Abstract
1. Inhibition and excitation of spontaneous phrenic nerve discharges in response to stimulation of the sinus, glossopharyngeal, aortic and superior laryngeal (SLN) nerves has been investigated in cats.
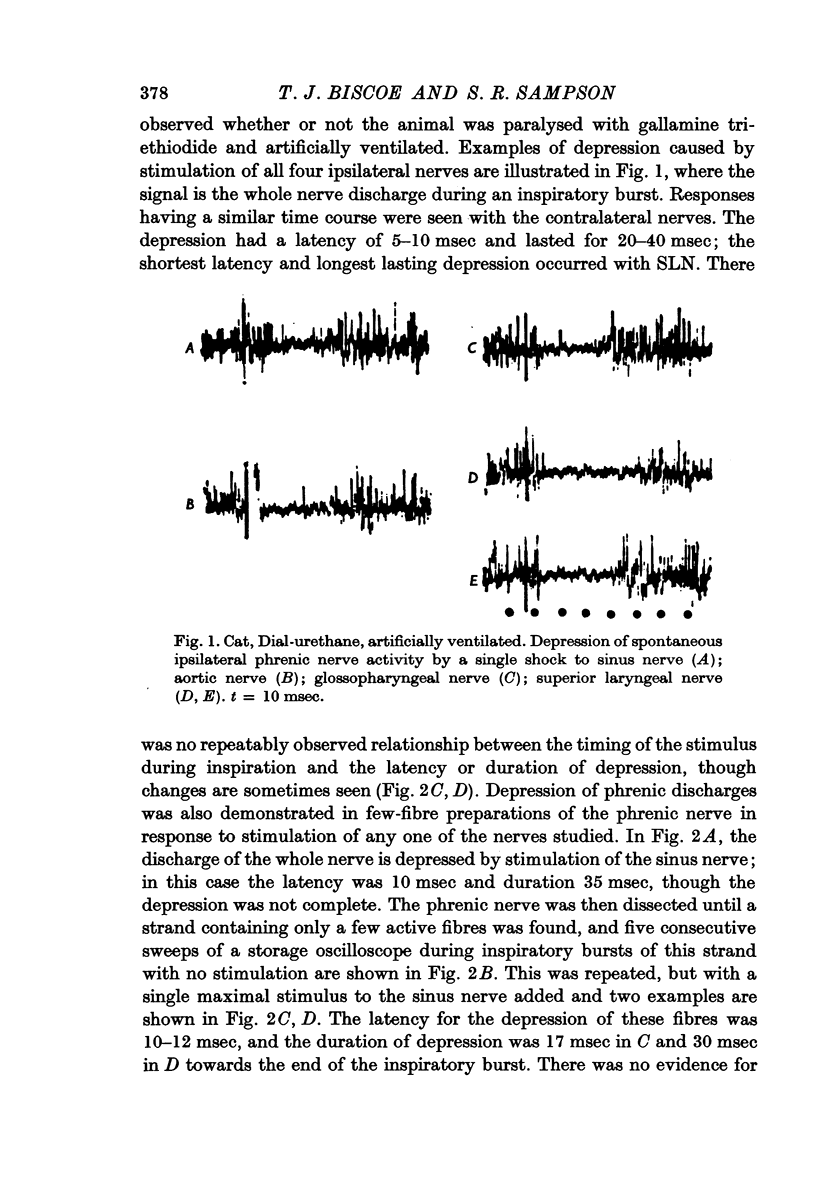
2. The inhibition, in response to a single shock, had a latency of 5-10 msec and lasted for 20-40 msec; the response to SLN stimulation was the most prolonged.
