Abstract
1. Effects of mecamylamine on the spontaneous discharge rate of afferent fibres of carotid body chemoreceptors in vivo and their responses to ACh, NaCN, HCl and hypoxia were studied in sixteen cats.
2. Cats were anaesthetized with sodium pentobarbitone, paralysed with gallamine triethiodide and artificially ventilated. Chemoreceptor excitants were injected into the common carotid artery; mecamylamine was given intravenously.
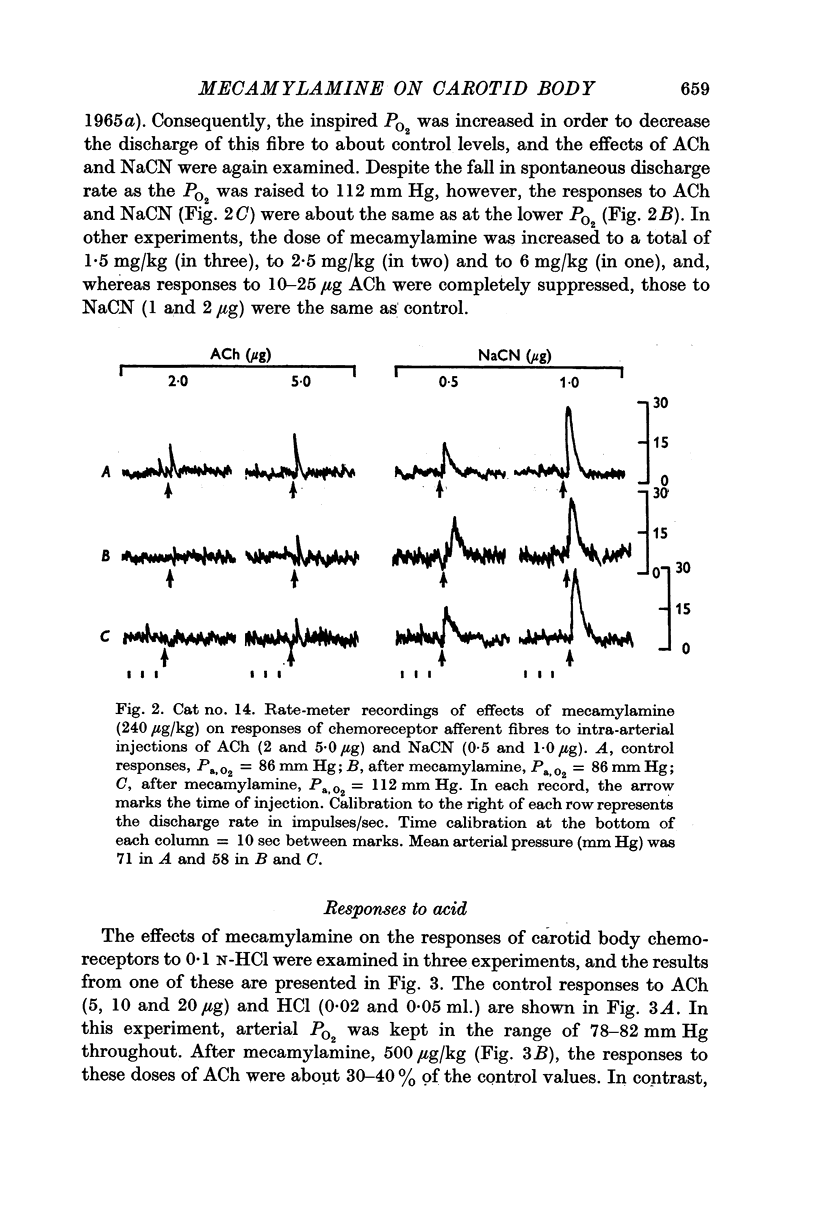
