Abstract
Gene vmrA, cloned from Vibrio parahaemolyticus, made Escherichia coli resistant to 4prime;,6-diamino-2-phenylindol, tetraphenylphosphonium chloride, acriflavine, and ethidium bromide. VmrA belongs to the DinF branch of MATE family efflux transporters. VmrA catalyzed acriflavine efflux and showed Na+/drug transporter activity because the addition of tetraphenylphosphonium to Na+-loaded cells caused Na+ efflux.
Drug resistance in bacterial cells is currently a serious clinical problem. In particular, it is extremely difficult to treat patients infected with multidrug-resistant bacteria. There are several mechanisms of drug resistance in bacterial cells, including degradation or modification of the drug, alteration of the target, or efflux of the drug from cells. However, the presence of multidrug efflux pumps in bacterial cells is a major cause of multidrug resistance. Large numbers of multidrug efflux pumps have been reported in numerous bacteria (19). Once a bacterium acquires certain multidrug efflux pump(s), or if a silent or weak multidrug efflux pump is activated, then the cell instantly becomes resistant to many antimicrobial agents. Thus, it is important to investigate multidrug efflux pumps in bacteria to gain insight into multidrug resistance in bacteria. Gene cloning, expression, and biochemical characterization are useful approaches to the understanding of multidrug efflux pumps.
V. parahaemolyticus is a slightly halophilic marine bacterium and is one of the major causes of food poisoning in Japan and many other countries (14). This microorganism requires Na+ for its growth (2). Energy metabolism and energy coupling in membranes of this microorganism are unique (21, 26). Cells of V. parahaemolyticus utilize an electrochemical potential of Na+ across the membrane as one of the major driving forces for energy-dependent membrane processes (1, 26). Interestingly, cells of V. parahaemolyticus show some natural resistance to some (or many) antimicrobial agents (unpublished observation). Thus, we were interested in multidrug efflux pumps of V. parahaemolyticus.
Previously, we reported the gene cloning and characterization of NorM, a member of new class of a multidrug efflux pump, from V. parahaemolyticus (16), and we reported that NorM is a Na+-driven Na+/drug antiporter (15). Here we report the gene cloning and characterization of a new multidrug efflux pump, VmrA, from V. parahaemolyticus and that VmrA is a member of a novel class of Na+/drug antiporters.
Host strain E. coli KAM32 and gene cloning.
It has been reported or suggested that E. coli cells possess many multidrug efflux pumps and putative multidrug efflux pumps (19). The major multidrug efflux pump in E. coli is the AcrAB system (10). We previously constructed a mutant strain of E. coli, KAM3, which lacks AcrAB and a restriction system (Δhsd [16]). The KAM3 strain was shown to be very useful for the cloning of multidrug efflux pumps from other bacteria (12, 13, 16). We also reported that YdhE from E. coli was a multidrug efflux pump and a homologue of NorM from V. parahaemolyticus (16). We later found that NorM was a Na+-driven Na+/drug antiporter (15). Also, we found that YdhE was a Na+/drug antiporter (unpublished results). Most multidrug efflux pumps in E. coli are H+-driven H+/drug antiporters. Thus, for the analysis within E. coli cells of Na+-dependent multidrug efflux pumps derived from other bacteria, it is desirable to use an E. coli mutant lacking YdhE, in addition to the AcrAB, as a host cell. Thus, we tried to construct a mutant lacking YdhE from KAM3. Previously, we cloned the ydhE gene into pBR322 and obtained pMEC2 (16). An NcoI fragment was removed from the ydhE gene in pMEC2 by NcoI digestion and self-ligation, and pMDEC2 was obtained. The disrupted ydhE region was transferred to a delivery vector pKO3 (9) and pKOEY2 was obtained. The pKOEY2 plasmid was introduced into KAM3 cells; chromosomal ydhE was replaced with the disrupted ydhE gene by homologous recombination (9), and strain KAM32 was obtained. Disruption of the ydhE gene in the chromosome of KAM32 was confirmed by the Southern blot hybridization method (24) (data not shown). We observed a slight reduction in the MICs of norfloxacin, kanamycin, and streptomycin in KAM32 cells compared with KAM3 cells (data not shown). These results were consistent with our previous results that introduction of the ydhE gene into KAM3 cells slightly increased the MICs of norfloxacin, kanamycin, and streptomycin (16). This indicated that KAM32 was a useful host for the cloning of multidrug efflux pumps from other organisms.