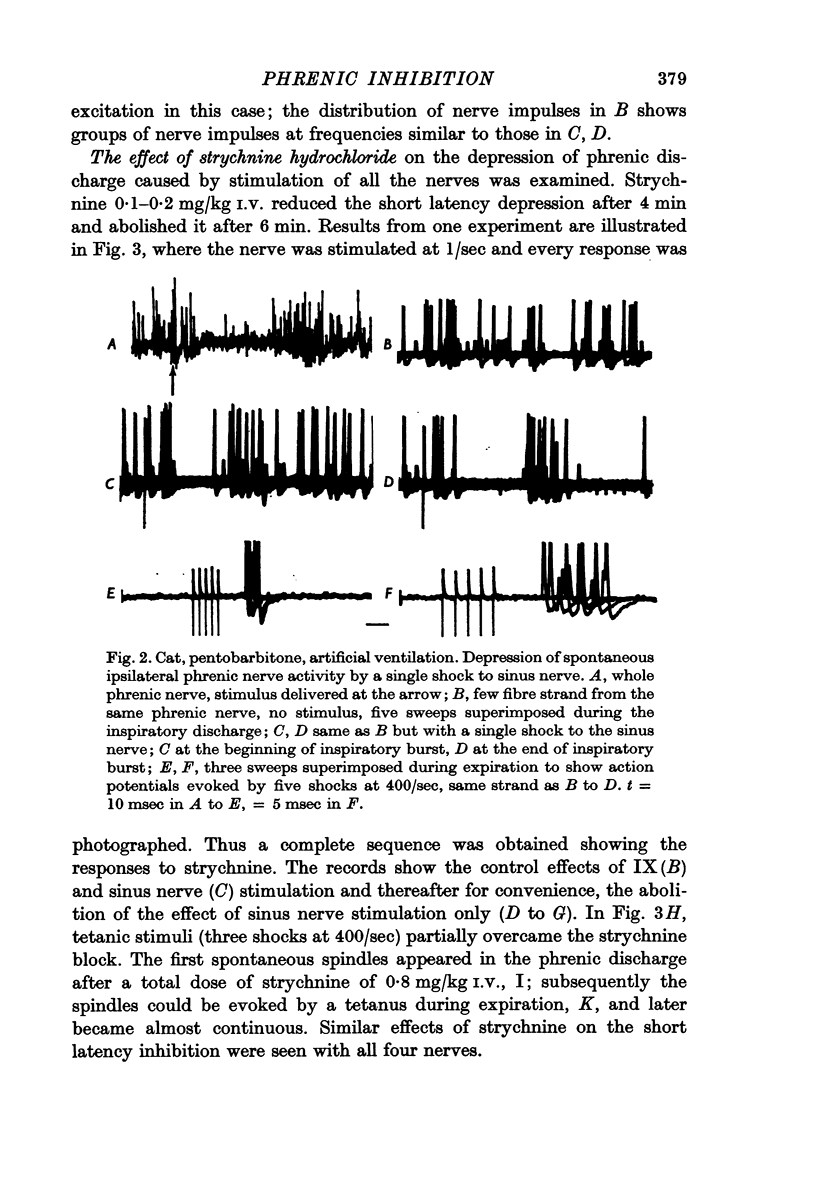
3. Excitation of phrenic motoneurones also occurred and was seen either before or after the end of the inhibition of the inspiratory burst and sometimes also during expiration.
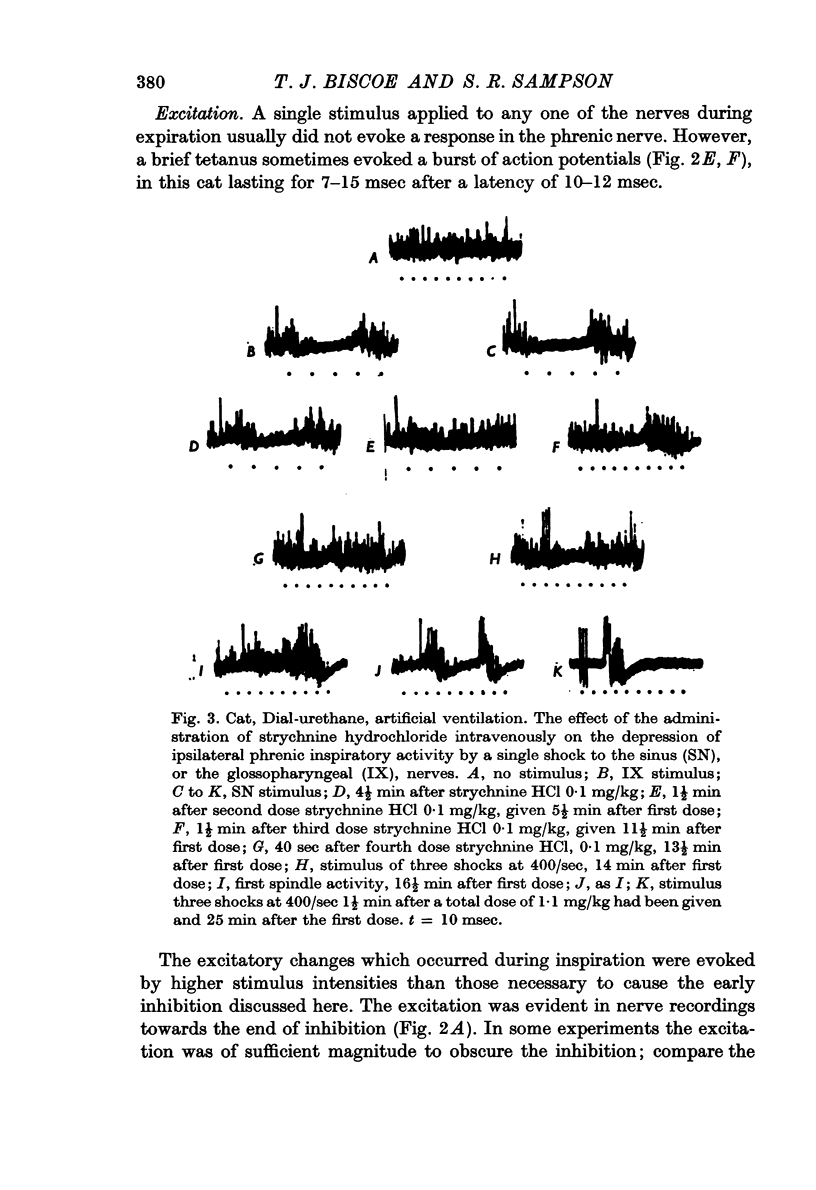
4. Intravenous strychnine blocked the inhibition.