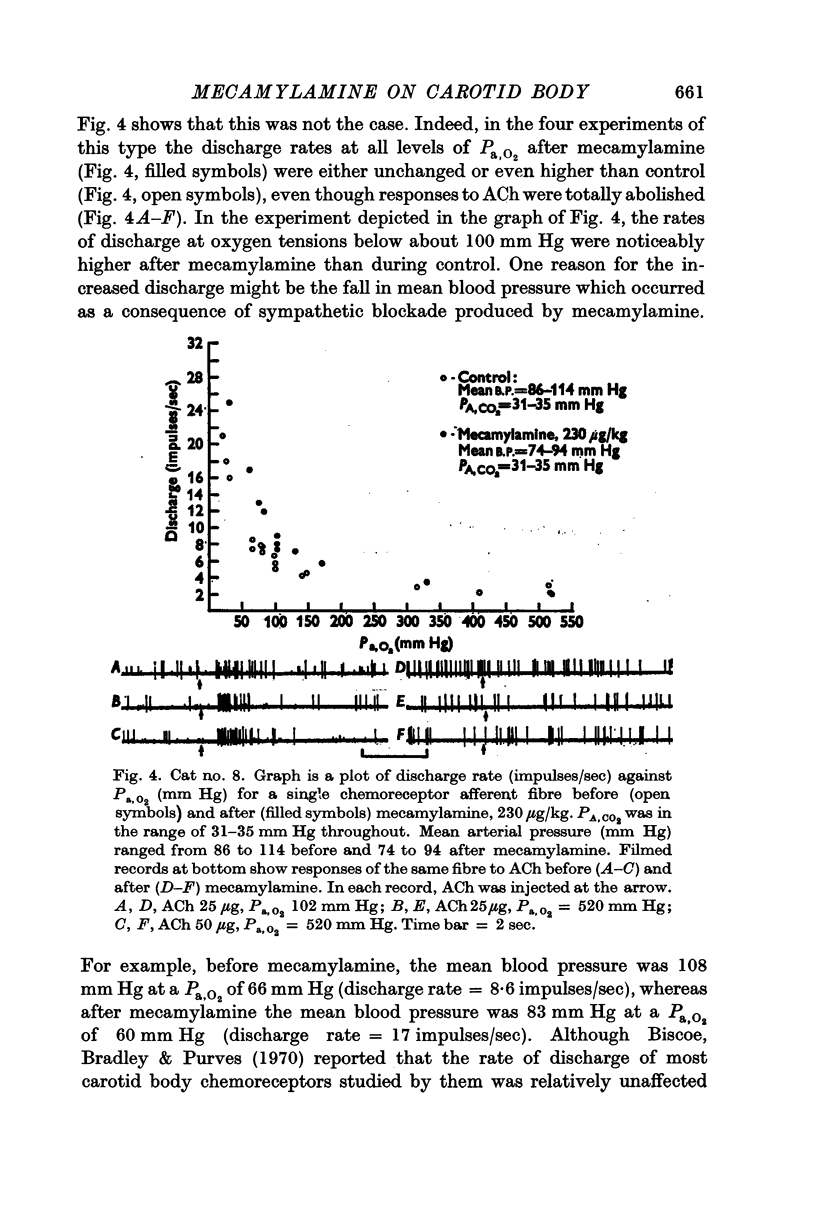
3. Mecamylamine, 230 μg/kg or greater, failed to diminish either the rate of spontaneous discharge of carotid body chemoreceptors at high arterial oxygen tensions (greater than 130 mm Hg), or the responses of these receptors to NaCN (0·5-25 μg), HCl or hypoxic blood.
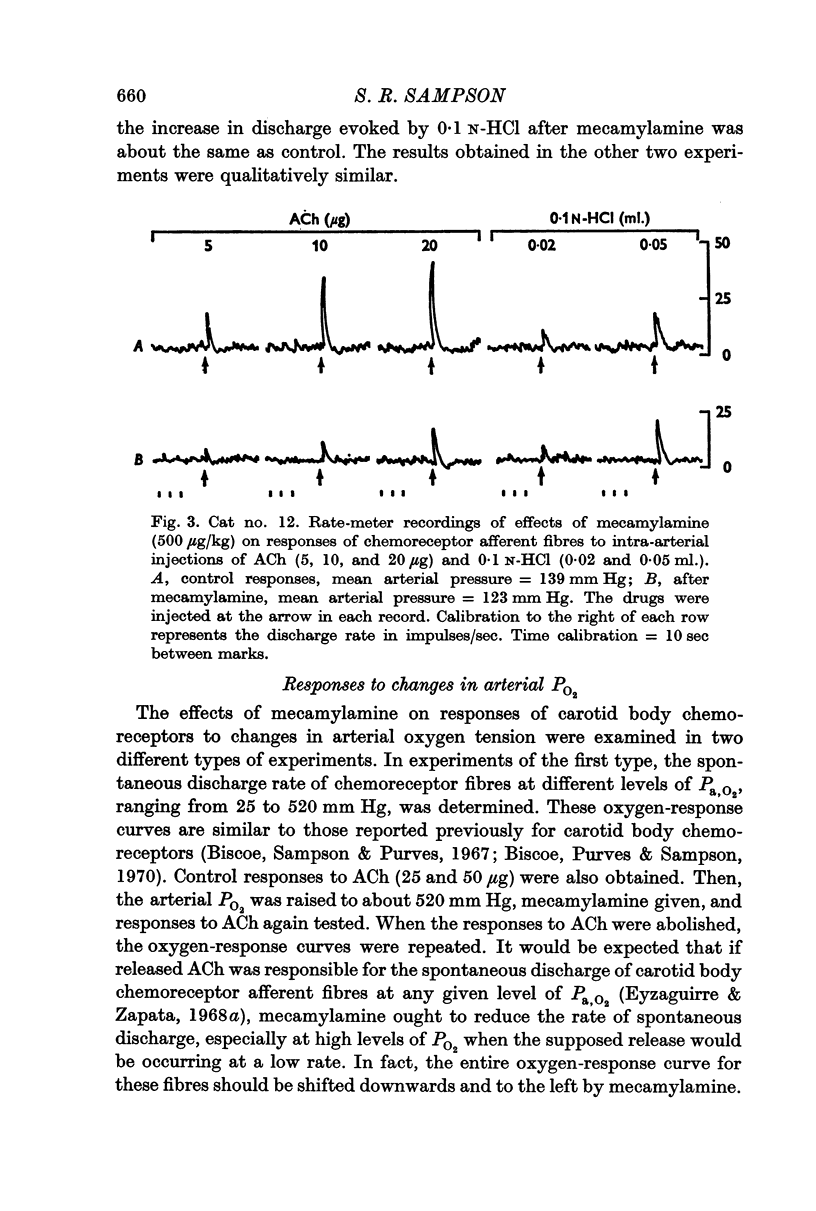
4. Responses of chemoreceptor afferent fibres to ACh (1·0-50 μg) in the same preparations were either completely abolished or considerably reduced by mecamylamine.