By using strain KAM32 as a host, plasmid pBR322 as a vector, and V. parahaemolyticus AQ3334 (26) as a source of chromosomal DNA, a gene responsible for ethidium bromide resistance was cloned from V. parahaemolyticus as follows. Cells of V. parahaemolyticus were grown in Luria-Bertani medium (11). Chromosomal DNA was prepared from cells of V. parahaemolyticus by the method of Berns and Thomas (3). The DNA was partially digested with Sau3AI, and fragments of 4 to 10 kbp were separated by sucrose density gradient centrifugation. The DNA fragments were ligated into pBR322 (which had been digested with BamHI and dephosphorylated with bacterial alkaline phosphatase) by using T4 DNA ligase. Competent cells (6) of E. coli KAM3 were transformed with the ligated hybrid plasmids and were spread onto agar plates containing L broth (8), 10 μg of ethidium bromide/ml, 60 μg of ampicillin/ml, and 1.5% agar. The plates were incubated at 37°C for 24 h, and the clones formed were picked up. Plasmids contained in the transformants were isolated, reintroduced into KAM3 cells, and spread onto the same plates again. The plates were incubated at 37°C for 24 h. Plasmids contained in the retransformants were prepared. Restriction patterns of selected 24 plasmids were compared, and they were classified into four groups. KAM32 cells harboring one of the groups formed the largest colonies. We further analyzed this group of plasmids (10 plasmids). It seemed that the resistance system encoded by the gene carried by this group of plasmids was the major system for ethidium resistance, as judged based on the size of colonies formed on plates containing ethidium bromide. We picked a plasmid carrying the shortest DNA insert, pVCJ6.
Drug specificity.
We tested drug specificity with KAM32 cells harboring plasmid pVCJ6. Table 1 shows that KAM32/pVCJ6 is more resistant not only against ethidium bromide but also against 4prime;,6prime;-diamino-2-phenylindole (DAPI), tetraphenylphosphonium chloride (TPPCl), and acriflavine compared with the KAM32 control. The structures of these compounds are different. Thus, it seems that plasmid pVCJ6 carries a gene(s) responsible for multidrug resistance. On the other hand, cells of KAM32 and KAM32/pVCJ6 showed indistinguishable susceptibilities to other antimicrobial agents tested, such as norfloxacin, tetracycline, erythromycin, streptomycin, chloramphenicol, and so on (Table 1).
TABLE 1.
Drug specificity in VmrA
| Drug | MIC (μg/ml)
|
|
|---|---|---|
| KAM32 | KAM32/pVCJ6 | |
| DAPI | 0.25 | 128 |
| TPPCl | 8 | 128 |
| Acriflavine | 2 | 32 |
| Ethidium bromide | 4 | 16 |
| Chloramphenicol | 0.5 | 0.5 |
| Norfloxacin | 0.03 | 0.03 |
| Rhodamine 6G | 8 | 8 |
| Tetracycline | 0.5 | 0.5 |
| Erythromycin | 4 | 4 |
| Streptomycin | 2 | 2 |
| Sodium deoxycholate | 2 | 2 |
| Sodium dodecyl sulfate | 0.1 | 0.11 |
Cells of V. parahaemolyticus AQ3334 showed considerable resistance against ethidium bromide, DAPI, TPPCl, and acriflavine (MICs of 16 to 256 μg/ml) and susceptibility to norfloxacin, tetracycline, and erythromycin (MICs of 0.12 to 0.5 μg/ml). Thus, VmrA may be functional in cells of V. parahaemolyticus AQ3334.
Sequence analysis.