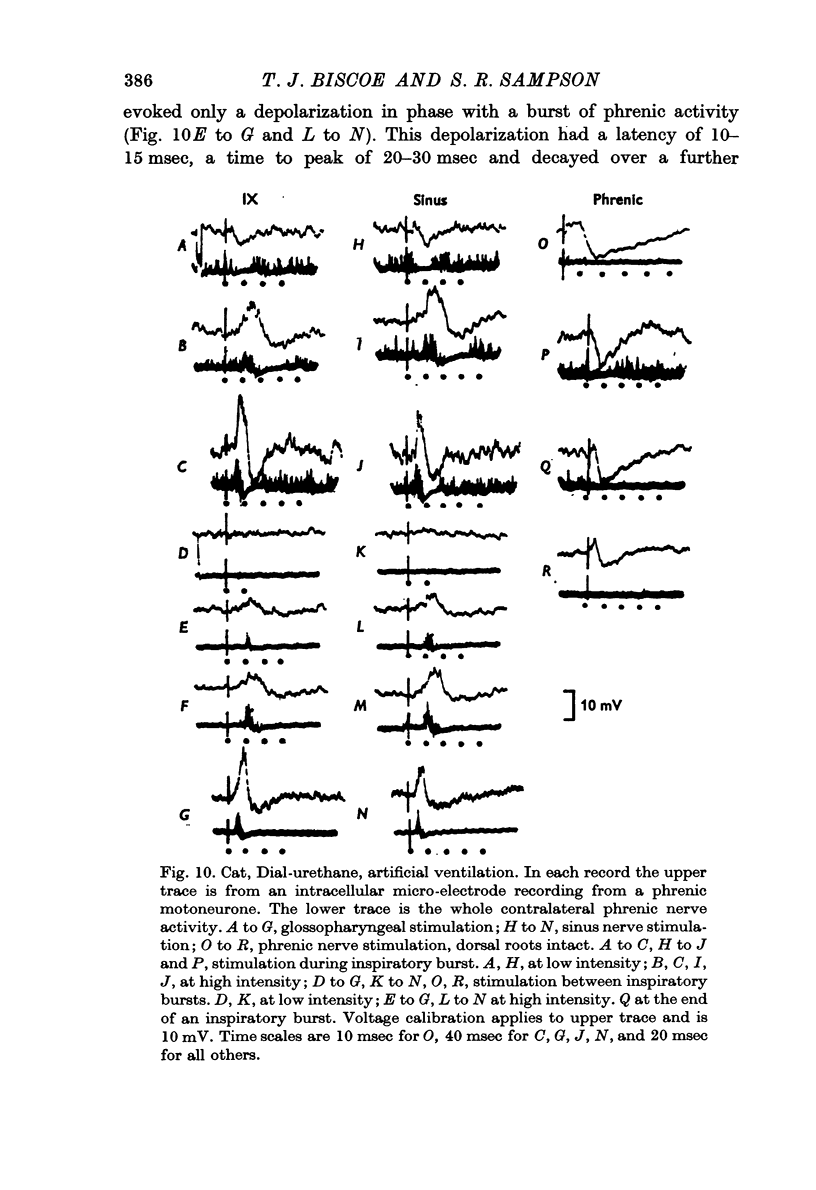
5. Intracellular recording from phrenic motoneurones showed that hyperpolarization was evoked by each nerve during the central phrenic depolarizing potential (CPDP) but only rarely in the interval between these potentials.