5. These data do not support the hypothesis of a cholinergic mechanism for the initiation of chemosensory discharges in the carotid body, either at rest or in response to stimuli such as NaCN, acid or hypoxia.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT G., TYLER C., ZAIMIS E. Mecamylamine and its mode of action. Lancet. 1957 Aug 3;273(6988):218–222. doi: 10.1016/s0140-6736(57)91598-2. [DOI] [PubMed] [Google Scholar]
- BYCK R. The effect of hexamethonium on the carotid chemoreceptor response to nicotine and cyanide. Br J Pharmacol Chemother. 1961 Feb;16:15–22. doi: 10.1111/j.1476-5381.1961.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Bradley G. W., Purves M. J. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol. 1970 May;208(1):99–120. doi: 10.1113/jphysiol.1970.sp009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Lall A., Sampson S. R. Electron microscopic and electrophysiological studies on the carotid body following intracranial section of the glossopharyngeal nerve. J Physiol. 1970 May;208(1):133–152. doi: 10.1113/jphysiol.1970.sp009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Lall A., Sampson S. R. On the nerve endings associated with the carotid body glomus cells of the cat. J Physiol. 1969 Feb;200(2):131P–132P. [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Rhythmical and non-rhythmical spontaneous activity recorded from the central cut end of the sinus nerve. J Physiol. 1968 May;196(2):327–338. doi: 10.1113/jphysiol.1968.sp008510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Spontaneous activity recorded from the central cut end of the carotid sinus nerve of the cat. Nature. 1967 Oct 21;216(5112):294–295. doi: 10.1038/216294a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Stimulus response curves of single carotid body chemoreceptor afferent fibres. Nature. 1967 Aug 5;215(5101):654–655. doi: 10.1038/215654a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W. The effect of a ganglion-blocking drug, hexamethonium, on the response of the cat's carotid body to various stimuli. J Physiol. 1952 Nov;118(3):373–383. doi: 10.1113/jphysiol.1952.sp004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of electrical stimulation on the frequency of chemoreceptor discharges. J Physiol. 1965 Jun;178(3):438–462. doi: 10.1113/jphysiol.1965.sp007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J Physiol. 1965 Jun;178(3):410–437. doi: 10.1113/jphysiol.1965.sp007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H., Taylor J. R. Presence of acetylcholine and transmitter release from carotid body chemoreceptors. J Physiol. 1965 Jun;178(3):463–476. doi: 10.1113/jphysiol.1965.sp007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Zapata P. Pharmacology of pH effects on carotid body chemoreceptors in vitro. J Physiol. 1968 Apr;195(3):557–588. doi: 10.1113/jphysiol.1968.sp008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Zapata P. The release of acetylcholine from carotid body tissues. Further study on the effects of acetylcholine and cholinergic blocking agents on the chemosensory discharge. J Physiol. 1968 Apr;195(3):589–607. doi: 10.1113/jphysiol.1968.sp008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical differentiation of types of cholinesterases and their localizations in tissues of the cat. J Pharmacol Exp Ther. 1950 Oct;100(2):158–179. [PubMed] [Google Scholar]
- Neil E., O'Regan R. G. Effects of sinus and aortic nerve efferents on arterial chemoreceptor function. J Physiol. 1969 Jan;200(1):69P–71P. [PubMed] [Google Scholar]
- STONE C. A., TORCHIANA M. L., NAVARRO A., BEYER K. H. Ganglionic blocking properties of 3-methylaminoisocamphane hydrochloride (mecamylamine): a secondary amine. J Pharmacol Exp Ther. 1956 Jun;117(2):169–183. [PubMed] [Google Scholar]
- Sampson S. R., Biscoe T. J. Efferent control of the carotid body chemoreceptor. Experientia. 1970 Mar 15;26(3):261–262. doi: 10.1007/BF01900082. [DOI] [PubMed] [Google Scholar]