We constructed a series of deletion plasmids carrying various portions of the DNA insert in plasmid pVCJ6. The DNA insert in plasmid pVCJ6 was digested with several restriction endonucleases and subcloned into pSTV28 (a vector plasmid carrying chloramphenicol resistance marker [TaKaRa Co.]). The resulting hybrid plasmids were introduced into KAM32 cells, and the transformants were tested for sensitivity or resistance to ethidium bromide. Thus, plasmids pVCJ60, pVCJ61, pVCJ62, pVCJ63, pVCJ64, pVCJ69, and pVCJ7 were obtained (Fig. 1). The presence of DNA inserts in the plasmids was confirmed by single or double digestion with restriction enzymes. We tested the drug susceptibility in cells of KAM32 harboring each plasmid (Fig. 1). Thus, we localized the gene responsible for ethidium resistance to a short DNA region. Next, we determined the nucleotide sequence (22) of this region by using a DNA sequencer (ALF Express; Pharmacia Biotech).
FIG. 1.
Plasmids and restriction maps of cloned V. parahaemolyticus DNA containing the vmrA gene. Physical maps of the DNA inserts derived from the V. parahaemolyticus chromosome in pVCJ6 and its derivatives are shown. Restriction sites determined in pVCJ6 are shown. The growth capabilities of E. coli KAM32 cells harboring each plasmid in L medium containing 10 μg of ethidium bromide/ml are shown on the right. +, Cells grew; −, cells did not grow. The position and direction of the vmrA gene revealed by sequencing are shown at the bottom.
The nucleotide sequence data reported in this study has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB063282.
We found an open reading frame preceded by a Shine-Dalgarno sequence (23) in this region and designated the open reading frame vmrA (vibrio’s multidrug resistance). Several promoter-like sequences (18, 20) were present upstream from vmrA. The vmrA gene consisted of 1,341 nucleotides, with a deduced polypeptide (VmrA) consisting of 447 amino acid residues with a calculated molecular mass of 49 kDa. VmrA was very rich in hydrophobic residues, indicating that the protein was an integral membrane protein.
Hydropathy analysis by the method of Eisenberg et al. (5) revealed that VmrA possessed 12 hydrophobic regions that may be transmembrane domains (data not shown).
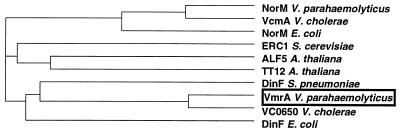
We searched for amino acid sequence homology between VmrA and the reported sequences in a protein sequence database (SwissProt). We found one hypothetical protein (VC0650) suggested from the genome sequence of V. cholerae, which showed high sequence similarity (identity, 63%; similarity, 77%) with VmrA (7). Judging from the overall sequence similarity, VmrA is classified as a member of MATE family drug efflux pumps (4). Brown et al. classified the MATE family into three subfamilies (clusters) (4). Figure 2 shows a dendrogram for representatives of the three subfamilies of the MATE family. Subfamily 1 includes NorM, subfamily 2 includes ERC1, and subfamily 3 includes DinF (4). Judging from the dendrogram, we believe that the VmrA is a member of subfamily 3 (DinF subfamily), which has not so far been shown to contain drug efflux transporters.
FIG. 2.
Dendrogram for three subfamilies of the MATE family. The dendrogram was obtained by using CLUSTAL W and Decypher of Stanford University computer system (http://dna.stanford.edu/projects.html). Classification of the MATE family into three subfamilies (clusters) is from Brown et al. (4). NorM bolongs to the subfamily 1, ERC1 belongs to the subfamily 2, and DinF belongs to the subfamily 3 (DinF subfamily).
Acriflavine efflux.