6. When the CPDP was suppressed, hyperpolarization could sometimes be evoked.
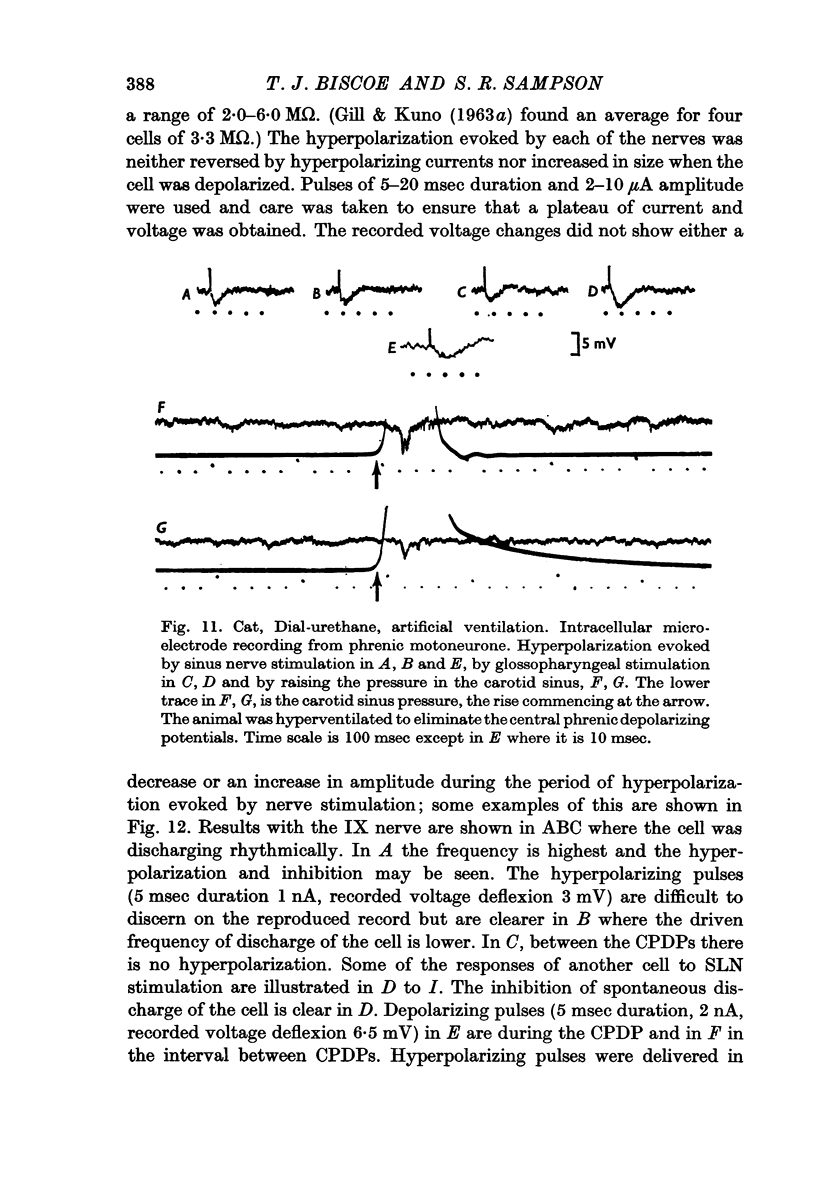
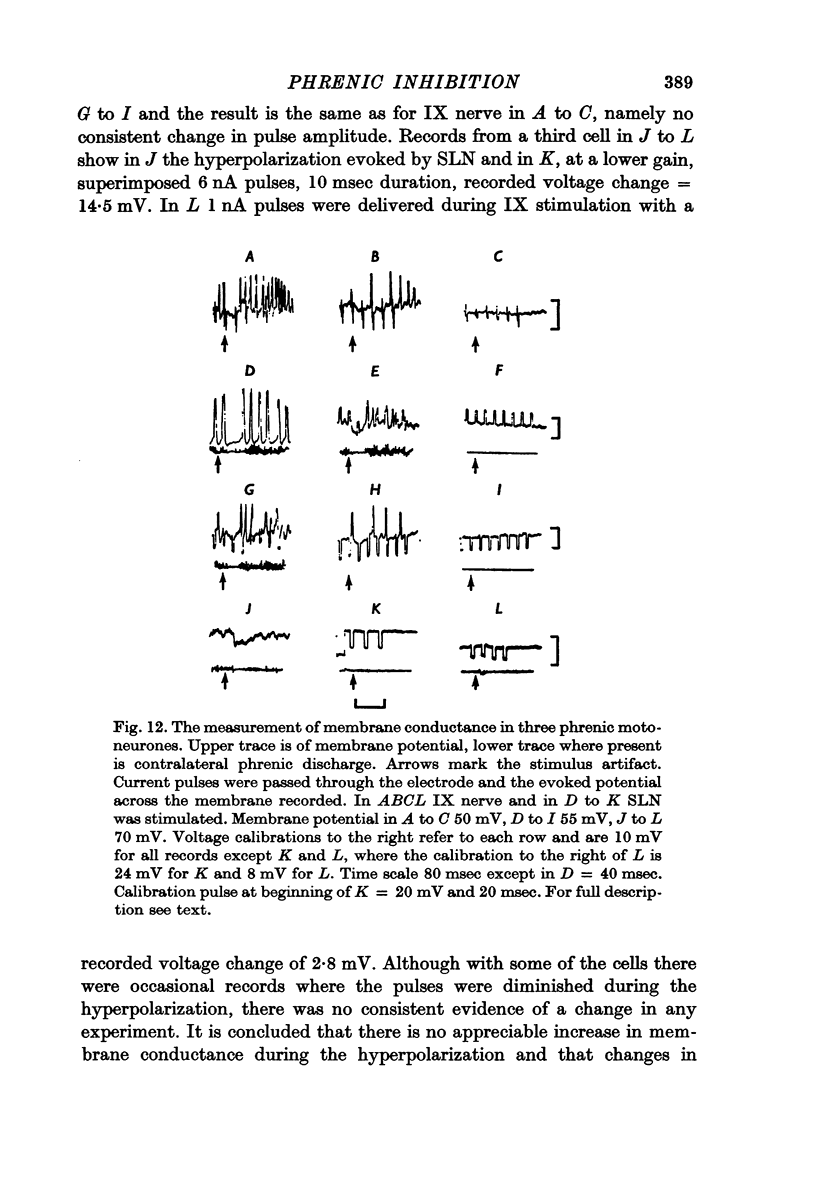
7. There were no changes in amplitude or time course of hyperpolarization during the passage of current through the cell membrane. No change in membrane conductance could be shown by passing current pulses.
