebacterial membrane lipids. J. Bioenerg. Biomembr. 24:555–566. [DOI] [PubMed] [Google Scholar]
- 33.Stith, B. J., J. Hall, P. Ayres, L. Waggoner, J. D. Moore, and W. A. Shaw. 2000. Quantification of major classes of Xenopus phospholipids by high-performance liquid chromatography with evaporative light scattering detection. J. Lipid Res. 41:1448–1454. [PubMed] [Google Scholar]
- 34.Sugahara, K., T. Okumura, and I. Yamashina. 1972. Purification of β-mannosidase from a snail, Achatina fulica, and its action on glycopeptides. Biochim. Biophys. Acta 268:488–496. [DOI] [PubMed] [Google Scholar]
- 35.Swain, M., J. R. Brisson, G. D. Sprott, F. P. Cooper, and G. B. Patel. 1997. Identification of β-l-gulose as the sugar moiety of the main polar lipid Thermoplasma acidophilum. Biochim. Biophys. Acta 1345:56–64. [DOI] [PubMed] [Google Scholar]
- 36.Uda, I., A. Sugai, Y. H. Itoh, and T. Itoh. 2000. Characterization of caldarchaetidylglycerol analogs, dialkyl-type and trialkyl-type, from Thermoplasma acidophilum. Lipids 35:1155–1157. [DOI] [PubMed] [Google Scholar]
- 37.Uda, I., A. Sugai, K. Kon, S. Ando, Y. H. Itoh, and T. Itoh. 1999. Isolation and characterization of novel neutral glycolipids from Thermoplasma acidophilum. Biochim. Biophys. Acta 1439:363–370. [DOI] [PubMed] [Google Scholar]
- 38.Wieslander, A., A. Christiansson, L. Rilfors, A. Khan, L. B.-A. Johansson, and G. Lindblom. 1981. Lipid phase structure governs the regulation of lipid composition in membranes of Acholeplasma laidlawii. FEBS Lett. 124:273–278. [Google Scholar]
- 39.Yang, L. L., and A. Haug. 1979. Structure of membrane lipids and physico-biochemical properties of the plasma membrane from Thermoplasma acidophilum, adapted to growth at 37°C. Biochim. Biophys. Acta 573:308–320. [DOI] [PubMed] [Google Scholar]
- 40.Yasuda, M., H. Oyaizu, A. Yamagishi, and T. Oshima. 1995. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl. Environ. Microbiol. 61:3482–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]