We tested whether VmrA is really a drug efflux pump. Some fluorescent antimicrobial agents such as ethidium bromide are commonly used for the measurement of efflux via multidrug efflux pumps (15). We tried to investigate whether VmrA is really a drug efflux pump by using fluorescent substrates. Although ethidium seemed to be a substrate for the VmrA system as described above, we were able to detect only small changes in fluorescence intensity of ethidium when an energy inhibitor such as CCCP (carbonyl cyanide m-chlorophenylhydrazone) was added to the assay mixture (data not shown). However, our results with ethidium clearly indicated that VmrA was a drug efflux pump. It was hard to characterize VmrA with ethidium as a substrate because of the low efflux activity. Thereafter, we tried to use DAPI and acriflavine as fluorescent probes, both of which seemed to be fairly good substrates of VmrA (Table 1). We found it difficult, however, to clearly measure accumulation and efflux of DAPI, which seemed to be the best substrate for VmrA (Table 1). On the other hand, the use of acriflavine gave us good results, as shown below.
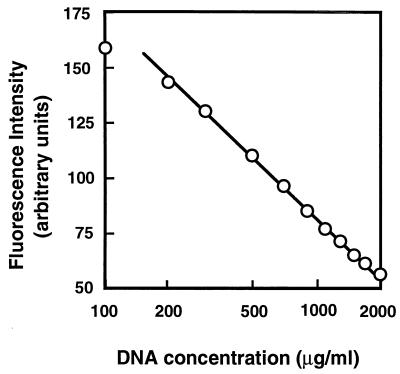
In contrast to what we observed with ethidium, the addition of DNA to an acriflavine solution decreased the fluorescence intensity (Fig. 3). This suggests that the binding of acriflavine to DNA decreases the fluorescence intensity of acriflavine. There was a good correlation between DNA concentration (exponential) and the decrease in fluorescence intensity. Thus, it is anticipated that the accumulation of acriflavine in cells elicits the binding of acriflavine to DNA and results in a decrease in fluorescence. Efflux of acriflavine from cells decreases intracellular acriflavine and will cause dissociation of acriflavine from DNA and will result in an increase in the fluorescence.
FIG. 3.
Effect of DNA concentration on the fluorescence intensity of acriflavine. The assay mixture contained modified Tanaka medium (25) (Na+ salts were replaced with K+ salts) and 1.25 μg of acriflavine/ml. DNA was added to the assay mixture at the indicated concentrations, and the fluorescence of acriflavine was measured at the excitation wavelength of 468 nm and the emission wavelength of 499 nm.
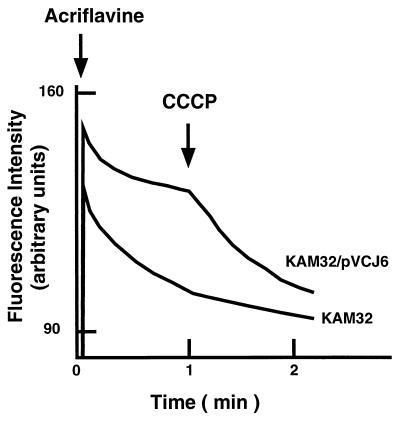
As shown in Fig. 4, we observed a clear decrease in acriflavine fluorescence when CCCP was added to a cell suspension of KAM32/pVCJ6, indicating that the accumulation of acriflavine took place after the addition of CCCP. On the other hand, we observed little change in fluorescence caused by the addition of CCCP with cells of KAM32 (Fig. 4). The final levels of the fluorescence intensities after the addition of CCCP were similar in the two strains, indicating that the accumulation levels of acriflavine in both strains are similar under deenergized conditions. An important point is that the acriflavine accumulation level in cells of KAM32/pVCJ6 was much lower than that in cells of KAM32. This indicates that cells of KAM32/pVCJ6, but not of KAM32, possess energy-dependent acriflavine efflux activity. Therefore, we conclude that VmrA is an energy-dependent drug efflux pump.
FIG. 4.
Acriflavine efflux-accumulation assay. Cells of E. coli KAM32 or KAM32/pVCJ6 were grown in L medium (8) to the late exponential phase of growth under aerobic conditions at 37°C. The cells were then harvested, washed with the modified Tanaka medium (25) supplemented with 2 mM MgSO4, and suspended in the same medium supplemented with 15 mM NaCl. After preincubation at 25°C for 4 min, acriflavine (1.25 μg/ml) was added to initiate the assay, and the fluorescence intensity was monitored as indicated in the legend for Fig. 2. A H+ conductor CCCP (30 μM) was added at time point indicated by a downward arrow to deenergize the membrane. The downward deflection in the pattern indicates accumulation of acriflavine in the cells.