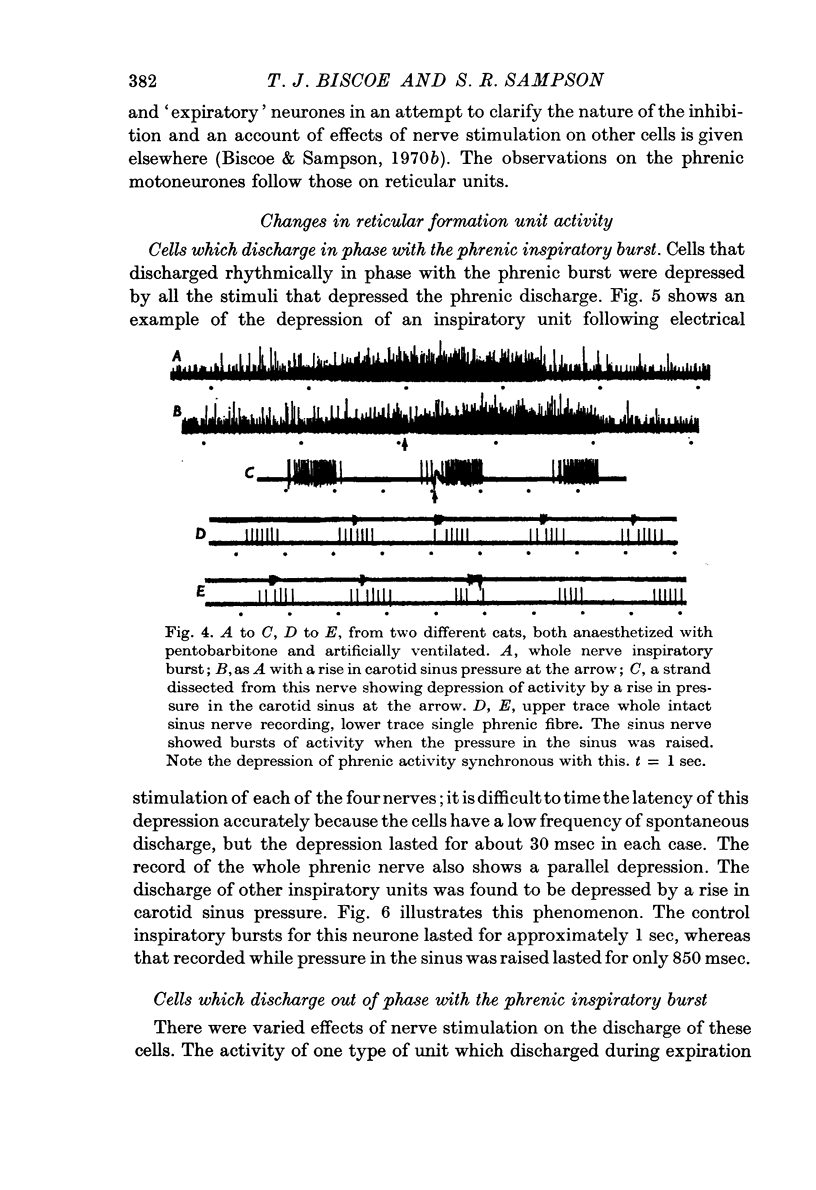
8. Raising the pressure in the carotid sinus also depressed phrenic activity and evoked a hyperpolarization whilst the CPDP was suppressed.
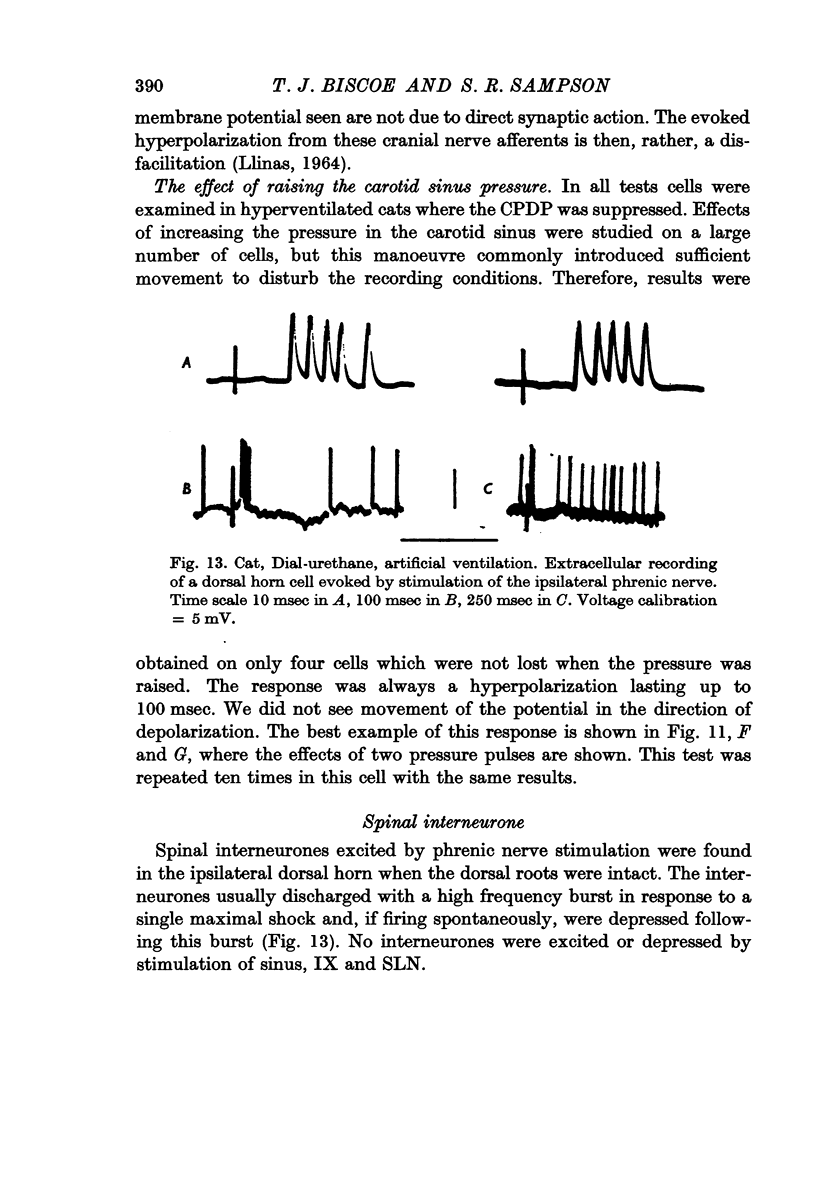
9. Inspiratory interneurones in the brain stem were suppressed by nerve stimulation and by a rise in carotid sinus pressure.
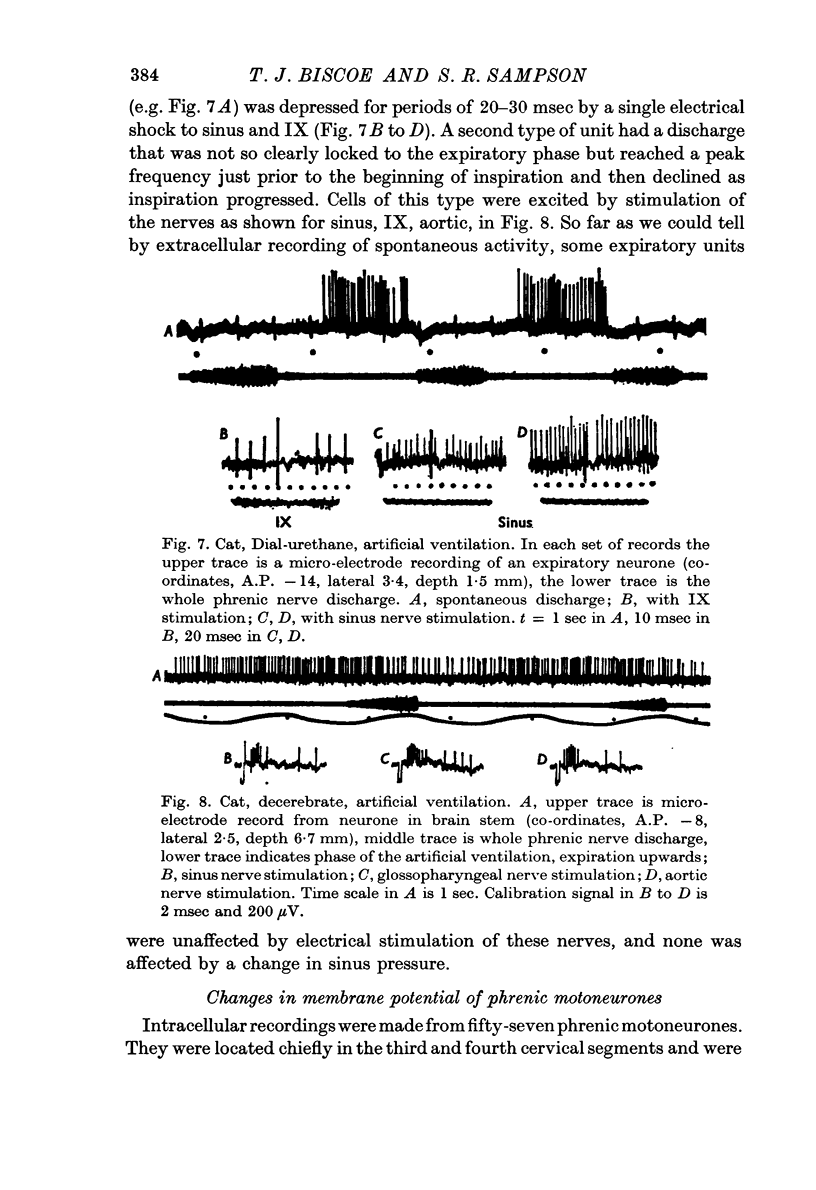
10. Expiratory interneurones in the brain stem were both excited and suppressed by electrical stimuli and unaffected by a change in carotid sinus pressure.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON F. D., BERRY C. M. An oscillographic study of the central pathways of the vagus nerve in the cat. J Comp Neurol. 1956 Nov;106(1):163–181. doi: 10.1002/cne.901060106. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Field potentials evoked in the brain stem of the cat by stimulation of the carotid sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol. 1970 Aug;209(2):341–358. doi: 10.1113/jphysiol.1970.sp009168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Responses of cells in the brain stem of the cat to stimulation of the sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol. 1970 Aug;209(2):359–373. doi: 10.1113/jphysiol.1970.sp009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R. Pharmacological studies on feline Betz cells. J Physiol. 