We tested whether the observed energy-dependent efflux of acriflavine from cells via VmrA was stimulated with Na+. NaCl stimulated the efflux activity, and increasing the concentrations of NaCl up to 15 mM resulted in increasing efflux activity (data not shown). The efflux activity decreased at concentrations of NaCl higher than 20 mM. Efflux activity of VmrA was very weak in the absence of NaCl. The addition of LiCl (15 mM) instead of NaCl to the assay mixture resulted in some stimulation of the efflux activity, and the addition of KCl (15 mM) resulted in no stimulation (data not shown). Thus, we conclude that VmrA is an Na+(Li+)-dependent efflux pump.
VmrA is an Na+/drug antiporter.
We reported previously that NorM of V. parahaemolyticus is an Na+/drug antiporter (15). We tested whether VmrA is also an Na+/drug antiporter. First, we loaded cells with Na+ to test this possibility. E. coli cells possess the inducible Na+/melibiose symporter, MelB (17, 27). Cells of E. coli KAM32/pVCJ7 (possessing vmrA) and of KAM32/pSTV28 (control) were grown in the presence of melibiose to induce MelB expression. Methyl-β-d-galactopyranoside (MβGal), a substrate of MelB (28), was added to the cell suspension to elicit accumulation of Na+ in the cells (Fig. 5). After a plateau level of Na+ accumulation was attained due to the symport of MβGal and Na+, TPPCl (a substrate of VmrA) was added to the cell suspension. Strong efflux of Na+ was elicited in KAM32/pVCJ7 cells, and a slight efflux was observed with KAM32/pSTV28 cells (Fig. 5). These results indicate that VmrA is a Na+/drug antiporter. It is suggested from these results that cells of E. coli KAM32 possess slight Na+/TPP+ antiport activity, which is due to neither AcrAB nor YdhE. Thus, it is likely that there is a weak Na+-coupled drug efflux pump(s) in E. coli other than YdhE. It should be noted that other substrates for the VmrA than TPPCl gave unfavorable effect on the Na+-electrode.
FIG. 5.
Na+ efflux from Na+-loaded cells elicited by an inwardly directed TPP+ gradient. Cells of E. coli KAM32/pSTV28 or E. coli KAM32/pVCJ7 were grown in the modified Tanaka medium supplemented with 1% tryptone, 10 μg of chloramphenicol/ml, and 10 mM melibiose at 30°C under aerobic conditions. It is important to grow cells at this temperature because MelB of E. coli K-12 strains is labile at 37°C (17, 27). Cells were harvested at the late exponential phase of growth, washed twice with 0.1 M morpholinepropanesulfonic acid-tetramethylammonium hydroxide (TMAH; pH 7.0), and resuspended in the same buffer. A portion of this suspension (0.5 ml) was diluted with 2.5 ml of 0.1 M Tricine-TMAH (pH 8.0; ca. 20 mg of protein/ml). NaCl was added to give a final concentration of 33 μM. Cells were incubated at 30°C in a plastic vessel with rapid stirring, and water-saturated N2 gas was introduced continuously to maintain anaerobic conditions. An Na+-electrode (Radiometer, Copenhagen, Denmark) and a reference electrode were put into the vessel (28). The first arrow indicates when an anaerobic solution of MβGal (final concentration, 5 mM) was added to the cell suspension to elicit Na+ uptake into cells. The second arrow indicates when an anaerobic solution of TPPCl (final concentration, 0.5 mM) was added to the assay mixture. Upward deflection represents the uptake of Na+, and downward deflection represents the efflux of Na+. Calibration was carried out by the addition of known amounts of NaCl.
The drug efflux pumps described thus far utilize either an electrochemical potential of H+ across cell membrane (H+/drug antiporter) or ATP as energy sources (19). NorM of V. parahaemolyticus was found to be an Na+/drug antiporter, which was the first example of this in the biological world (15). VmrA is the second example of an Na+/drug antiporter.
Recently, we cloned a gene very similar to the vmrA gene from chromosomal DNA of V. cholerae non-O1, nonhalophilic bacterium (M. Nazmul Huda et al., unpublished data). The gene seemed to correspond to a hypothetical VC0650 of V. cholerae O1 El Tor (7), a nonhalophilic bacterium. Thus, it seems that the Na+-driven multidrug efflux pump VmrA is not specific for halophilic V. parahaemolyticus.
The present study is the first report that shows that a member of subfamily 3 (DinF subfamily) of the MATE family is a drug efflux transporter.
Acknowledgments
We thank Manuel Varela of Eastern New Mexico University for critical reading of the manuscript.
REFERENCES
- 1.Atsumi, T., L. McCarter, and Y. Imae. 1992. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature 355:182–184. [DOI] [PubMed] [Google Scholar]
- 2.Baumann, P., and R. H. W. Schubert. 1984. Family II: Vibrionaceae veron, p.516–550. In S. T. Williams, M. E. Sharpe, and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 3.Berns, K. L., and C. A. J. Thomas. 1965. Isolation of high molecular weight DNA from Haemophilus influenzae. J. Mol. Biol. 11:476–490. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M. H., I. T. Paulsen, and R. A. Skurray. 1999. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31:394–395. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg, D., E. Schwarz, M. Komaromy, and R. Wall. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125–142. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580. [DOI] [PubMed] [Google Scholar]
- 7.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennox, E. S. 1955. Transduction of linked genetic characters of host by bacteriophage P1. Virology 1:190–206. [DOI] [PubMed] [Google Scholar]
- 9.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45–55. [DOI] [PubMed] [Google Scholar]
- 11.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual, p.68. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Masaoka, Y., Y. Ueno, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2000. A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J. Bacteriol. 182:2307–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miwatani, T., and Y. Takeda. 1976. Food poisoning due to Vibrio parahaemolyticus in Japan, p.22–25. In T. Miwatani and Y. Takeda (ed.), Vibrio parahaemolyticus, a causative bacterium of food poisoning. Saikon Publishing Co., Tokyo, Japan.
- 15.Morita, Y., A. Kataoka, S. Shiota, T. Mizushima, and T. Tsuchiya. 2000. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182:6694–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prestidge, L. S., and A. B. Pardee. 1965. A second permease for methyl-thio-β-d-galactoside in Escherichia coli. Biochim. Biophys. Acta 100:591–593. [DOI] [PubMed] [Google Scholar]
- 18.Pribnow, D. 1975. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc. Natl. Acad. Sci. USA 72:784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putman, M., V. H. van, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reseberg, M., and D. Court. 1979. Regulatory sequence involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319–353. [DOI] [PubMed] [Google Scholar]
- 21.Sakai, Y., Y. Tamao, T. Shimamoto, H. Hama, M. Tsuda, and T. Tsuchiya. 1989. Cloning and expression of the 5prime;-nucleotidase gene of Vibrio parahaemolyticus in Escherichia coli and overproduction of the enzyme. J. Biochem. 105:841–846. [DOI] [PubMed] [Google Scholar]
- 22.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shine, J., and L. Dalgarno. 1974. The 3prime;-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503–517. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka, S., S. A. Lerner, and E. C. C. Lin. 1967. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J. Bacteriol. 93:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchiya, T., and S. Shinoda. 1985. Respiration-driven Na+ pump and Na+ circulation in Vibrio parahaemolyticus. J. Bacteriol. 162:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchiya, T., J. Lopilato, and T. H. Wilson. 1978. Effect of lithium ion on melibiose transport in Escherichia coli. J. Membr. Biol. 42:45–59. [DOI] [PubMed] [Google Scholar]
- 28.Tshchiya, T., and T. H. Wilson. 1978. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr. Biochem. 2:63–79. [DOI] [PubMed] [Google Scholar]