1966 Sep;186(1):121–138. doi: 10.1113/jphysiol.1966.sp008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E., Reis D. J. Distribution of carotid sinus and depressor nerves in cat brain stem. Am J Physiol. 1968 Feb;214(2):269–276. doi: 10.1152/ajplegacy.1968.214.2.269. [DOI] [PubMed] [Google Scholar]
- Decima E. E., von Euler C. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiol Scand. 1969 May-Jun;76(1):148–158. doi: 10.1111/j.1748-1716.1969.tb04459.x. [DOI] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. EXCITATORY AND INHIBITORY ACTIONS ON PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:274–289. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLINAS R. MECHANISMS OF SUPRASPINAL ACTIONS UPON SPINAL CORD ACTIVITIES. DIFFERENCES BETWEEN RETICULAR AND CEREBELLAR INHIBITORY ACTIONS UPON ALPHA EXTENSOR MOTONEURONS. J Neurophysiol. 1964 Nov;27:1117–1126. doi: 10.1152/jn.1964.27.6.1117. [DOI] [PubMed] [Google Scholar]
- Megirian D. Vestibular control of laryngeal and phrenic motoneurons of cat. Arch Ital Biol. 1968 Dec;106(4):333–342. [PubMed] [Google Scholar]
- Miura M., Reis D. J. Electrophysiological evidence that carotid sinus nerve fibers terminated the bulbar reticular formation. Brain Res. 1968 Jul;9(2):394–397. doi: 10.1016/0006-8993(68)90247-3. [DOI] [PubMed] [Google Scholar]
- PORTER R. UNIT RESPONSES EVOKED IN THE MEDULLA OBLONGATA BY VAGUS NERVE STIMULATION. J Physiol. 1963 Oct;168:717–735. doi: 10.1113/jphysiol.1963.sp007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition induced by vagal afferent volleys. J Neurophysiol. 1967 Sep;30(5):964–981. doi: 10.1152/jn.1967.30.5.964. [DOI] [PubMed] [Google Scholar]
- SAMPSON S., EYZAGUIRRE C. SOME FUNCTIONAL CHARACTERISTICS OF MECHANORECEPTORS IN THE LARYNX OF THE CAT. J Neurophysiol. 1964 May;27:464–480. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson S. R., Biscoe T. J., Campion P. D. Effects of sinus nerve stimulation on activity of phrenic motoneurones. Nature. 1968 May 18;218(5142):680–681. doi: 10.1038/218680a0. [DOI] [PubMed] [Google Scholar]
- Sampson S. R., Biscoe T. J. Electrical potentials evoked in the brain stem by stimulation of the sinus nerve. Brain Res. 1968 Jul;9(2):398–402. doi: 10.1016/0006-8993(68)90248-5. [DOI] [PubMed] [Google Scholar]


